Study on ionic liquids based novel method for separation and purification of silkworm pupa protein
Wen Zhng,Shoqi Yng,Bozeng Ren,Xingmei Lu,Ci Ji,*
a Beijing Key Laboratory of Ionic Liquids Clean Process,CAS Key Laboratory of Green Process and Engineering,State Key Laboratory of Multiphase Complex Systems,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
b Zhengzhou Institute of Emerging Industrial Technology,Zhengzhou 450000,China
c Key Laboratory of New Materials and Facilities for Rural Renewable Energy of Ministry of Agriculture and Rural Affairs,College of Mechanical & Electrical Engineering,Henan Agricultural University,Zhengzhou 450002,China
d School of Chemical Engineering,Zhengzhou University,Zhengzhou 450001,China
Keywords:Silkworm pupa protein Ionic liquids Protein backbone Silkworm pupa Separation
ABSTRACT Silkworm pupa protein (SPP) that obtained by traditional method usually had a high fat content,which would impose restrictions on the further use of SPP.Herein,various functionalized ionic liquids (ILs)were used to extract SPP from silkworm pupae,the structure-performance relationship of ILs with their SPP separation performance were explored at the same time.The research showed that the maximum extraction yield of SPP was up to 62.6% with less than 0.5% low fat content by using 1-ethyl-3-methylimidazolium chloride ([Emim]Cl),when the dissolution experiment was conducted at 90°C for 24 h with ethanol bath as the regeneration solvent.Comparing with the structure of raw material,the regenerated SPP maintained the native protein backbone.Meanwhile,all regenerated SPP showed a decreased crystallinity,which also exhibited decreased fraction of the α-helix comparing to that β-sheet united with coil random structures.
The silkwormBombyx moriL.had been used for silk production for about 5000 years [1].As the byproduct of silk reeling process,large quantity of silkworm pupae were commonly used as livestock feed and farm fertilizer with low-value utilization due to lack of further processing technology [2].Since dried silkworm pupa is generally composed of 50%-65% protein,23%-30% fat,4%-8%polysaccharide and rich trace elements [3,4],extracting the main component SPP from silkworm pupae had been getting a remarkable increase of interest.SPP is a kind of natural protein with high value owing to its reasonable and balanced amino acid ratio,good bioactivity and biocompatibility and low toxicity.It is suitable for textiles,pharmaceutical intermediate,health care products [5] surfactants [6],food [7] and cosmetics [8].However,it was not easy to extract pure protein from silkworm pupa.The recalcitrance of this protein,which was almost insoluble and hard to degrade in water and most organic solvents,could directly affect the extraction efficiency [9].This was due to its high density of hydrogen bonds and tight packing of theα-helices andβ-sheets structure [10].Obviously,in order to optimally utilize the pupae material and obtain purified protein,a clean and efficient technology was urgently needed.
Ionic liquids (ILs),they were composed of cations and anions,had been used to efficiently dissolve and extract purified materials from natural biomass such as wood,corn stalk and crustacean shell [11,12].Meanwhile,many reports had published about the application of ILs in dissolving natural protein materials such as wool [13],cocoon silk [14],feather and skin [15].Phillipset al.[16] found that silkworm silk could be dissolved in ILs,for example [Bmim]Cl,which could disrupt the hydrogen bonding network in crystalline domains of silk fibroin.The crystal structure of regenerated film from [Bmim]Cl solution was affected by coagulation bath and methanol bath would induce the formation ofβ-sheet structure.Recently,3% choline hydroxide IL (CH-IL) solution was used as the SPP extraction solvent [17].64.52% of protein recovery rate with 90.45% of protein content could be achieved at 40 °C for 1 h.However,owing to the unstable characteristic of CH-IL,more functional ILs were still needed for efficient separation of SPP from silkworm pupa.As reported by Zhenget al.[18],the dissolution capability of wool keratin in ILs was significantly affected by interaction between cations and anions.In such a case,theα-helix andβ-sheet structures of wool keratin reformed through regeneration.The above performance of ILs could be ascribed to high polarity of the ionic structure.The cation of IL,acted as electron acceptor,complexed with the carbonyl oxygen in protein chain,while the anion associated with hydrogen atom of amino [19].The interaction would disrupt the hydrogen bonds within and between macromolecules,resulting in the dissolution of protein [9].The ability of ILs to disrupt hydrogen bonding provides great possibilities for the separation of SPP from silkworm pupae.
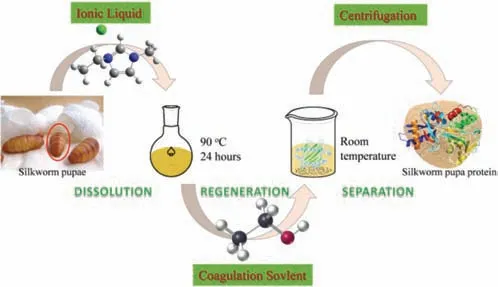
Fig.1.SPP extraction process from silkworm pupae by using ILs.
In this work,an imidazolyl-based IL process was explored for the separation and extraction of SPP from silkworm pupae (Fig.1).The process conditions like dissolution time and temperature,as well as coagulation solvent,were investigated for optimum choice.The structure-performance relationship of ILs in such application was also systematically investigated.The properties and structures of the regenerated protein were characterized by fourier transform infrared spectroscopy (FT-IR),X-ray diffraction analysis (XRD),solid-state13C nuclear magnetic resonance spectroscopy (13C NMR)and thermogravimetric analysis (TGA).
According to our research,ILs had the ability to dissolve silkworm pupae.The qualitative detection of IL solution was then conducted to demonstrate the hypothesis that both the fat and protein,the mainly components of dried silkworm pupae,could be dissolved by ILs.Four kinds of ILs including1-allyl-3-methylimidazoliumchloride([Amim]Cl),1-octyl-3-methylimidazoliumchloride([Omim]Cl),1-ethyl-3-methylimidazoliumchloride([Emim]Cl)and1-ethyl-3-methylimidazolium bromine ([Emim]Br) were used to perform the experiments.Considering the stability and viscosity of ILs,the temperature was arranged at 80°C.The dissolution was stopped after 24 h according to the mixture appearance,which was observed under a light microscope.
By directly testing IL solutions with Coomassie brilliant blue G-250 method,the color change of Coomassie blue staining solution(Fig.S1 in Supporting information) confirmed that SPP was dissolved in these ILs.The ILs solutions were also extracted with ethyl ether and then analyzed with ESI MALDI-TOF MS.As the results presented in Fig.S2 and Table S1 (Supporting information),the species in the ethyl ether solution mainly consisted of five kinds of fatty acids including linolenic acid,oleic acid,palmitic acid,linoleic acid and stearic acid.It confirmed that ILs could dissolve the fat in silkworm pupae [20].
According to the results above,ILs had the ability to dissolve both the protein and fat in silkworm pupae.After silkworm pupa was dissolved into ILs,it was possible to regenerate the protein by simply pouring the biomass/IL solution into excessive amounts of coagulation solvent.As fat could also be dissolved by ILs,the fat content of regenerated protein was affected by experimental conditions.
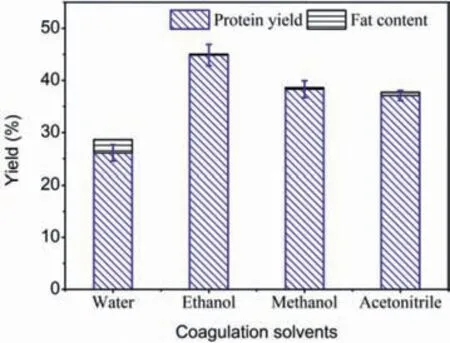
Fig.2.Effect of coagulation solvents on the separation of SPP.
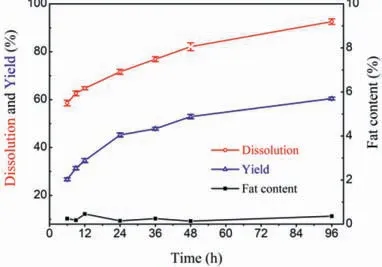
Fig.3.Effect of dissolution time on the separation of SPP.
To compare the role of different coagulation solvents in the extraction of protein,a series of molecular solvents were explored for[Amim]Cl solution.Solvents like water,methanol,ethanol and acetonitrile were proved that which could effectively precipitate the protein as light-yellow product,while aprotic acetone and ethyl acetate failed,owing to the formation of droplets,which were immiscible with their respective coagulation bath and resulting in the protein were not able to coagulate.
Besides,the experimental results in Fig.2 indicated that the fat contents of protein regenerated by methanol,ethanol,acetonitrile and water were 0.3%,0.2%,0.6% and 2.6%,respectively.It was likely due to the first three baths could dissolve much fatter than water,thus much more fat was dissolved in the coagulation bath rather than sticking to the regenerated protein [17].Meanwhile,the data also suggested that the amount of protein extracted from ethanol bath could reach 44.9%,higher than those from other baths.It could be deduced that the stronger polarity of methanol and acetonitrile was unfavorable for the SPP precipitation under certain conditions.Therefore,ethanol was chosen as optimum precipitator for regeneration of SPP in such IL-based process.
To test the effect of dissolution time,0.5 g raw material was added to 10 g [Amim]Cl and dissolved at 80°C.After the dissolution processes,the solution was centrifuged for separation of SPP.The IL solutions were treated with ethanol,the precipitates were washed with ethanol for three times.It was observed that both the dissolution percentage and protein yield increased with the dissolution time (Fig.3).When dissolution time increased from 6 h to 96 h,the dissolution rate of silkworm pupa increased from 58.6% to 92.6%,and the protein yield increased from 26.7% to 60.4%.Noteworthily,the fat contents of the regenerated products kept below 0.5%.The residues in all dissolution cases could attribute to that the complex and compact structure of silkworm pupae powder prevented ILs invading into its interior structure [17].In addition,the yields increased more sharply when the dissolution time increased from 6 to 24 h comparing with that from 24 h to 96 h.This could be attributed to the results of more perfect crystalline structure in residue and the increased viscosity of protein solution,resulting in the increase of mass transfer resistance along with time[17].Taking the yield and dissolution time into consideration,an appropriate dissolution time of 24 h was chosen to evaluate the effect of other process variables on the extraction of protein.
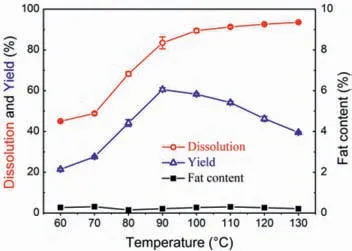
Fig.4.Effect of temperature on the separation of SPP.
The effect of dissolution temperature was also explored by using [Amim]Cl for 24 h.The regeneration of protein was also conducted through precipitation by ethanol with centrifuge separation process.As clearly indicated in Fig.4,with the temperature increasing from 60 to 130°C,the dissolution rate increased from 45.0% to 83.5% and the yield of protein increased from 21.5% to 60.6%,and then decreased to 39.5%.The increase of temperature was favorable for dissolution.High temperature could reduce the viscosity of IL,thus enhancing the diffusion capacity and solubility.However,too high of dissolution temperature would lead to more serious degradation of the macromolecular proteins and the lower molecular weight degradation products remained in ILs after the regeneration process.In addition,the fat contents of each experiment were under 0.5%.According to the results above,choosing 90°C as an optimum temperature for further experiments was advantageous for the extraction of protein.
By integrating the optimum choices for the separation of SPP based on IL,the process of dissolution and regeneration of SPP was established.After dissolving the pupae powder in an IL at 90°C for 24 h,the IL solution was treated with ethanol,precipitating the regenerated materials which were recovered by centrifugal separation process.
The previous research had proved that the ILs characteristics could significantly affect their dissolution and regeneration properties of biomass [17,21].Herein,various cations and anions compositions of ILs were used to perform the studies.
The anion structure of IL was of great importance on the extraction of protein,and the direct effects had been studied in this work.Considering the commercial availability and stability,a series of ILs containing the same [Emim]+cation and a variety of anions were selected to conduct the experiments.As displayed in Fig.5,for each IL,there was an obvious difference between dissolution rate and the protein yield because of the small molecular protein from the original silkworm pupae and the partly degradation of SPP in dissolution process.Such protein would probably retain in ILs after the regeneration process.Besides,the fat contents of the protein regenerated from other ILs were all less than 0.5%.The data summarized in Fig.5 also illustrated that the ability of ILs for the extraction of protein increased in the following order:NTf2-<DCA-<Br-<OAc-<DMP-<DEP-<Cl-.It was found that physicochemical properties of ILs had significant impact on both the dissolving power of ILs and yield of regenerated protein,such as the hydrogen bond basicity (β),viscosity and molar volume (Table 1) of ILs.The yields of protein regenerated from these ILs were all higher than 40% except [Emim]NTf2and [Emim]DCA.The protein yields for the two latter ILs were only 3.3% and 4.3%,which were too low for the measurement of fat content.
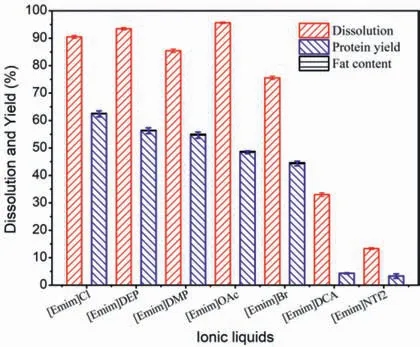
Fig.5.Effects of the ILs anions on the separation of SPP.
[Emim]NTf2and [Emim]DCA withβvalves less than 0.5 were less effective agents,indicating thatβvalve played the major role in dissolution process although the lower viscosity was favorable for mass transfer.This was in agreement with the report that the hydrogen bond basicityβwas able to express the ability of accepting hydrogen bonds [28,29].Chloridion had a smaller size and a stronger polar than bromide ion and it was more competent to participate in breaking down hydrogen bonds in silkworm pupae powder,thus resulting in that [Emim]Cl had a more efficient extracting effect (62.6%) than [Emim]Br (44.3%).The ILs with phosphate anions,such as [DEP]-and [DMP]-,were proved to possess tremendous potentiality due to their highβvalues of 1.0.In this work,the dissolution rates of silkworm pupae at 90°C in[Emim]DEP and [Emim]DMP were 93.4% and 85.4%,respectively,the yields could reach 56.3% and 54.7%,respectively.[Emim]OAc also had a highβvalue of 1.1,it had confirmed which had a strong power for dissolving chitin and cellulose because of its low viscosity and low melting point [12,30].In this study,[Emim]OAc exhibited the strongest ability of dissolving the pupa powder as expected,with the dissolution rate reached up to 95.6% at 90°C for 24 h.However,the yield of extracted protein was only 48.5%,which was much less than that from [Emim]Cl (62.6%).It could be deduced that the stronger basicity of OAc-made it more efficient at breaking the intra- and intermolecular hydrogen bonds of protein than Cl-[17,31],but it also caused more degradation of protein at the same time.
Since Cl-showed the best extracting performance for SPP,a series of ILs composed of the Cl-anion and different alkyimidazolium cations were applied to explore the structure-performance relationship of cations.Cation was proved to play an important role in the separation of SPP,but it had lesser influence than the anion of IL.As shown in Fig.6,the dissolution rate and yield decreased with the increasing length of alkyl chain on the methyl imidazolium cation.[Emim]Cl led to the maximum dissolution of 90.5% and a highest protein yield of 62.4%,while [Omim]Cl provided the lowest dissolution of 72.9% and a lowest protein yield of 42.5%.The decreased dissolution rate of silkworm pupae was likely due to the growing dynamic viscosity and higher bulkiness of cations (exhibited as molar volume,Table 2).The ILs with a long alkyl chain in cation had too high viscosity to dissolve more silkworm pupa powder.The bulkiness of cation would increase stereohindrance effect and weaken the hydrogen bonding between protein and ILs [35,36].In addition to molar volume,the dynamic viscosity of IL system was also found to be an influencing factor in limiting the dissolution process.[Amim]Cl also exhibited a good dissolution capability probably because of its low dynamic viscosity and short sidechain in its cation structure.Furthermore,the fat contents of the regenerated SPP were all below 0.5%.
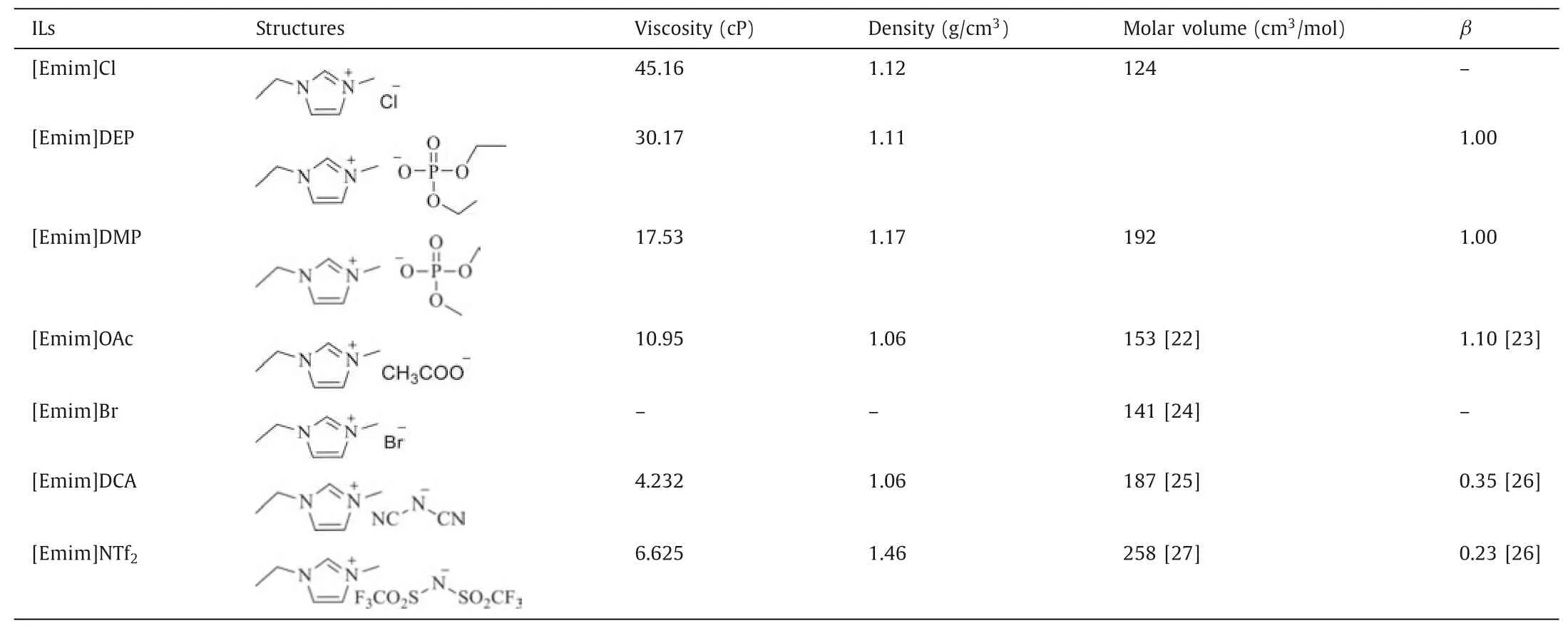
Table 1 The physicochemical properties of ILs.
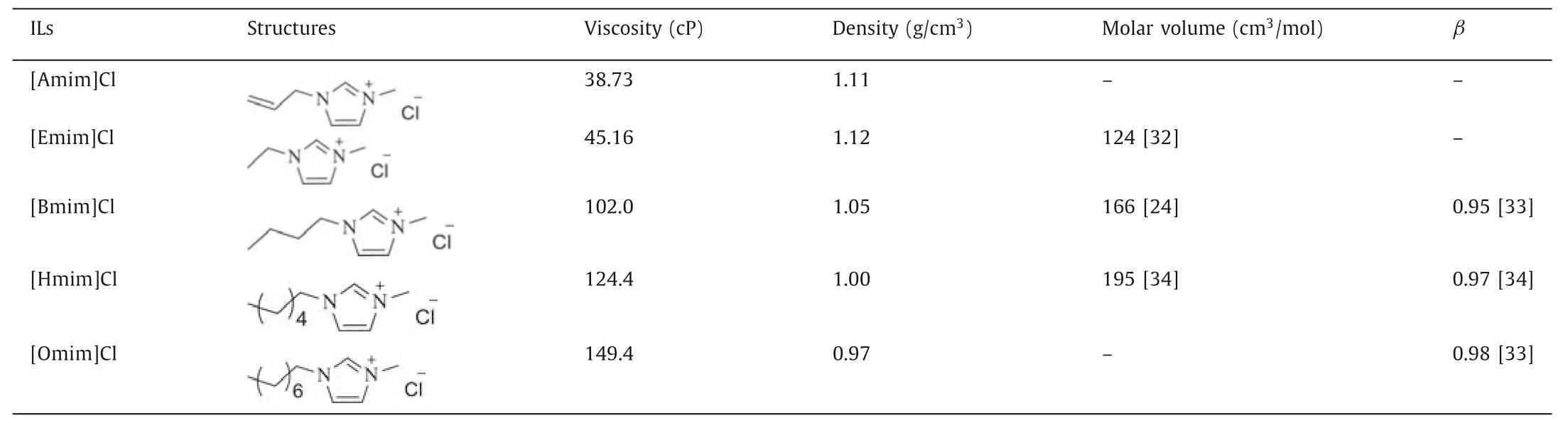
Table 2 Physicochemical properties of ILs containing Cl- as anion.
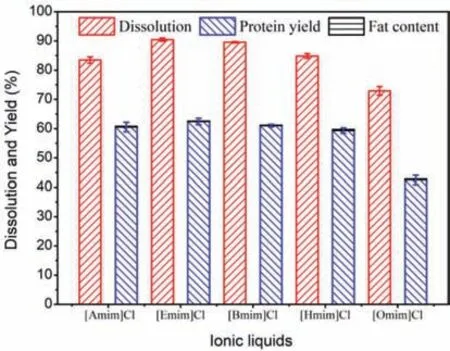
Fig.6.Effects of the IL cations on the separation of SPP.
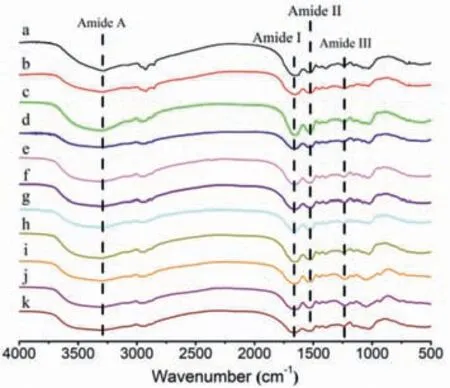
Fig.7.FT-IR of (a) silkworm pupa,obtained SPP materials by (b) typical method,(c) [Amim]Cl,(d) [Omim]Cl,(e) [Hmim]Cl,(f) [Bmim]Cl,(g) [Emim]Cl,(h) [Emim]Br,(i) [Emim]DEP,(j) [Emim]DMP,(k) [Emim]OAc.
The abovementioned SPP were characterized and made quality analysis by using FT-IR,XRD,solid state13C NMR and TGA methods.The FT-IR spectra of silkworm pupa and the materials obtained from typical method and protein/IL solutions were compared in Fig.7.In all these spectra,the characteristic absorption bands could be predominantly indexed to peptide bonds (-CONH)in protein,labeled as Amide A,Amide I,Amide II and Amide III.The peak at 3292–3300 cm-1was ascribed to N–H stretching vibration (Amide A).The Amide I at approximately 1652–1658 cm-1was related to C=O stretching vibration,with a strong absorption[17].Another strong absorption bond at 1527–1538 cm-1mainly represented N–H bending and C–N stretching vibrations (Amide II).The protein Amide III at 1228–1238 cm-1corresponded to the C–O and C–N stretching vibrations,N–H and O=C–N bending vibrations[17].There was no obvious difference between the spectra of raw material and obtained SPP from different methods.The close similarity of these spectra indicated that the main structure of protein was maintained after the dissolution and regeneration processes.
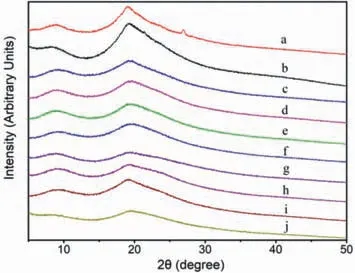
Fig.8.XRD patterns of (a) silkworm pupa; obtained SPP from (b) traditional method,(c) [Emim]Cl,(d) [Bmim]Cl,(e) [Hmim]Cl,(f) [Omim]Cl,(g) [Emim]Br,(h)[Emim]DEP,(i) [Emim]DMP,(j) [Emim]OAc.
In order to further compare the characteristics of regenerated protein products,the crystal structures of raw material and the proteins regenerated from typical method and IL method were examined by XRD.As shown in Fig.8,the raw material displayed a small 2θpeak was at about 27°,which was indexed as a peak of the crystal structure in chitin.The 2θpeak at about 9° was mostly contributed from theα-helix structure of protein and the band at around 20° was mainly indexed asβ-sheet structure [16,37].The absence of peak at 27° in spectra of regenerated materials confirmed the high purity of regenerated protein [16,37].All the samples showed typical diffraction patterns ofα-helix andβ-sheet,which meant the maintenance of the two types of structures in regenerated protein.It could also clearly observed thatβ-sheet showed a higher intensity thanα-helix in all spectra.However,each regenerated protein exhibited a decrease in the intensity ofβ-sheet comparing with raw material or obtained protein from the typical method.It indicated that the crystalline structure of protein in raw material had been disrupted during dissolving process.As is known,the stability and resistance to dissolution of SPP are the consequences of the tight packing of theα-helices andβ-sheets that presented in the polypeptide structure.Meanwhile,the destruction ofβ-sheet structure and the decrease of crystallinity contributed to the dissolubility and the further application of SPP.
Solid state13C NMR was used to further study the local structure of regenerated protein.The13C NMR results were collected in Fig.9.All the spectra of regenerated protein showed similar characteristic peaks to that of raw material,indicating that the feature structures of SPP were maintained after the dissolution and regeneration process.There were several distinct regions as shown in Fig.9.The maximal chemical shift at about 172 ppm was attributed to the C=O present in protein.The broader peak at about 50–54 ppm was due toα-carbon,while the signal at 38–40 ppm was the contribution fromβ-carbon mainly in leucine.The peaks with lower chemical shifts at 19 ppm,24 ppm and 29 ppm were assigned to alkyl groups of the side chains [38,39].
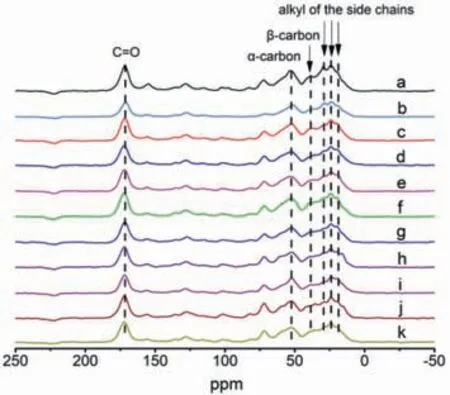
Fig.9.The 13C NMR spectra of (a) silkworm pupa; obtained SPP from (b) traditional method,(c) [Amim]Cl,(d) [Omim]Cl,(e) [Hmim]Cl,(f) [Bmim]Cl,(g) [Emim]Cl,(h)[Emim]DEP,(i) [Emim]DMP,(j) [Emim]OAc,(k) [Emim]Br.
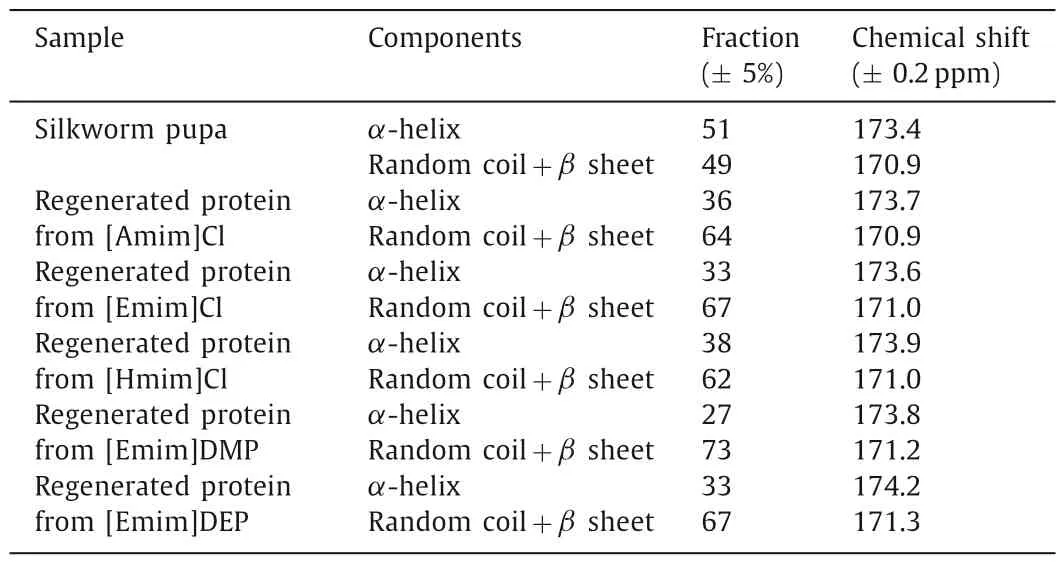
Table 3 Silkworm pupa and the obtained SPP secondary structure analysis.
It was known that the secondary structure of protein is constituted ofα-helix andβ-sheet [40].They had a slightly difference in chemical shift of the C=O group when measured by13C NMR [38].For the purpose of further assessing the changes in regenerated protein,the C=O peak divided into two peaks at about 171 ppm and 174 ppm as displayed in Fig.10.Chemical shift in the region of~174 ppm could be ascribed toα-helix structure,while the peak at~171 ppm was related to bothβ-sheet and random coil structures.By peak-differentiating and imitating the line shape of C=O,the corresponding fraction ofα-helix andβ-sheet combining with random coil random could be calculated for each sample.As recorded in Table 3,it was clear that each protein product displayed a decrease in fraction of theα-helix and an increase in fraction of theβ-sheet combining with random coil comparing with the raw material.The results proved that IL disrupted the crystalline structure of the protein in raw material during the dissolving process,and further promoted the formation ofβ-sheet and random coil structures during the regeneration process.These results were consistent with the results of SPP XRD spectra analysis.
The thermodynamic stabilities of silkworm pupa and the obtained proteins were analyzed by using TGA method (Fig.S3 in Supporting information).Two weight loss stages could be found in all spectra.The weight loss of raw material and regenerated protein was about 7% at near 100°C,which could be attributed to the evaporation of residual water in the samples.The decomposition temperatures (Td) of SPP were between 200 °C and 400 °C as shown in Table S2 (Supporting information).It showed that the obtained SPP materials had better thermal stability than silkworm pupa material (Tdof silkworm pupa material about 257 °C) [16,37].
Comparing with the reported alkali solubilization–acid precipitation strategy [17],this work presented high SPP extraction effi-ciency with 90.5% silkworm pupa dissolution and 62.6% SPP yield by using [Emim]Cl at 90 °C for 24 h.There was no traditional inorganic acid and alkali used in this work,it also showed an environmental strategy that conscious alternative to traditional solvents.In addition,Zenget al.reported a method for extraction SPP by using choline hydroxide (CH-IL) [17],the SPP extraction rate is 64.52% with 90.45% protein content at 40 °C for 1 h.It exhibited a similar result for the SPP extraction efficiency with our research work that 62.6% SPP extraction rate.However,the protein content of obtained SPP is up to 99.5% in this work,higher purity of SPP could be obtained comparing with the CH-IL strategy.
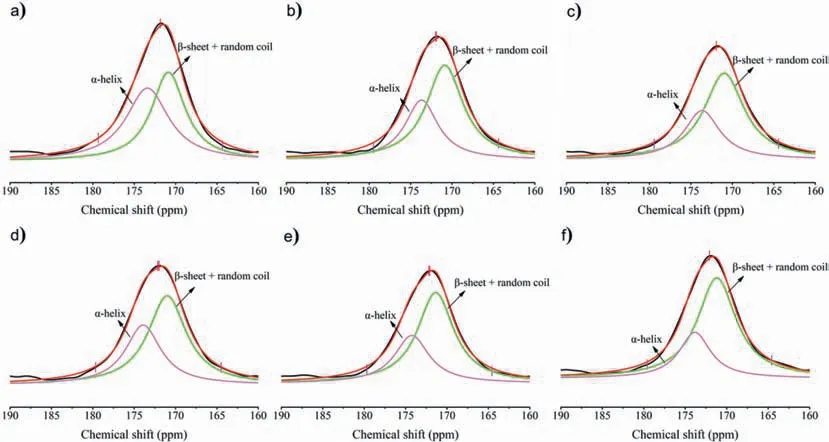
Fig.10.The 13C NMR spectra of (a) silkworm pupa and the regenerated protein from (b) [Amim]Cl,(c) [Emim]Cl,(d) [Hmim]Cl,(e) [Emim]DEP,(f) [Emim]DMP solutions.
In conclusion,efficient separation of SPP could be achieved by using [Emim]Cl at 90 °C for 24 h with ethanol as the regeneration solvent in this work.The research proved that the cations and anions of ILs both had an important influence on the separation of SPP from silkworm pupa,while the anions of ILs played a more significant role than cations.ILs with high hydrogen bond basicity (β),short alkyl sidechain in cations and low viscosity had a better efficiency for separation of SPP.The obtained SPP materials showed a decreased polypeptide chain,crystallinity and the fraction ofα-helix,but with little chemical changes of composition.This work provided one kind of efficient strategy for separation of SPP from silkworm pupa by using ILs at mild conditions,which showed good scale application potential for the utilization of silkworm pupae materials by using IL-based method.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported financially by the National Natural Science Foundation of China (Nos.81673400 and 21878292).We thank Prof.Suojiang Zhang (Institute of Process Engineering,Chinese Academy of Sciences) for helpful discussion.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.04.072.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
