Screening promising TM-doped CeO2 monolayer for formaldehyde sensor with high sensitivity and selectivity
Zhouho Zhu,Hengcong To,Jingbin Fu,Yingtng Zhou,Jin Guo,*,Chunyng Zhi,e,*
a School of Petrochemical Engineering & Environment,Zhejiang Ocean University,Zhoushan 316022,China
b School of Port and Transportation Engineering,Zhejiang Ocean University,Zhoushan 316022,China
c College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310058,China
d Key Laboratory of Key Technical Factors in Zhejiang Seafood Health Hazards,Zhejiang Ocean University,Zhoushan 316022,China
e School of Materials Science and Chemical Engineering,Ningbo University,Ningbo 315211,China
Keywords:Cerium oxide Density functional theory Formaldehyde Metal doping
ABSTRACT Developing convenient,fast-response and high-performance formaldehyde detection sensor is significant but challenging.Herein,two CeO2 phases (Fm¯3m and P42/mnm),three facets (CeO2(100),CeO2(110) and CeO2(111)) and three adsorption sites (top,bridge and hollow) are selected as substrate to interact with formaldehyde.Twenty-eight candidated transition metals (TM) are doped on CeO2 surfaces to investigate the performance of detecting formaldehyde by density functional theory.It shows that (i) CeO2 in a cubic fluorite structure with the space group Fm¯3m is suitable for formaldehyde adsorption compared with P42/mnm; (ii) TM-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr) are considered as candidated materials to absorb formaldehyde ascribed to lower adsorption energies.The d-band center,partial density of states,charge density difference and electron localization function are employed to clarify the mechanism of TM-doped CeO2 improving the performance of formaldehyde adsorption.It obviously displays that TM doped CeO2(100) changes the d orbit and rearranges electrons resulting in the superior ability to the adsorbed formaldehyde.This work provides theoretical guidance and experimental motivation for the development of novel formaldehyde sensor based on metal oxide semiconductor materials.
Formaldehyde (HCHO) is one kind of colorless poisonous volatile organic compounds (VOC) synthesized by the oxidation of methanol and widely used as an antiseptic,disinfectant and histologic fixative [1].It is unavoidably released from artificial building decoration materials including coatings,paints,and resins [2].Previous reports have shown that high-dose exposure increases the risk of acute poisoning and long-term exposure could lead to chronic poisoning or even cancer [3].The International Agency for Research on Cancer (IARC) classified HCHO as a Group I carcinogen[4,5].In addition,the World Health Organization (WHO) set the HCHO exposure limit as 0.08 ppm within 30 min [6].Thus,the development of HCHO detection technology is great of urgent to daily life and industrial applications.The traditional techniques for the detection of HCHO include gas chromatography [7],electrochemistry [8] and spectrophotomete [9].However,these detection techniques show many disadvantages,such as bulky equipment and complex detection steps [10].Therefore,it is significance to develop convenient,fast-response and high-performance HCHO sensor.
Compared to bulky equipment,gas sensors based on metal oxide semiconductor (MOS) has recently attracted more attention owing to small size,low cost and facile operation [11–13],such as CeO2,SiO2,Al2O3and TiO2[14].Among them,CeO2,featured by remarkable redox properties associated with facile conversion of Ce4+to Ce3+and strong interaction with metal,is widely investigated [15].Many researchers have reported the excellent performance of CeO2in the detection of HCHO based on experiment results.Shahidet al.[16] prepared CeO2polyhedral nanostructures with highly exposed surface area.The result was shown that gas sensing response to HCHO is better than that of other target gasses at 150 ppm.Zhanget al.[17] provided ultra-thin CeO2nanosheets by a simple low-temperature hydrothermal method.It exhibited a fast response to 5–400 ppm HCHO vapor.D.S.Danielet al.[18] proposed doping Zn ions into CeO2films and observed an obvious HCHO response in a lower detection (0.5 ppm) at 32°C.The above works have proved that CeO2is a potential metal oxide material for HCHO detection.However,these reports are obtained through prolonged trial and error,bulky equipment and complex experimental procedures.Therefore,it is of great significance to propose a simple method to improve the ability of CeO2to detect HCHO instead of the traditional experiment.
Density functional theory (DFT) [19] is a computational quantum mechanical modeling method used in physics,chemistry and materials science.It is widely used to explore materials for the detection of HCHO.For example,Denget al.[20] studied the electronic properties of TM (TM=Ni,Pt,Ti,Pd) doped MoS2and the adsorption of HCHO on these monolayer 2D structures by DFT,shows that TM-doped can substantially improve the sensitivity towards HCHO.[21] Jinget al.[22] used the density functional theory with Hubbard U correction (DFT+U) to study the effect of Au doping CeO2on the adsorption HCHO.The authors believed that Au doping promoted the activation of surface oxygen and promoted the adsorption of HCHO.Hence,as a detection descriptor,adsorption can be employed to describe the performance of HCHO adsorption sensor.TM-doped CeO2to improve the adsorption capacity of HCHO by using DFT is considered to be an effective measure to improve the ability to detect HCHO.However,to the best of our knowledge,there is still a lack of relevant reports.
Inspired by above reports,two CeO2phases (Fm¯3m andP42/mnm),three facets (CeO2(100),CeO2(110) and CeO2(111)) and three adsorption sites (top,bridge and hollow) are proposed to elucidate stability for HCHO adsorption.Afterwards,twenty-eight candidated TMs are doped on CeO2to simulate the adsorption performance of HCHO.It is found that CeO2in a cubic fluorite structure with the space group of Fm¯3m is favorable to HCHO adsorption compared with the CeO2(P42/mnm) base on DFT simulation.TM-CeO2(TM=Au,Hf,Nb,Ta,Zr) are considered as candidated materials for absorbing HCHO due to their lower adsorption energies than the other twenty-five TM-CeO2.Finally,the d-band center,partial density of states and charge density difference are employed to explain the mechanism of improving the adsorption capacity of HCHO.The simulation solid date reveals that the improved performance of CeO2of adsorbing HCHO by doping TMs ascribed to the electron rearrangement and hybridization of Ce and TMs orbitals.The present work provides a beneficial guidance for exploring practical applications of the transition metal-doped CeO2as superior HCHO detection materials.
All the density functional theory calculations are carried out in the ViennaAb-initioSimulation Package (VASP) [23],and VASPKIT[24] is used to post-process the data calculated by VASP.The generalized gradient approximation (GGA) is used with Perdew-Burke-Ernzerhof (PBE) [25,26] exchange- correlation functional.The Projector augmented-wave (PAW) method is used to deal with the core electrons,and the valence electrons are described by a plane wave basis set with a cutoff energy of 450 eV.The adsorption energy and electronic properties are calculated byk-point grid(1×1×1) and (4×4×1) Monkhorst-Pack grids,respectively.In order to avoid the interaction between periodic structures,the vacuum layer is set to 15 ˚A,and the convergence criteria of force and energy are set to 0.03 eV/˚A and 10-5eV,respectively.
The adsorption energy (Eads) of HCHO adsorbed on CeO2surface that measures the strength of the interaction between HCHO and the surface,is calculated as
whereEtotal,EsubstrateandEHCHOrepresent the total energy of CeO2adsorbing HCHO,the energy of CeO2substrate and the energy of HCHO molecules in vacuum,respectively.It is be considered adsorption energies below -0.5 eV to be ‘strong’,or chemisorbed,while adsorption energies above -0.5 eV to be ‘weak’,or physisorbed in this work [27].
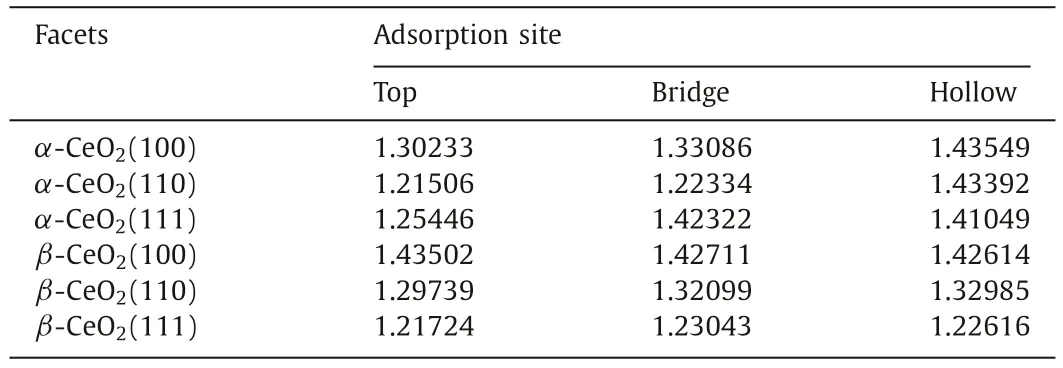
Table 1 C–O bond length of HCHO adsorbed on α-CeO2 and β-CeO2 surfaces.
The binding energy (Ebinding) of TMs doped CeO2is calculated as follows:
Among them,Etotal,Etotal-TMandETMrepresent the energy of TM doped CeO2,the energy of TM doped CeO2without TM,and the energy of a metal atom in vacuum,respectively.The negative value ofEbindinindicates that the doping of TM is an exothermic reaction.In general,the more negative their values are,the more stable the adsorption is.
In order to simulate the sensitivity of selected TM-α-CeO2(100)(TM=Au,Hf,Nb,Ta,Zr),characteristics between sensor response and the current-voltage are calculated based on the nonequilibrium Green’s function formalism of the SMEAGOL package [28].The double-zeta basis with a cut off energy of 600 Ry and generalized gradient approximation of Perdew-Burke-Ernzerhof were used.The Brillouin zone is set on 1×8×10 and 1×10×10 Monkhorst-Packk-meshes in the electrode and transport calculations,respectively.
Two CeO2phases,noted asα-CeO2andβ-CeO2,are employed to simulate.As shown in Fig.S1a (Supporting information),α-CeO2in a cubic fluorite structure with the space grouping Fm¯3m[29].Ce is surrounded by eight nearby O,while O is surrounded by four nearby Ce.The lattice parameters area=b=c=5.411 ˚A,α=β=γ=90° As shown in Fig.S1b (Supporting information,β-CeO2with the space group isP42/mnmand the lattice constant isa=b=5.135 ˚A,c=3.636 ˚A,α=β=γ=90° in order to compare withα-CeO2.The low index surfaces of CeO2are CeO2(100),CeO2(110) and CeO2(111) [30,31].The surfaces ofα-CeO2andβ-CeO2before and after optimization are demonstrated in Figs.S1c-f (Supporting information) and Figs.S1g-l (Supporting information),respectively.According to previous report,the most favorable configuration for HCHO adsorption over stoichiometric oxides is normal to the surface with the oxygen bound to the metal cation[32].The three sites (top,bridge and hollow) of HCHO adsorbed vertically onα-CeO2andβ-CeO2surfaces above Ce atom are selected in Fig.S2 (Supporting information).
After structural optimization,eighteen stable models are obtained.The adsorption energies and C–O bond length (d) of HCHO are used to evaluate the adsorption stability of HCHO on three facets.As describe in Table 1 and Figs.1a and b,the lowest adsorption energies of HCHO onα-CeO2(100),α-CeO2(110),α-CeO2(111),β-CeO2(100),β-CeO2(110),β-CeO2(111) are -2.99 eV,-1.49 eV,-1.56 eV,-3.67 eV,-1.50 eV and -1.35 eV,respectively.It is considered that adsorption energies below -0.5 eV is to be‘strong’,or chemisorbed [27].All of them are lower than -0.5 eV,indicating that the adsorption of HCHO on CeO2belongs to chemical adsorption.It is worth noting that their corresponding bond lengths are longer than the other two adsorption sites.For instance,adsorption energies ofα-CeO2(100) on three sites are hollow (-2.99 eV)>bridge (-1.51 eV)>top (-1.12 eV).Their corresponding C–O bond length are hollow (1.44 ˚A)>bridge (1.33 ˚A)>top (1.30 ˚A).The more negative adsorption energy and the longer bond distance with interacting of CeO2surfaces and HCHO indicate higher performance to adsorb HCHO.Therefore,the orders of adsorption capacity of HCHO on CeO2areα-CeO2(100)>α-CeO2(111)>α-CeO2(110) andβ-CeO2(100)>β-CeO2(110)>β-CeO2(111).The hollow site for HCHO ofα-CeO2(100) and the top site for HCHO ofβ-CeO2(100) are employed in the following simulation.
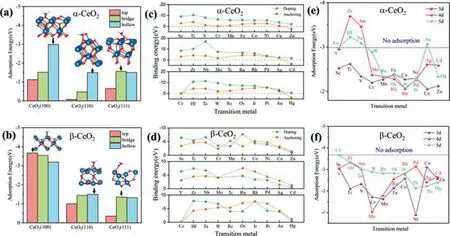
Fig.1.Adsorption energies of HCHO at three adsorption sites (top,bridge and hollow) of (a) α-CeO2 and (b) β-CeO2.Binding energies of TM doping at two sites of (c) α-CeO2(100) and (d) β-CeO2(100).Yellow and green spots indicate that TMs are doped on anchoring site and doping site,respectively.Adsorption energies of HCHO adsorbed by (e) TM-α-CeO2 and (f) TM-β-CeO2 (TM=Sc,Ti,V,Cr,Mn,Fe,Co,Ni,Cu,Zn,Y,Zr,Nb,Mo,Ru,Rh,Pd,Ag,Cd,Hf,Ta,W,Re,Os,Ir,Pt,Au,Hg).
In order to explore the favorable adsorption sites to TMs doping on CeO2,28 candidate TMs (TM=Sc,Ti,V,Cr,Mn,Fe,Co,Ni,Cu,Zn,Y,Zr,Nb,Mo,Ru,Rh,Pd,Ag,Cd,Hf,Ta,W,Re,Os,Ir,Pt,Au,Hg) are designed to investigate their binding energies at anchoring site and doping site of CeO2.The structural models are displayd in Fig.S3 (Supporting information).According to the comparison of binding energies (Figs.1c and d),it is show that (i) for TM-α-CeO2(100),most of TMs are apt to be doped at the doping site,while Pt and Au are more squint towards be doped at the anchoring site.For TM-β-CeO2(100),TMs before the VIB group of the elements periodic table are apt to be adsorbed at the doping site.However,the TMs after the VIIB group of the elements periodic table are more inclined to be adsorbed at the anchoring site(except Fe); (ii) TMs doping is an exothermic reaction in virtue of their binding energies below 0 eV (except for IIB group TMs since they with no incomplete d shell and their electronic properties are not active).In the following simulation,doping site for TM-α-CeO2(100) is employed to adsorb HCHO.As for TM-β-CeO2(100),we use sites that TMs are more inclined to doping.
To explore the adsorption capacity of TM dopedα-CeO2(100)andβ-CeO2(100) to with HCHO,their adsorption energies and electronic properties are calculated.The calculated results of adsorption energies are displayed in Figs.1e and f.Among them,the adsorption energies of five TM dopedα-CeO2(100) are lower thanα-CeO2(100).They are Zr-α-CeO2(-3.70 eV),Nb-α-CeO2(-3.47 eV),Hf-α-CeO2(-3.23 eV),Ta-α-CeO2(-3.09 eV) and Auα-CeO2(-3.07 eV),respectively.It indicates that the adsorption capacity of CeO2for HCHO is slightly enhanced owing to five TMs doped.However,the adsorption energies of TM dopedβ-CeO2(100) are higher thanβ-CeO2(100),indicating thatα-CeO2(100) is more suitable for adsorbing HCHO.The transition states and the structural parameters of HCHO adsorb at TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr) are displayed in Figs.S4 and S5(Supporting information),respectively.Thed(Ce-O) and A(H–C-H)of TM-α-CeO2(100) are smaller thanα-CeO2(100).It is consistent with the result of adsorption energies.
Partial density of states (PDOS) and d-band center are used to further explain the mechanism that TM dopedα-CeO2(100) increases the CeO2adsorption capacity of HCHO.As shown in Fig.2a,the d orbitals of TMs and the d orbitals of Ce hybridized obviously from -6 eV to -4 eV.The d orbitals of Ce have obviously changed indicating that TM doped changes the d orbit of Ce,rearranges the electrons of the Ce,result in TMs can be stably doped on the surface of CeO2.According to above results,it is concluded that the adsorption performance of HCHO onα-CeO2(100) is stronger than HCHO onβ-CeO2(100).
The d-band center of Ce is calculated using the following method (Eq.3):
In general,the shift of d-band center away from the Fermi level will increase the filling of the antibond state,thus weakening the adsorption of the adsorbent,otherwise it will enhance the adsorption of the adsorbent [33].In our results,the d-band of TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr) are closer to the Fermi level thanα-CeO2(100),illustrating that TM doped increases the bonding state and led to the enhancement of the interaction between Ce and HCHO.Similarly,we find that the fermi level of HCHO adsorbed TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr) have a red shift(Fig.2b).Therefore,TM doped decreases the free energy ofα-CeO2(100),resulting in more stable structure [34].
The Charge density difference (CDD) is employed to further clarify the electron transfer mechanism of HCHO adsorbed on TMα-CeO2(100),it is calculated by Eq.4.
In the formula,ρTM-CeO2–HCHO,ρTM-CeO2andρHCHOare the charge densities of HCHO adsorbed on TM-α-CeO2(100),α-CeO2(100) and isolated HCHO molecule,respectively.As displayed in Fig.2c,the yellow and cyan areas denote the charge accumulation and loss,respectively.It reveals that the C atom of HCHO obtains electrons from two Ce atoms ofα-CeO2(100).After doping TM,the C atom with HCHO is inclined to obtain electrons from TM result in forming covalent bonds of C of TM.Additionally,electron localization function (ELF) maps confirm that HCHO has a strong bonding with TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr) but a weak interaction withα-CeO2(100) (Fig.3).Hence,the TM doped promote the adsorption of HCHO on TM-α-CeO2(100).
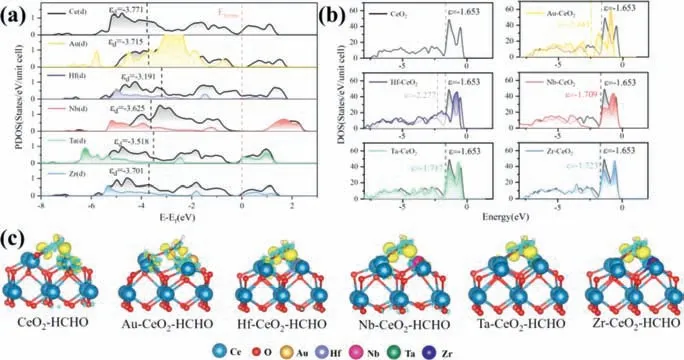
Fig.2.(a) PDOS of HCHO adsorb at TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr).0 eV represents Fermi level.The black lines represent the D-band of Ce in HCHO adsorb at TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr).(b) The DOS of HCHO adsorb at TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr).(c) The charge density difference of TM-α-CeO2(100)(TM=Au,Hf,Nb,Ta,Zr).
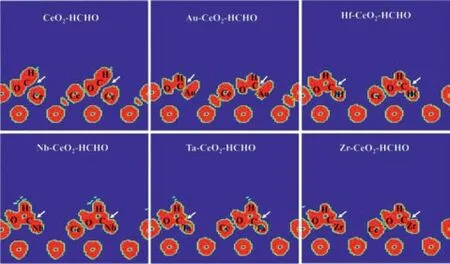
Fig.3.Electron localization function of HCHO adsorb at TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr).The white arrow points to the adsorption site of CeO2 surface and HCHO,and the connection of TMs to HCHO indicates strong adsorption capacity.The saturation value is 0.1.
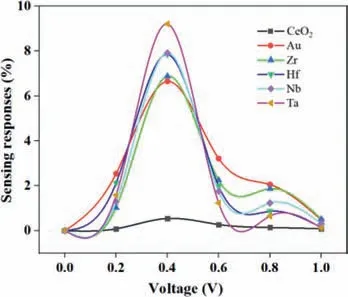
Fig.4.Current-voltage characteristics along the TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr) directions for sensing responses of HCOH.
Fig.4 shows the current-voltage characteristics along the TMα-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr) after the adsorption of a HCHO molecule.When a voltage of 0.4 V is applied,the sensing response is the highest for HCHO for all of transport directions.As an applied voltage increases,the sensing response follows the order,Au>>Zr>Nb>Hf>Ta>substrate.Therefore,at an applied voltage of 0.4 V,it is concluded that Au-α-CeO2(100) shows excellent sensitivity performance than other TM-α-CeO2(100) sensor.
In conclusion,we proposed two CeO2phases (Fm¯3m andP42/mnm),three facets (CeO2(100),CeO2(110) and CeO2(111)) and three adsorption sites (top,bridge and hollow) as substrate models.DFT calculation reveals that (i) The hollow site for HCHO ofα-CeO2(100) and the top site for HCHO ofβ-CeO2(100) are considered to be suitable for the adsorption of HCHO by comparing adsorption energies; (ii) TM-α-CeO2(100) (TM=Au,Hf,Nb,Ta,Zr)are screened as candidate materials to absorb HCHO on account of TM doped improves the performance of adsorbing HCHO; (iii) TM doped changes the d orbit,rearranges the electrons of Ce,causesα-CeO2(100) with lower free energy result in TM doped improves the performance ofα-CeO2(100) for adsorbing HCHO; (iv) C atom gets electrons and forms covalent bond with TM contribute to improve the performance of surfaces for adsorbing HCHO.These findings would provide a route to design HCHO sensor materials with superior performance.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work,there is no professional or other personal interest of any nature or kind in any product,service and/or company that could be construed as influencing the position presented in,or the review of,the manuscript entitled.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.22005269); the NSFC-Zhejiang Joint Fund for Integration of Industrialization and Diversification (No.U1809214);the National Natural Science Foundation of Zhejiang Province(No.LQ21B030007) and the Science and Technological program of Ningbo (No.2021S136)
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.04.074.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
