Synthesis of a glucose conjugate of pristimerin and evaluation of its anticancer activity
Fn Yng,Jie Zhng,Jicheng Li,Weno Ye,c,*,Ang Li,*,Weiwei He,*
a Shanghai Key Laboratory of New Drug Design,School of Pharmacy,East China University of Science and Technology,Shanghai 200237,China
b State Key Laboratory of Bioorganic and Natural Product Chemistry,Center for Excellence in Molecular Synthesis,Shanghai Institute of Organic Chemistry,University of Chinese Academy of Sciences,Chinese Academy of Sciences,Shanghai 200032,China
c The Research Center of Chiral Drugs,Innovation Research Institute of Traditional Chinese Medicine,Shanghai University of Traditional Chinese Medicine,Shanghai 201203,China
Keywords:Glucose conjugation Warburg effect Pristimerin Extracellular stability
ABSTRACT Taking advantage of the Warburg effect in cancer cells,glucose conjugation has emerged as a useful strategy for targeted delivery of anticancer agents.Pristimerin is a naturally occurring triterpenoid that displays potent but non-selective cytotoxicity.We developed a convergent and modular approach to construction of glucose-payload conjugates featuring copper-mediated azide-alkyne cycloaddition and prepared a glucose conjugate of pristimerin through this approach.The anticancer activity of this conjugate was evaluated in cancer cells and normal cells; however,the selectivity toward cancer cells was not significantly improved.We then examined the extracellular stability of the conjugate and found that its ester linkage was cleaved rapidly in Dulbecco’s Modified Eagle’s Medium at 37°C,which resulted in the release of pristimerin.In fact,the inorganic components in this medium were sufficient to induce the cleavage.Given that the subtle difference between intrinsic stability and extracellular stability of the conjugate linker is often underappreciated,this work highlights the importance of the latter in the development of target-selective conjugates.
Most cancer cells rely on aerobic glycolysis,rather than mitochondrial oxidative phosphorylation favored by normal cells,to generate energy for cellular processes.This phenomenon is known as the Warburg effect [1–3].The high rate of aerobic glycolysis in cancer cells requires a significant increase in glucose uptake [2].Glucose transporters (GLUTs),a family of membrane proteins that facilitate the transport of glucose across the plasma membrane,are overexpressed in a wide range of cancer cells [4].Of note,some glucose analogues can also be recognized by GLUTs [5].For instance,2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG),a radiolabeled glucose analogue,is taken up by cancer cellsviaGLUTs (in particular GLUT1 and GLUT3) at a markedly higher rate than by normal cells,which forms the basis of tumor imaging by positron emission tomography (PET) in clinical oncology [3].Based on a similar mechanism of action,glucose conjugation has been considered a promising strategy for targeted delivery of anticancer agents [6].The glucose conjugates of a number of cytotoxic compounds,including those of ifosfamide [7],duocarmycin SA [8],and paclitaxel[9,10],exhibited improved target selectivity and water solubility.
Tripterygium wilfordiiis a Chinese herb known natively as“Thunder God Vine”.A series of hydrophobic terpenoids with potent but non-selective cytotoxicity have been identified from this species,including triterpenoids pristimerin [11,12] and celastrol(tripterine) [13] (1 and 2,Fig.1) and diterpenoid triptolide [14] (3).Despite considerablein vitroandin vivostudies,the lack of selectivity toward cancer cells,coupled with the poor water solubility,hampered the evolution of these natural products into anticancer drug candidates [12].Of note,Liu,Pomper,and co-workers developed a glucose conjugate of 3,which displayed significantly improved selectivity and water solubility compared to the natural product itself [15].Inspired by this work and in accordance with our continued interest in triterpenoids [16–18],we devised a glucose-pristimerin conjugate (4,Fig.1) to overcome the problems of 1.This structure embodied the following considerations.(a)From the payload perspective,esterification of the enol could be a suitable method for the modification of 1.The C3 hydroxyl group of 1 had proved critical for its biological activities [19,20].Capping this hydroxyl group might help to reduce the side effects of 1 in the extracellular environment.Upon entry into cells,the conjugate would be cleaved at the ester linkage by cellular esterases,releasing the payload.Therefore,this conjugate was expected to serve as a prodrug to some content [10,15].(b) From the saccharide perspective,theβ-glucoside should remain recognizable for GLUT1 [5].(c) From the linker perspective,the 1,4-disubstituted 1,2,3-triazole moiety would arise from an azide fragment and an alkyne fragment; copper-mediated 1,3-dipolar cycloaddition [21–25] could be used for convergent and modular assembly of this type of conjugate.Herein,we report the synthesis of 4 and evaluation of its cytotoxicity in cancer cells and normal cells; however,the selectivity toward the former was not significantly improved.The unexpected cleavage of the ester linkage of 4 in the cell culture medium highlights the importance of extracellular stability of the conjugate.
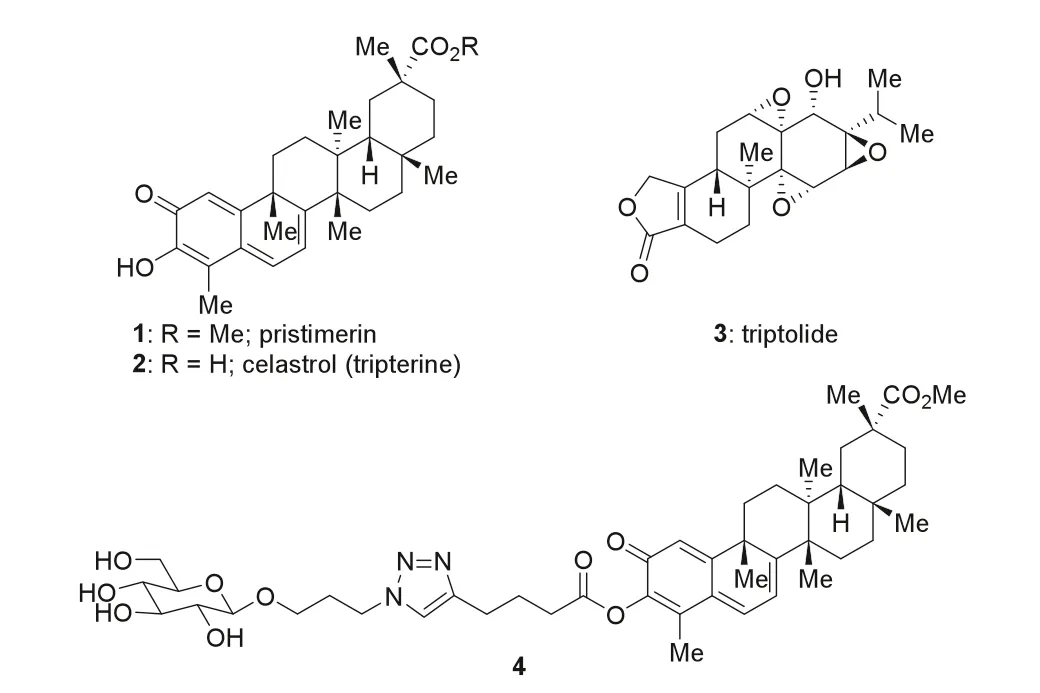
Fig.1.The structures of anticancer terpenoids pristimerin,celastrol (tripterine),and triptolide (1–3) and the devised structure of a glucose conjugate of pristimerin (4).
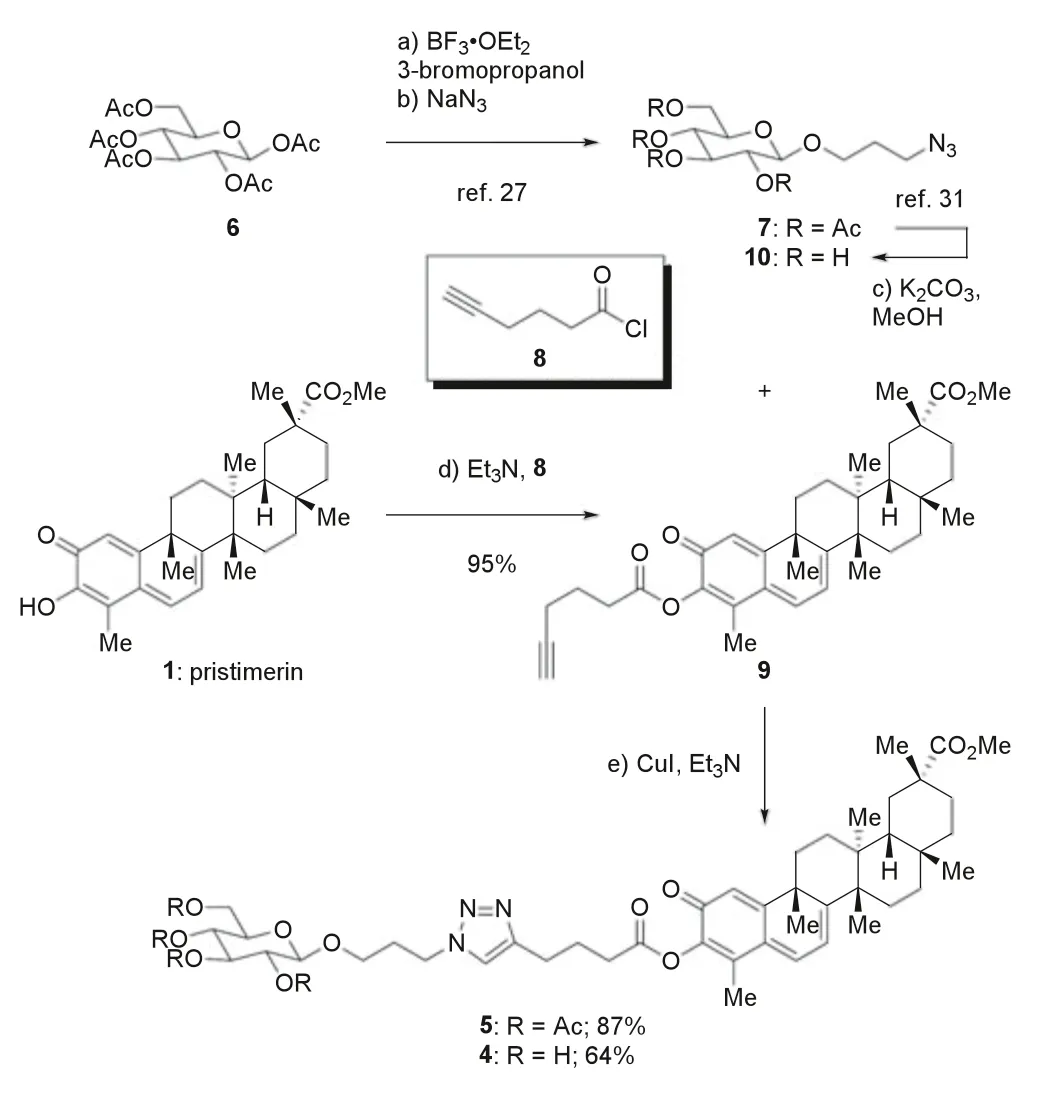
Scheme 1.Preparation of glucose conjugate 4 through a convergent and modular approach.
Following our experience with the synthesis of hybridaphniphylline B [26],we first prepared tetraacetate 5 as a plausible precursor of 4 (Scheme 1).On one hand,β-D-glucose pentaacetate(6) underwent a known two-step sequence of glycosylation and substitution to give compound 7,via3-bromopropylβ-D-glucosidetetraacetate [27] as an intermediate.On the other hand,treatment of 1 with acyl chloride 8 in the presence of Et3N provided enol ester 9 with excellent efficiency [28].Under the 1,3-dipolar cycloaddition conditions (CuI,Et3N) modified from those used in our one-pot triflate elimination-cycloaddition protocol [29],5 was obtained from 7 and 9 in good yield.However,global deacetylation of 5 resulted in undesired cleavage of the enol ester,even under the mild conditions (Et3N,MeOH,water) used for our synthesis of hybridaphniphylline B [26,30].We then had recourse to fully deacetylated precursor 10 derived from 6 (Scheme 1) [31].To our delight,the [3+2] cycloaddition reaction of 10 and 9 proceeded smoothly to afford target molecule 4.From a broad perspective,these efforts established a convergent and modular approach to preparation of glucose-payload conjugates.
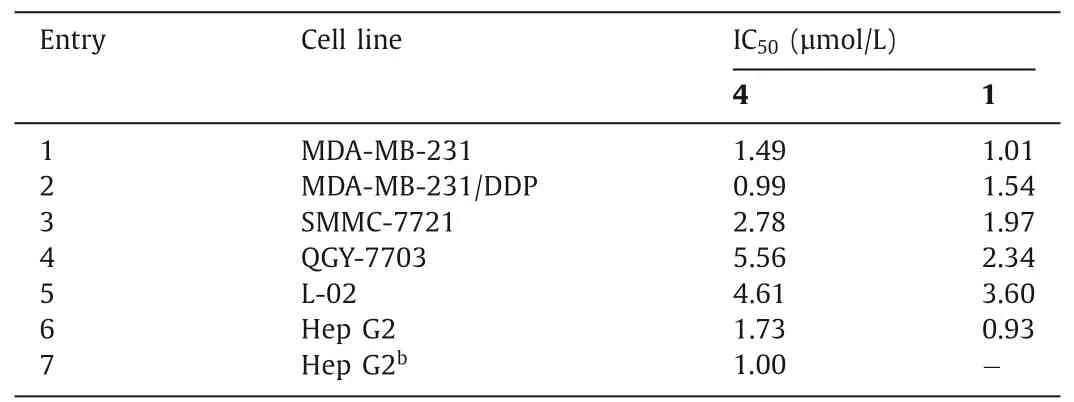
Table 1 The cytotoxicity of compound 4 against cancer cells and normal cells.a
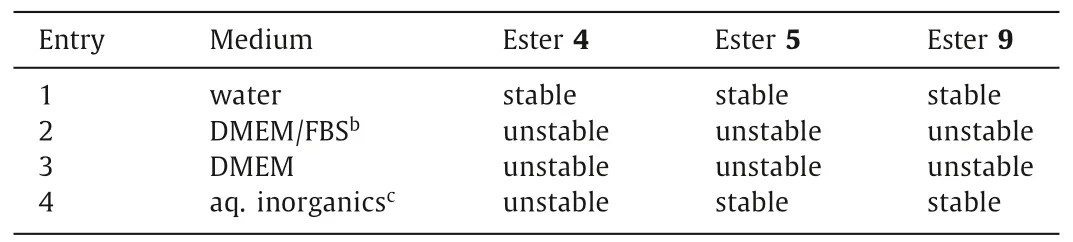
Table 2 The extracellular stability of enol esters 4,5 and 9.a
We then evaluated the cytotoxicity of 4 in a number of human cancer cell lines and a human fetal hepatocyte line (L-02),using 1 as a control (Table 1 and Fig.S1 in (Supporting information).This glucose conjugate displayed potent inhibitory activity against triple-negative breast cancer (TNBC) MDA-MB-231 and cisplatinresistant MDA-MB-231 (MDA-MB-231/DDP) cells and hepatocellular carcinoma (HCC) SMMC-7721 and QGY-7703 cells (Table 1,entries 1–4).However,its cytotoxicity against L-02 cells (entry 5) was not reduced as expected.The significant similarity between the cytotoxicity profiles of 4 and 1 implied that the glucose conjugation did not improve the selectivity toward cancer cells.Intrigued by the cause of this unsatisfactory selectivity,we investigated whether 4 could be effectively delivered into cancer cellsviaGLUTs.Frommer and colleagues reported that HCC Hep G2 cells rely predominantly on GLUT1 for glucose uptake [32].If 4 enters Hep G2 cellsviaGLUT1,WZB117 [33],a GLUT1 inhibitor,should be able to antagonize the anticancer effect of this glucose conjugate [15].However,when Hep G2 cells were co-treated with 4 and WZB117,the potency of the former remained unaffected (entry 6versusentry 7).These results suggested that 4 was not effectively taken up by cancer cells and might have been hydrolyzed extracellularly to release 1.
We continued to examine the extracellular stability of 4,5,and 9 (Table 2),all of which possess enol ester motifs.A series of media containing these compounds were incubated at 37°C for 4 h.Not surprisingly,all the compounds were stable in water (Table 2,entry 1),which verified the intrinsic stability of their ester linkages.However,Dulbecco’s Modified Eagle’s Medium (DMEM) with and without Fetal Bovine Serum (FBS) both resulted in the release of 1 from the compounds (entries 2 and 3).This may be attributable to the ester cleavage mediated by amine nucleophiles in the media.Interestingly,the inorganic components in DMEM (including CaCl2and MgCl2; see Supporting information for details) were found to be sufficient to induce the cleavage of the ester linkage of 4 but not to affect 5 and 9 (entry 4).We postulated that aquated Ca2+or Mg2+might be transiently enriched by the glucose moiety of 4viaits multiple hydroxyl groups and then serve as a local promoter of the enol ester hydrolysis.
In summary,we developed a convergent and modular approach to constructing glucose conjugates of anticancer agents and prepared glucose-pristimerin conjugate 4 through this approach.Compared to the payload (1),this conjugate did not exhibit significantly improved selectivity toward cancer cells as expected.Further investigations revealed that the inorganic salts in DMEM were sufficient to induce the cleavage of its ester linkage and the release of 1 in the extracellular environment.This work points to the need for proper evaluation of extracellular stability in the development of glucose-payload conjugates as well as other types of target-selective conjugates.The study of the next-generation glucose conjugates of 1 is underway in our laboratories.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This paper is dedicated to Prof.Guo-Qiang Lin.This work was supported by Ministry of Science and Technology (National Key Research and Development Program of China,No.2018YFA0901900),National Natural Science Foundation of China(Nos.21931014,U2002221,21772225,21572064,81502956 and 21621002),Chinese Academy of Sciences (Strategic Priority Research Program,No.XDB20000000; International Partner Program,No.121731KYSB20190039; Key Research Program of Frontier Sciences,No.QYZDB-SSW-SLH040),Science and Technology Commission of Shanghai Municipality (Nos.20430713400,17XD1404600 and JCYJ-SHFY-2022–005).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.04.036.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
