Bouquet-like uranium-containing selenotungstate consisting of two different Keggin-/Anderson-type units with excellent photoluminescence quantum yield
Mengyun Cheng,Hiying Wng,Yufeng Liu,Jingwen Shi,Mingqi Zhou,Weixin Du,Dongdi Zhng,*,Guoping Yng,*
a Henan Key Laboratory of Polyoxometalate Chemistry,College of Chemistry and Chemical Engineering,Henan University,Kaifeng 475004,China
b Jiangxi Province Key Laboratory of Synthetic Chemistry,Jiangxi Key Laboratory for Mass Spectrometry and Instrumentation,East China University of Technology, Nanchang 330013,China
Keywords:Polyoxometalates Selenotungstates Uranium Synthesis Photoluminescence
ABSTRACT We present the synthesis,characterization and photoluminescence properties of uranium-containing selenotungstate,[(UO2)3(SeO3)3Na5(H2O)6(SeW6O21)(SeW9O33)3]21–,which was isolated by a one-pot reaction of uranium nitrate with sodium tungstate and sodium selenite in a pH 5.2 aqueous solution at 90°C.In this study,the effect of the introduction of lone-electron pair containing heteroatoms on the structure is demonstrated,a three-layered heterometallic {Se3U3Na5} cluster is encapsulated by two different anionic building block units: three trivacant Keggin {B-α-SeW9O33} and one Anderson {SeW6O21}.To our knowledge,the {Se3U3Na5} cluster has never been observed in the polyoxometalate chemistry.The solidstate photoluminescence properties and lifetime decay behaviours of the title compound (1) have been measured at room temperature,and the photoluminescence spectrum displays the characteristic emission bands of corresponding uranyl cations.In addition,the photoluminescence quantum yield of 1 is 72%,which is almost three times that of starting material UO2(NO3)2·6H2O (27%).By using this strategy,we envision that an increasing number of assemblies with ‘open’clusters may be designed and obtained,in which the exposed oxygen atoms show strong affinity towards metal ions,providing new opportunities to generate bigger clusters or to tune existing properties.
Actinides (Ac),known as f-block elements because their 5felectron shells are gradually filled as the atomic number increases,present similar physical and chemical properties and play a major role in societally beneficial technologies [1–3].Compared to 3d transition metal ions,5f ions display high coordination numbers and offer different redox chemistry,resulting in unique modes of bond forming reactivity.However,our understanding of actinide chemical bonding and electronic structure is far from complete,which is mainly due to the lack of variety of data to some extent.
Polyoxometalates (POMs) [4,5],a large family of anionic metal oxide clusters,possess a rich structural diversity and have been proven to be ideal inorganic ligands for the incorporation of a large number of cations from the elements of the periodic table[6–14].In this respect,lacunary heteropolyoxometalates (HPOMs)have gained great interest [15–17],largely due to nucleophilic oxygen centres at the vacant site.Such vacant sites show strong affinity towards metal ions,which could help to understand the chemical and electronic interactions of metals with POM units [18,19].Within this class,the use of lone-electron pair containing pyramidal heteroatoms can have a profound effect on the structure of the resulting cluster.This is because the non-bonding,but stereochemically active lone pair of electrons generally restrict the formation of plenary structures,which is prone to yielding intrinsically new assemblies and further incorporating extra electrophilic linkers to generate larger clusters [20–24].
It is important to note that,from the viewpoint of structure,the Ac-POM assemblies can be divided into two families,namely actinide-based peroxide POMs and POM-based actinide complexes.The former emerged in 2005 [25] and has been rapidly developed during the past decade.The chemistry of these uranyl peroxide clusters has been already highlighted by Nyman,Burns and Loiseauet al.in several reviews [26–28].In the family of POMbased uranium clusters (POMUCs),although the pioneer work of can be traced to 1970s [29],the incorporation of uranyl cations within POM moieties has been much less explored [30,31].
It is noteworthy that the reported POMUCs are mainly based on the polyoxotungstates (POT) units,but much fewer results on analogues of Mo and Nb,which may be due to the large number of pre-existing polyvacant POT species.Up to now,only two polyoxomolybdate-based ([UIVMo12O48]8–and [NaUIV(Mo6P4O31H7)2]n5n-) [32] and two polyoxoniobate-based ([(Nb7O22H2)4(UO2)7(H2O)6]22–and [(UO2)(H2O)]3[Nb46(UO2)2O136H8(H2O)4]24–)[33] uranium clusters reported in the literature.Representative publications on POTUCs over the last decade in the literature include Keggin-type-based sandwich-type clusters [34–36],cyclic trimeric assemblies [37],and star-like hexameric compound [38],as well as two wheel-shaped uranium-containing 48-tungsto-8-phosphates [39,40].
We also note that,from the point of view of synthesis,almost all these reported POMUC species are synthesized by using readily formed polyvacant POM precursors as starting materials.Consequently,the obtained POMUCs can be easily retain its mother building block structural integrity,which,in turn,leads to difficulties in forming unprecedented structures.Inspired by this,including our ongoing interest in POM chemistry,we set about attempting to investigate the incorporation of uranyl ions into POM frameworks,which are in-situ formed by using composing elements as starting materials in combination with the heteroatom template to give rise to the new building block units (BBUs) with unsaturated nucleophilic oxygen atoms to construct even bigger clusters.Herein,we present a strategy of constructing a unique “bouquet”-like molecular assembly,[(UO2)3(SeO3)3Na5(H2O)6(SeW6O21)(SeW9O33)3]21–(1a),which was isolated as a hydrated mixed sodium-potassium salt,K1.5Na12.5[H7Na5(H2O)6(UO2)3(Se4W6O30)(SeW9O33)3]·26H2O(1).It is also the first time that the unprecedented triple-layered{Se3U3Na5} cationic core was encapsulated by three trivacant selenotungstate (ST) units and held together at the “bottom” by a further Anderson fragment.In addition to the structural studies,compound 1 has also been characterized in the solid state by singlecrystal X-ray diffraction,as well as thermogravimetric and elemental analyses.Moreover,photoluminescence quantum yield (PLQY)of compound 1 is 72%,which is more than twice that of the highest value (33%) [35] ever reported in POMUC chemistry.
As mentioned in the introduction,only a couple of POMUCs have been described in recent two decades (see Table S1 in Supporting information for a summary).Notably,most of the reported POMUCs were obtained by reaction of readily formed POM precursors ({XW9/11}X=Si/As,{P8W48} or {Nb6}) with different sources of uranium (U(SO4)2·6H2O,UO2(NO3)2·6H2O,UCl4,Li4UO2(O2)3or UO2(CH3COO)2·2H2O),but,rare start from the use of simple materials.In 2006,Popeet al.reported three examples of sandwichtype POMUCs stabilizing two seven-coordinate uranium atoms,which are formed by the reaction of a neutralized (pH 7–7.5)solution of WO42-and HAsO42-with an UO22+solution [41].Lately,one of our co-authors has also successfully used the similar strategy to bind up to six uranium cations within two [B-α-SbW9O33]9-units,which was synthesized by reaction of SbCl3,NaOAc·3H2O,Na2WO4·2H2O,H3PO4and UO2(NO3)2·6H2O in aqueous solution of the reaction system [35].
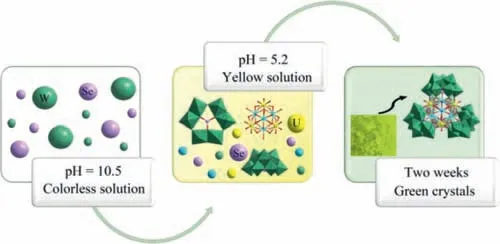
Scheme 1.Possible formation process of compound 1.
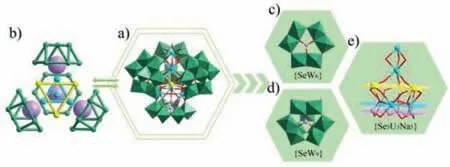
Fig.1.(a) Combined polyhedral/ball-and-stick representation of polyoxoanion 1a.(b) Simplified view of 1a.(c) {SeW6} fragment.(d) {SeW9} segment.(e) {Se3U3Na5}cluster.Color code: Se,lavander; W,green; U,yellow; Na,sky blue; O,red.
Anionic templates such as SO32–,AsO33–or SeO32-can direct the linking of WO6octahedra to access new POM clusters with novel architectures.Recently,we have been involved in a quest to investigate the interaction of template heteroatoms in the presence of H2O2with sodium tungstate,potassium niobate or potassium tantalate,and reported several series of uncommon POM BBUs [42–45].In effort to develop our studies,we use POM assemblies,in-situformed with presence of heteroatom templates,to bind the uranyl UO22+cationic group.Very recently,we reported the preparation of the first example of uranium-containing ST cluster [10].To demonstrate the utility of this methodology and in hope of generating highly complex nanoclusters,extension of this work has led to a unique tetrameric structure 1a (Scheme 1).Compound 1 was obtained by a reaction of Na2WO4and Na2SeO3and UO2(NO3)2·6H2O at 90 °C.As we know,the reaction is quite sensitive to synthetic parameters,such as pH and concentration.First,the molar ratio of Na2WO4and Na2SeO3is one of the key factors,crystal 1 can only be gained with the molar ratio of 10:1,which is obviously higher than the W/Se molar ratio (ca.5:1) of the title compound.This may be due to the formation of the white powder samples during the crystallization process.Second,if the pH is below 5,some powder precipitates was formed,while the pH is above 6,an unreported dimeric cluster was formed.Therefore,compound 1 can be obtained at pH 5–6 range.The reported experimental procedure in Supporting Information represents the highest yielding syntheses.
Single-crystal X-ray structure analysis of compound 1 reveals that it crystallizes in a triclinic system with the space groupP-1 (Table S2 in Supporting information).Polyanion 1a consists of two parts that are linked together by an unprecedented ‘threeparallel-layer’ cluster with mixed Se/U/Na atoms (Figs.1a and b,Fig S1 in Supporting information).The bottom part is one Anderson-type fragment [SeW6O21]2–({SeW6},Fig.1c) with an average Se-O bond length of 1.718 ˚A,and the other parts are three equivalent segments [B-α-SeW9O33]8–({SeW9},Fig.1d) that are fused together by three U linkers to give cyclic trimeric species{(SeW9O33)3(UO2)3}({U3W27},Fig.2).The molecular structure of 1a displays two pertinent features: the presence of two different kinds of ST units at the same time (three {SeW9} and one{SeW6}),which has not yet been observed together in union;and the presence of ‘three-parallel-layer’ heterometallic cluster,[(UO2)3(SeO3)3Na5(H2O)6]5+({Se3U3Na5},Fig.1e),which is ligated by three {SeW9} segments and supported by one {SeW6} fragment from the bottom.To our knowledge this is the first ‘three-parallellayer’heterometallic core of this kind encapsulated by POM ligands.
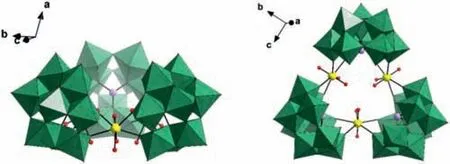
Fig.2.Combined polyhedral/ball-and-stick representation of the trimeric {U3W27},highlighting the two different viewing directions.Color code: Se,lavander; W,green; U,yellow; O,red.
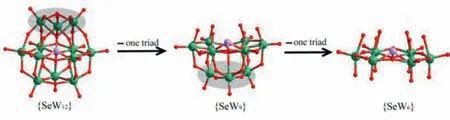
Fig.3.Ball-and-stick representation of parent {SeW12} cluster (left),trivacant{SeW9} (middle) and {SeW6} (right) species,highlighting the transformation from{SeW12} to {SeW9} and then to {SeW6} by removing three edge-sharing {WO6} octahedra successively.Color code: Se,lavander; W,green; O,red.
It is widely acknowledged that trivacant {SeW9} can be classified as derivative of the corresponding plenary {SeW12} Keggin structure by removing three edge-sharing octahedra (Fig.3),and thus it contains six unsaturated oxygen atoms,having the potential for further coordination.However,{SeW9}-based derivatives have only seldom been observed in POM structures [46–50].In 2001,Kortzet al.reported a dimeric polyanion,[(α-SeW9O33)2Cu3(H2O)3]10–,in which two {α-SeW9O33} units joined by three equivalent Cu2+ions [46].Subsequently,Cronin and coworkers presented a series of nanoscale STs by using SeO32–as a template linker [47–49].Very recently,Zhaoet al.reported several rare-earth-embedded ST derivatives [50].
The Anderson-type {SeW6} fragment contains three edgesharing {W2} groups and a capping SeO3ligand,in which three{W2} groups are linked together in a corner-sharing mode.It should be noted this is the second example of {SeW6} cluster in POM chemistry.In {SeW9} segment,if three additional {WO6} octahedra from three different {W3O13} triads are further removed,then {SeW9} segment turns into {SeW6}’species (Fig.S2 in Supporting information).The obvious difference between {SeW6} and{SeW6}’is that all the sixμ2-oxo atoms linking the {WO6} octahedra in {SeW6} appear on the same side of the {W6} plane (Fig S3 in Supporting information,bottom),whereas those in the {SeW6}’species are distributed alternatively above and below the plane (Fig S3,up).Crucially,this kind of unit is unique because it can be coordinated another six metal centres to the equatorial {W6} ring.
Interestingly,the three Na and Se centres are alternatively distributed because of the bond lengths.The three sodium ions with longer bond lengths ranging from 2.344(13) ˚A to 2.616(11) ˚A are connected to two terminal oxo ligands of two {WO6} octahedra from adjacent {W2} groups (Na3 to Na5),while each of Se centres with shorter bond lengths varying from 1.679(10) to 1.723(10)˚A is linked to two terminal oxo ligands from each {WO6} octahedra within one {W2} group,resulting in a beautiful crown-shape structure {Se3Na3W6} (Fig.S4 in Supporting information).Even though the concept of directing effect of lone-pair-containing heteroatoms template is not entirely new,the observation of {SeW9}and {SeW6} species in one single architecture is still significant because it may offer a pathway for the formation of unique POM architectures,demonstrating unusual clusters can be generated by interaction of the composing elements without the need for hydrolysis of pre-existing parent clusters.
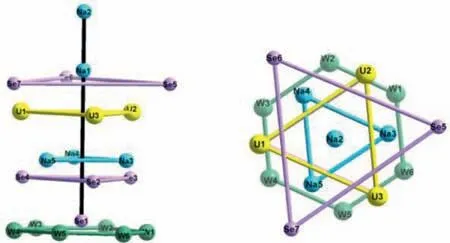
Fig.4.Representation of the heterometallic core,highlighting the parallel planes that are perpendicular to the Na2-Na1-Se1 artificial line (black).Color code: Se,lavander; W,green; U,yellow; Na,sky blue.
One rather novel aspect of the structure is the ‘triple-layered’heterometallic core {Se3U3Na3},which represents an unprecedented assembly on different fronts.First,the distances of M···M are almost identical,and the angles are nearly 60° (M=Se,Na,U,Fig S5 and Table S3 in Supporting information),leading to an approximate equilateral triangle.Secondly,if the {W6} ring in {SeW6}is designated as the bottom plane,then the three {M3} planes are almost parallel to it with the dihedral angles ranging from 0.2° to 0.6° (Table S4 in Supporting information).Not only do the three central heteroatoms in {SeW9} segments form a similar triangle ring.This ring is also parallel with the {W6} ring (Fig.S6 in Supporting information).Thirdly,the artificial line connecting Na2,Na1 and Se1 centres can be regarded as a vertical axis of these five planes,both in that the Na2-Na1-Se1 bond angle is 179.8° but also in that the angles between these planes and this line are all approximately 90° (Fig.4 and Table S5 in Supporting information).More interestingly,these four triangle rings can be viewed as a layer-by-layer assembly with a 60° rotation (Fig.S7 in Supporting information),just like ‘lego’bricks that it should take advantage of perfect grasp to maintain its stability.To our knowledge,this is the first branch motif of this kind encapsulated by POM ligands,and it is also the first time that Keggin and Anderson ST units have been observed together within one discrete molecule.
All of the W,U and Se ions are six-,seven- and threecoordinate,adopting usual octahedral,pentagonal-bipyramidal and triangular configuration,respectively,while the sodium centres exhibit three different configurations (Fig.S8 in Supporting information).Each of U atoms is coordinated by four terminal O atoms from two neighbor {SeW9} segments,one doubly-bridging O atom linking axial sodium and {U3} plane,one quadruple-bridging O atom linking three planes,and one terminal oxo group to form a pentagonal bipyramidal coordination geometry (Fig.S9 in Supporting information).
Bond valence sum (BVS) calculations [51] are carried out on all the W,Se,U and O centres,and the results indicate that the oxidation states of all W,Se and U centres are +6,+4 and +6,respectively (Table S6 in Supporting information).In addition,three terminal oxygen atoms and three bridging oxygen atoms of the Na1 ion are in the range of 0.17–0.45,suggesting aqua ligands (Fig.S10 and Table S7 in Supporting information).However,the results of elemental analysis suggest that there are only 12.5 sodium and 1.5 potassium cations,which means there must be some additional protons in 1a.We think they are disordered in the structure,which is common in POM chemistry [52–55].In addition,the phase purity of compound 1 was determined by comparing the experimental PXRD patterns with the simulated XRD patterns from singlecrystal structure analysis of the samples (Fig.S11 in Supporting information),which are also characterized by IR,Raman and TG(Figs.S12–S16 in Supporting information).
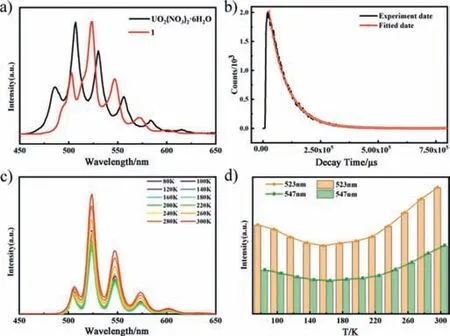
Fig.5.(a) Solid-state fluorescence emission spectra of compound 1 and UO2(NO3)2·6H2O.Excitation wavelength 353 nm.For better comparison,we normalized emission spectrum of UO2(NO3)2·6H2O.(b) PL decay lifetime trace of compound 1 with single-exponential fit curve.(c) Emission map of spectra recorded from 80 K to 300 K.(d) Integrated up-conversion intensities at 523 and 547 nm versus temperature.
Owing to the symmetry and antisymmetric vibration modes of UO22+,endowing them promising photoluminescence (PL) properties [56].Therefore,compound 1 are supposed to exhibit outstanding photoluminescence properties.Under the excitation at 353 nm,the solid-state PL emission spectrum of compound 1 in the range of 450–650 nm displays five characteristic emission peaks centered at 503,523,547,573 and 601 nm respectively corresponding to the S11→S00and S10→S0ν(ν=1–4) electronic transitions of uranium ions [57].However,the positions of these five peaks were obviously redshifted withca.15 nm by comparison with those of UO2(NO3)2·6H2O (Fig.5a).This is probably because of changes in coordination mode from six-coordinate (octahedral) to sevencoordinate (pentagonal-bipyramidal) [40],resulting in the variation in the number of donor atoms in equatorial plane of the uranium coordination sphere,a factor which is known to affect the band positions [58].The PL decay lifetime curve of compound 1 was also collected under the excitation at 353 nm,which can be fitted with the first-order exponential functionI=Aexp(-t/τ) with the lifetime (τ) 81.5 μs and the pre-exponential factor (A) 2150 (Fig.5b).Furthermore,the overall emission colors of compound 1 and the mother material UO2(NO3)2·6H2O are evaluated in terms of the standard CIE chromaticity diagram,which can be easily seen to be the similar green for both of them,corresponding to the CIEx,ycoordinate values of (0.21,0.68) and (0.20,0.60) (Fig.S17 in Supporting information),respectively.
As known,the PLQY is highly dependent on the competition rate between radiation (kr) and non-radiation (knr),the lower the intensity ratio ofknr/kr,the higher PLQY.As shown in Table S8,the ratio value for compound 1 and UO2(NO3)2·6H2O is 0.39 and 2.7,respectively.The significantly lower value ofknr/krthat of directly indicates that compound 1 should have a noteworthy higher PLQY than that of UO2(NO3)2·6H2O,which is in accordance with the experimental results.The PLQY of solid-state compound 1 (72%) is almost three times that of UO2(NO3)2·6H2O (27%).To our knowledge,the highest value ever reported in POMUC chemistry is 33%[35].
The temperature-dependent emission spectra (Fig.5,Figs.S18 and S19 in Supporting information) of compounds 1 and UO2(NO3)2·6H2O were also investigated between 80 and 300 K to evaluate the potential application in temperature sensing.As shown in Figs.5c and d and Fig.S18,the rate of non-radiative transfer is promoted by thermal energy increases with the temperature increasing in the range of 80–160 K,leading to non-radiative decays and thus reflecting in a decrease in fluorescence.However,further increase of the temperature,the emission intensity begins to increase,as a result of the excitation relaxation dominated radiative decay [59,60].
In summary,we have successfully obtained the first example of POMUC cluster consisting two different selenotungstate units,[(UO2)3(SeO3)3Na5(H2O)6(SeW6O21)(SeW9O33)3]21–(1a),from the reaction of the starting materials in the presence of the heteroatom templates.In the scope of POMUC chemistry two aspects deserve special interest,first polyanion 1a comprises three trivacant Keggin{SeW9} and one Anderson {SeW6} building block units at the same time,and second the unique “triple-layered” heterometallic cluster{Se3U3Na5} is also firstly observed in POM chemistry.Additionally,the high PLQY of compound 1 indicates the potential application of compound 1 in light-emitting materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.22071045 and 22001034),Excellent Youth Science Fund Project of Henan Province (No.202300410042),and the Open Fund of the Jiangxi Province Key Laboratory of Synthetic Chemistry (No.JXSC202008).Dongdi Zhang thanks for the start-up fund for Distinguished Professor of Henan University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.02.015.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
