The protective effects of a D-tetra-peptide hydrogel adjuvant vaccine against H7N9 influenza virus in mice
Ked Chen,Xioxin Wu,Qingjing Wng,Ying Wng,Hiyn Zhng,Shui Zho,Chonn Li,Zhiwen Hu,Zhimou Yng,1,*,Lnjun Li,*
a Shulan International Medical College,Zhejiang Shuren University,Hangzhou 310015,China
b State Key Laboratory for Diagnosis and Treatment of Infectious Diseases,Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases,National Clinical Research Center for Infectious Diseases,The First Affiliated Hospital,Zhejiang University School of Medicine,Hangzhou 310003,China
c Zhejiang Shuren College,Zhejiang Chinese Medical University,Hangzhou 310053,China
d State Key Laboratory of Medicinal Chemical Biology,Key Laboratory of Bioactive Materials,Ministry of Education,College of Life Sciences,Synergetic Innovation Center of Chemical Science and Engineering,and National Institute of Functional Materials,Nankai University,Tianjin 300071,China
e Jiangsu Center for the Collaboration and Innovation of Cancer Biotherapy,Cancer Institute,Xuzhou Medical University,Xuzhou 221004,China
Keywords:H7N9 influenza virus Hydrogel Adjuvant Vaccine Preventative effects
ABSTRACT Repeated waves of influenza virus H7N9 epidemics after 2013 have caused severe influenza in humans,with mortality reaching approximately 40%–50%.To prevent possible pandemics,the development of highly effective vaccines against influenza virus H7N9 is highly desired.In the present study,by taking advantage of the D-tetra-peptide adjuvant (GDFDFDY),we reported a simple method to prepare H7N9 vaccines.Naproxen (Npx),with good anti inflammatory and broad anti-viral effects,was employed as an N-terminal capping group to construct a hydrogel precursor,Npx-GDFDFDY.The hydrogel adjuvant was prepared using a routine heating cooling protocol and the final vaccine was ready after mixing with the split A/Zhejiang/DTID-ZJU01/2013 (H7N9) antigen by vortexing.Compared with the traditional Al(OH)3 adjuvant vaccine and the split vaccine,our hydrogel adjuvant vaccine showed the best preventive effects against H7N9 infection.A mechanistic study illustrated that higher antibody responses and variations in cytokine expression might account for its increased protective effects.Our strategy demonstrated the advantages of a peptide hydrogel adjuvant in the application of vaccines against H7N9 and demonstrated its potential application in vaccines against emerging threats from other viruses.
Influenza viruses exist widely in nature,and possess a fast variation rate and the possibility of cross infection between humans and other species,thus representing a huge challenge for human health [1].One report from the World Health Organization (WHO)showed that 5%–10% of adults and 20%–30% of children develop seasonal influenza every year,resulting in 3 million to 5 million inpatient cases and thousands of deaths worldwide [1].H7N9,as an avian influenza virus,has been observed to possess marked potential to cross avian-human barriers and cause severe disease in humans since its first appearance in China in 2013 [2–5].Pneumonia,acute respiratory distress syndrome,septic shock,multiple organ failure,and even death within 3–7 days are associated with H7N9 infections [3,5,6].Repeated epidemics of H7N9,accumulated mutations,and increasing affinity to human respiratory epithelial sialic acid receptors,along with the high mortality rate of patients infected with H7N9 (approximately 40%-50%),have increased public concerns about possible pandemics and prompted the development of effective methods to control and prevent the spread of the disease [5,7–13].
Among different methods used against influenza virus,vaccines are still the most feasible approach to defend against pandemics [14].Since the first outbreak of H7N9,different research groups have provided candidate vaccines and our group described the first recombinant H7N9 vaccine in China,comprising an A/Zhejiang/DTID-ZJU01/2013 (H7N9) split vaccine,using a reverse genetic technique [15].Furthermore,we evaluated its immunogenicity,protective effects,safety,and mechanismsviamixing it with different kinds of adjuvants [15].The results illustrated that adjuvant-combined vaccines,compared with H7N9 virus alone,presented greater performances in terms of elevating hemagglutination inhibition (HI),microneutralization (MN) and immunoglobulin G (IgG) antibody titers in response to antigens from H7N9,and different adjuvants could potentially induce significant variations in cytokine production and the immune response post-infection,highlighting the crucial roles of adjuvants in the preparation of vaccines against influenza virus [15–18].
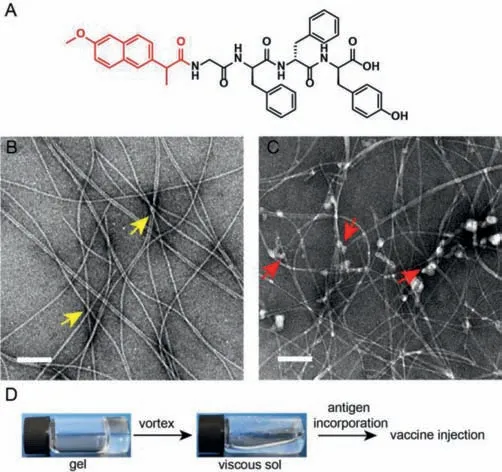
Fig.1.(A) The molecular structure of Npx-GDFDFDY.(B,C) Transmission electron microscopy (TEM) images of the hydrogel of Npx-GDFDFDY (B) and the final vaccine incorporated with H7N9 antigen (C),respectively.Representative morphologies of the hydrogel and binding mode of H7N9 antigen onto fibrils are shown by yellow and red arrows.The scale bars represent 200 nm.Npx,Naproxen.(D) Schematic illustration of preparation protocol of hydrogel adjuvant vaccine.
We previously developed a kind of D-type peptide adjuvant based on the short peptide sequence,GDFDFDY [19–24].In detail,the N-terminal aromatically capped GDFDFDY sequence can form a hydrogel and is suitable for the preparation of various vaccines copacked with different antigens by easy vortexing,including DNA,protein,and cell lysate-based vaccines [25–29].Adjuvants can elicit both humoral and cellular immune responses,and have been exploited to manufacture vaccines against tumors and viruses [25–31].Moreover,adjuvants modified with nonsteroidal anti inflammatory drugs (NSAIDs),such as flurbiprofen (Fbp),carprofen (Car),and naproxen (Npx),performed better than traditional aluminum adjuvants and naphthylacetic acid (Nap) modified GDFDFDY in producing IgG antibodies and cytokines,finally facilitating tumor elimination in mice [32].The modulation ability of these NSAIDmodified adjuvants against tumor associated inflammation was implied to contribute to their beneficial effects.In the present study,we endeavored to explore the potential application of NSAIDmodified adjuvants in the preparation of vaccines against H7N9 virus.Meanwhile,the underlying mechanism of the supramolecular peptide adjuvant was studied preliminarily to provide valuable insights into the development of H7N9 vaccines.
We chose aromatic Npx,representing not only antiinflammatory activity,but also broad spectrum antiviral activity,as the N-terminal capping group to develop the hydrogel precursor.The molecular structure is shown in Fig.1A.Meanwhile,we prepared the hydrogel of Npx-GDFDFDY (3 mg/mL) using a routine heating-cooling treatment,as reported previously [23,27,33].As shown in Fig.1B,Npx-GDFDFDY mainly consisted of uniform fibrils with a diameter of approximately 10 nm.Reticular morphology was also observed,implying a potential higher-dimensional crosslinked structure of the hydrogel.Meanwhile,the H7N9 antigen had a somewhat amorphous morphology,which might reflect its aggregation forms (Fig.1C).The hydrogel adjuvant vaccine was prepared by mixing the equal volumes of hydrogel and H7N9 antigen together and gently vortexing before injection (Fig.1D).Interestingly,H7N9 proteins were mainly located around the fibrils and the fibril surface might be the primary sites enriched for the antigens at the two dimensional scale (Fig.1C).
Fig.2.(A) Schematic illustration of immunization procedure.Six mice in each group were immunized for further evaluation.(B) Weight variations of mice infected with H7N9 virus (50 μL,1.0 × 106 TCID50/mL).Groups of mice were immunized with the indicated vaccines on days 0 and 14 before intranasal virus inoculation.The percent weight was calculated as the percentage of weight on the indicated days versus the weight on the initial day.(C–E) Weight variations of mice at 5 (C),6 (D) and 7 (E) days post infection.The asterisk (*) denotes a P value<0.05.
Next,we assessed the protective effects of the hydrogel adjuvant vaccine in a mouse model.BALB/c mice (19–21 g) were vaccinated twice on days 0 and 14 (Fig.2A).All animal experiments were conducted following the Guide for the Care and Use of Laboratory Animals of Zhejiang Province and the Ethics Committee of the First Affiliated Hospital,Zhejiang University School of Medicine.At 14 days after the second boost,the mice were challenged using A/Zhejiang/DTID-ZJU01/2013 (H7N9) virus (hereafter abbreviated as H7N9) and weight variations within next 7 days were recorded(Figs.2B–E) and used as the main index to evaluate mouse health illness after H7N9 infection and the protective effects of the different vaccines.The detailed compositions of vaccines and controls used are shown in Table S1 (Supporting information).The results showed that H7N9 infection caused illness and weight loss even at 1 day after virus inoculation,and the hydrogel adjuvant vaccine had the best ability to reverse this process.In detail,the body weights of uninfected mice (normal control group) increased by 13.7% ± 2.88% within 7 days (Fig.2E).Mice infected with H7N9 only (positive control) presented with mild respiratory distress and appetite suppression,and showed gradual weight loss over time.The loss of weight on 6thday reached 12.6% ± 4.85% (Fig.2D);however,a slight weight recovery was observed on the next day.Clearly,all vaccines showed some ability to shorten the weight loss period and enabled recovery of body weight after 4 days of infection,and the hydrogel adjuvant vaccine ranked the best one to recover the body weight of infected mice.Taken together,we concluded that the hydrogel adjuvant vaccine possessed the greatest protective effects against H7N9 infection among our tested vaccines.
Respiratory distress and lung damage of mice infected with H7N9 is one of the main symptoms [34].As shown in Fig.1D,at 1 week after infection of H7N9 virus,mice were sacrificed and lung tissues were evaluated using HE and IHC staining to reveal the effects of the hydrogel adjuvant vaccine on lung infection and damage from H7N9.As shown in Fig.S1 (Supporting information),compared with the uninfected mice (normal control) H7N9 infection (positive control) showed large lesions or multiple fused patchy lesions in lung tissues (HE panel),demonstrating the occurrence of multifocal interstitial inflammatory hyperemia (interstitial pneumonia) and lung exudative pathological changes.High infection levels were observed in the mice treated with H7N9-self(positive control),especially in the region of the bronchiolar epithelium (IHC panel).We observed that all vaccines could alleviate H7N9 infection and lung injury,appearing as lower levels of interstitial pneumonia (HE panel) and H7N9 infection (IHC panel).Together,these results suggested that the hydrogel adjuvant vaccine could effectively protect the lung from the virus infection and lung damage.
To evaluate the immune response after vaccines administration,we conducted series of antibody titer measurements using ELISA.Sera on day 14 after the second immunization and at day 7 after H7N9 inoculation were collected,and both HI and MN titers to indicate the specific antibody responses to prevent the hemagglutination caused by virus infection and virus-specific neutralizing antibodies against influenza viruses [35,36],respectively,were tested.The results showed that all vaccines (i.e.,the split vaccine,the Al(OH)3adjuvant vaccine,and the hydrogel adjuvant vaccine)could elicit potent antibody titers against H7N9 after the second boost or further virus challenge,and both the HI and MN titers showed similar variation tendencies (Fig.3).Meanwhile,titer variations before and after virus challenge showed that although virus infection could elicit further antibody production,mice immunized with different vaccines exhibited better performance in terms of antibody responses.Moreover,the hydrogel adjuvant vaccine induced the highest antibody titers after virus challenge,suggesting the strongest immune memory raised by hydrogel adjuvant vaccine and the importance of adjuvant selection for vaccine development against viruses.
To further characterize the immune responses,we evaluated the levels of cytokines and chemokines elicited by the various vaccines and virus challenge.Sera were collected after 1 week of H7N9 challenging and the levels of 23 cytokines and chemokines were determined,including interleukin (IL)-1α,IL-1β,IL-2,macrophage inflammatory protein 1-alpha (MIP-1α),IL-4,IL-3,IL-6,IL-5,IL-9,IL-10,IL-13,macrophage inflammatory protein 1-beta (MIP-1β),regulated upon activation,normally T-expressed,and presumably secreted (RANTES),interferon gamma (IFN-γ),keratinocytesderived chemokine (KC),granulocyte colony stimulating factor (GCSF),granulocyte-macrophage colony-stimulating factor (GM-CSF),interleukin 12 subunit P40 (IL-12P40),interleukin 12 subunit P70(IL-12P70),IL 17,EOTAXIN,tumor necrosis factor alpha (TNF-α)and monocyte chemoattractant protein-1 (MCP-1) (Fig.4).In most cases,no obvious differences were observed among the different groups.However,significant differences between individual groups were revealed in terms of IL-5,IL-6,IL-13,MIP-1β,IFN-γ,and GM-CSF.For IL-5,IL-6,and MIP-1β,it was clear that all vaccines elicited comparable expression levels,but their levels were higher than those of either the normal or positive control.Interestingly,the hydrogel adjuvant vaccine elicited the highest level of IFN-γand the lowest level of GM-CSF,which were very different from the other vaccines and controls.Meanwhile,variations of IL-13 levels in the different groups were more complicated; (1) mice without immunization and virus infection (normal control) expressed a negligible amount of IL-13; (2) mice infected by virus only (positive control) showed the highest expression of IL-13; (3) the expression levels of IL-13 after immunization with different vaccines were lower than that of the positive control,but higher than that of the normal control.The various cytokines and chemokines have complicated functions in immunotherapy,and we could not define their roles accurately here; however,the variations in their expression levels might account for the differences in the protective properties of the vaccines.
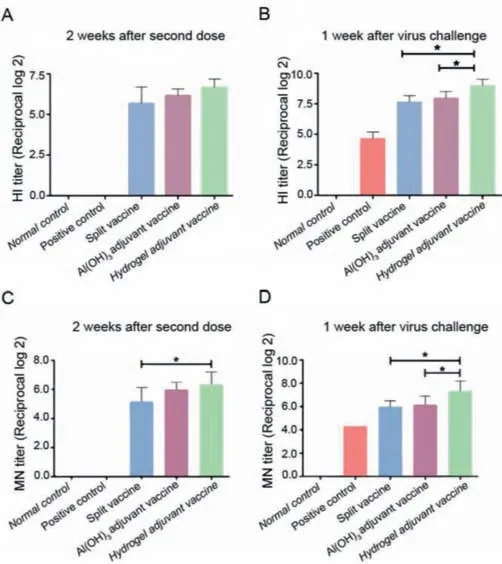
Fig.3.Hemagglutination inhibition (HI) and microneutralization (MN) titer measurements of sera from mice immunized with different agents at 2 weeks after the second boost (A,C) and at 1 week after virus inoculation (B,D),respectively.The asterisk (*) denotes a P value<0.05.
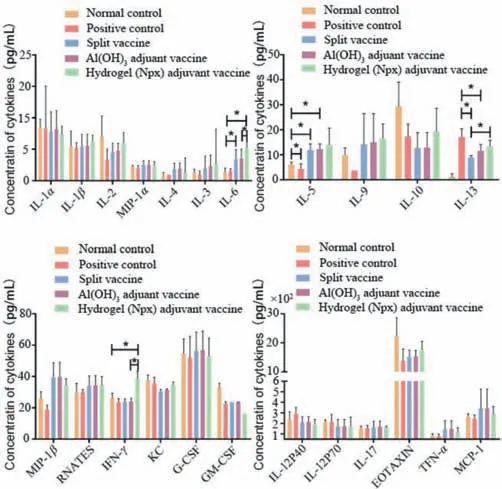
Fig.4.Expression levels of cytokines and chemokines in sera at after 7 days of virus inoculation.All groups of mice were pretreated according to the flowchart shown in Fig.1D.The asterisk (*) denotes a P value<0.05.
Vaccines remain the most effective strategy to prevent infectious diseases [37,38].As a significant component of vaccines,adjuvants can strengthen immune responses and are a research hotspot in vaccine development [39,40].Conventionally,Aluminum hydroxide is used in vaccine design because of its good safety; however,its relatively weak ability to evoke potent immune responses limits its application [41].Different kinds of nanomaterials with good biocompatibility were revealed to enable antigen incorporation,delivery,and sustained release,showing advantages to enhance antigen immunogenicity and develop vaccines.Therefore,short peptide hydrogels have attracted increased interest because their ease of design,biocompatibility,defined functional motifs,and good biodegradability [22,42–46].Moreover,short peptide hydrogels have proven to be powerful immune adjuvants that elicit strong immune responses.
In the present study,we used a D-tetra-peptide hydrogel adjuvant,i.e.Npx-GDFDFDY,to develop an H7N9 vaccine.By copacking with a split H7N9 antigenviavortexing,we prepared the D-tetrapeptide hydrogel adjuvant vaccine.We demonstrated that the hydrogel adjuvant vaccine elicited strong antibody responses,shortened the period of symptoms,and reversed illness after virus inoculation.Moreover,the hydrogel adjuvant showed a better performance than the traditional aluminum hydroxide adjuvant,implying its greater potential in vaccine development against viruses.
Cytokines mediate immune responses,and different levels of cytokines,such as IL-2,IL-6,IL-10,IL-13,and IFN-γ,are related to variations in immune responses,implying different immune mechanisms [47–50].In our tests,we found that the hydrogel adjuvant vaccine could increase the secretion of IFN-γ,IL-13,IL-9,IL-6,IL-5,and IL-2 at different levels compared with those of the positive or normal controls.IL-2 and IFN-γare mainly secreted by T helper 1 (Th1) immune cells and IL-5,IL-6,and IL-13 are secreted from Th2 immune cells; therefore,we hypothesized that the immune responses elicited by the hydrogel adjuvant vaccine involved both Th1 and Th2 cells.Meanwhile,the variations in cytokine types and levels in the hydrogel adjuvant vaccine group,compared with the aluminum hydroxide adjuvant vaccine group,suggested their different abilities to strengthen immune responses against viruses.
In summary,we successfully explored the application of the D-tetra-peptide adjuvant in the preparation of a vaccine to defend against H7N9 infection.We demonstrated that the hydrogel adjuvant vaccine could evoke strong antibody responses and presented good protective effects against virus infection in mice.Both Th1 and Th2 immune types might contribute to its protective immune responses.Moreover,the hydrogel adjuvant performs better in terms of antibody responses against H7N9 compared with the traditional aluminum hydroxide adjuvant,suggesting the potential of the hydrogel adjuvant to confront the shortages of aluminum hydroxide adjuvant and strengthen the protective effects of vaccines against H7N9 infection.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Chenyu Yang in the Center of Cryo-Electron Microscopy (CCEM),Zhejiang University for her technical assistance with the transmission electron microscopy.We really appreciate Wei Yao from Zhejiang Tianyuan Bio-Pharmaceutical Co.,Ltd.providing us the Al(OH)3adjuvant.We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach,California) for editing our manuscript.
We also acknowledge the support from the Medical and Health Science and Technology Program of Zhejiang Province,China (No.2020379356) and the China Postdoctoral Science Foundation (No.2020T130102ZX).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.04.044.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
