Zirconia prepared from UIO-66 as a support of Ru catalyst for ammonia synthesis
Chuanfeng Zhang,Siyu Shi,Biyun Fang,Jun Ni,Jianxin Lin,Xiuyun Wang,Bingyu Lin,Lilong Jiang
National Engineering Research Center of Chemical Fertilizer Catalyst,College of Chemical Engineering,Fuzhou University,Fuzhou 350002,China
Keywords:Ru catalyst ZrO2 Carbon Hydrogen adsorption-desorption Ammonia synthesis
ABSTRACT The development of effective Ru catalyst for ammonia synthesis is of important practical value and scientific significance because of the wide application of ammonia as a fertilizer and its promising applications in the renewable energy.Generally,ZrO2 was regarded as an inferior support for Ru catalyst used in ammonia synthesis.Here we prepare ZrO2 with monoclinic phase and carbon species from ZrCl4 following the preparation route of UiO-66 as well as ammonia treatment.Owing to the presence of a larger amount of hydrogen adsorption as well as the easier desorption of hydrogen species,the ill effect of hydrogen species on the nitrogen adsorption-desorption and ammonia synthesis can be effectively alleviated.The resulting ZrO2-supported Ru catalyst showed 4 times higher ammonia synthesis activity than the conventional Ru/ZrO2 obtained from zirconium nitrate.
Ammonia (NH3) has attracted worldwide interest because of its successful applications in as a fertilizer in crop production as well as its promising applications in the renewable energy [1–3].However,the high energy-consumption and high emission of ammonia production (Haber–Bosch process using Fe catalyst),which represents 1%–2% world’s energy every year and more than 1%CO2emission [4,5],impedes the widely application of ammonia in the future.Diverse strategies [6–11],including electrocatalysis,photocatalysis,plasma catalysis,have been used to develop the environmentally-benign ammonia synthesis process.Unfortunately,the efficiency of these methods is far below commercially viable levels [6,7],and Haber-Bosch process is still the only method for the large-scale ammonia production,and thus the continuing improvement in catalyst is necessary to lower the energy consumption [7,12–15].The replacement of Fe catalyst by carbon-supported Ru catalyst can decrease significantly the energy consumption of ammonia synthesis [16],but the application of Ru catalyst in ammonia synthesis is greatly inhibited by carbon loss,including carbon methanation and carbon oxidation [17,18].It is highly desirable to develop an effective Ru catalyst for ammonia synthesis,and oxide-supported Ru catalysts are supposed to be the promising candidates.
It has been reported that ZrO2was inferior supports for Ru catalysts of ammonia synthesis [19].However,the catalytic activity of oxide-supported metal catalyst could be improved by tuning the metal–support interaction [20–24].The N2dissociation is proposed to be the rate-determining step of ammonia synthesis [25–27],but other steps,including the hydrogen activation,the reactions of H and N atoms,as well as the desorption of ammonia or other unreacted gases (N2and H2),also can strongly affect the ammonia synthesis activity of Ru catalysts.Wuet al.found that owing to the higher mobility of hydrogen species,the polar MgO(111) supportported Ru catalysts showed higher ammonia synthesis activities than those supported on nonpolar MgO [28].Linet al.reported that the change in the alumina phase affected the desorption/desorption property of hydrogen species,leading to enhancement of ammonia synthesis activities for alumina-supported Ru catalysts [29].In the meantime,it has been found that various pretreatment methods,including of N2H4reduction [30],NaBH4treatment [31],CO activation [32] and sacrificial sucrose strategy [22],would affect the adsorption-desorption property and the desorption pathway of hydrogen species for ceria-supported Ru catalysts,resulting in enhancement of the ammonia synthesis rates.As a result,it is envisaged that ZrO2or other oxides might be able to be support candidate for Ru catalyst used in ammonia synthesis by optimizing of hydrogen adsorption-desorption property.
In this work,we prepared ZrO2from ZrCl4following the preparation route of UiO-66.The results showed that the ammoniatreated ZrO2prepared from ZrCl4contained carbon species and monoclinic ZrO2,the resulting Ru/ZrO2catalyst showed 4 times higher ammonia synthesis activity than the counterpart obtained from zirconium nitrate.The superior performance can be attributed to a larger amount of hydrogen adsorption as well as the easier desorption of hydrogen species,which resulted in the alleviation ill effect of hydrogen species on the nitrogen adsorptiondesorption and ammonia synthesis.This work confirms the possibility that the design of high active Ru catalyst for ammonia synthesis by the use of those oxides,which are traditionally not considered to be an ideal support for Ru catalyst.
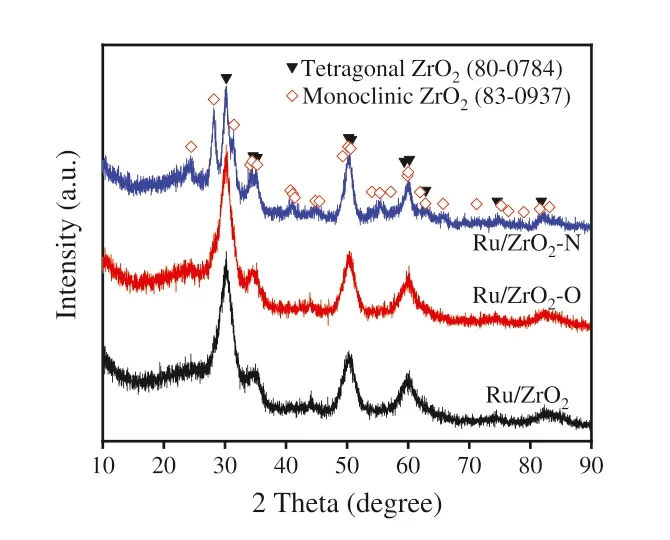
Fig.1.XRD patterns of the reduced Ru catalysts.
ZrO2–O and ZrO2–N were prepared from ZrCl4following a preparation route of UiO-66 [33],the resulting UiO-66 was calcined in air or NH3at 600 °C for 3 h to obtain ZrO2–O (air) or ZrO2–N (NH3).The traditional zirconia (ZrO2) was obtained by decomposing of ZrO2sol–gel solution [34].Ru was introduced by incipient wetness impregnation of zirconia with ruthenium(III) nitrosyl nitrate solution.The diffraction peaks of tetragonal zirconia at 2θ=30.3,34.6,35.3,50.3,50.7,59.4,60.2,62.9,74.6 and 81.8° can be found in the XRD patterns of Ru/ZrO2and Ru/ZrO2–O(Fig.1).However,besides the peaks of tetragonal zirconia,the new diffraction peaks at about 24.5,28.2 and 31.6°,which are assigned to the monoclinic zirconia (PDF #81–1314) [35–37],can be found in the XRD patterns of Ru/ZrO2–N.It can conclude that the tetragonal ZrO2is dominant phase in Ru/ZrO2and Ru/ZrO2–O,while there are the mixed-phases of tetragonal and monoclinic ZrO2in Ru/ZrO2–N.A similar conclusion on the difference in zirconia phases could be drawn from Rietveld analysis based on the as-prepared ZrO2supports (Fig.S1 in Supporting information).On the other hand,no characteristic diffraction peaks corresponding to Ru metal or Ru oxide are found,indicating that Ru species is well dispersed on support material.This result is in line with the observations of EDS mappings and TEM images (Figs.S2 and S3 in Supporting information).The results of temperature-programmed oxidation study of ZrO2–O and ZrO2–N show that there is a larger amount of carbon species on ZrO2–O than that on ZrO2–N (Fig.S4 in Supporting information).However,the differences in the amount of carbon species between Ru catalysts are slight because most unstable carbon would be removed during the heat treatment of Ru catalysts.As a consequence,elemental analysis shows that the carbon contents of Ru/ZrO2–O and Ru/ZrO2–N are 3.67 and 3.50 wt%,respectively (Table S1).A similar amount of carbon species remained on Ru/ZrO2–O and Ru/ZrO2–N is also confirmed by the result of Raman spectra (Fig.S5 in Supporting information),and two bands at 1345 (D band) and 1595 cm-1(G band),which are characteristic peaks of carbon [38–41],can be found in Raman spectra of Ru catalysts.The presence of carbon species leads to enhancement of the surface area for Ru catalysts,but no significant difference in the surface area can be found between Ru/ZrO2–O and Ru/ZrO2–N catalysts (Table S1 in Supporting information).Moreover,the Ru loadings are 5.55,5.28 and 5.37 wt% for Ru/ZrO2,Ru/ZrO2–O and Ru/ZrO2–H (Table S1),indicating that the variation in catalytic performances of Ru catalysts cannot be fully attributed to the change in Ru content.
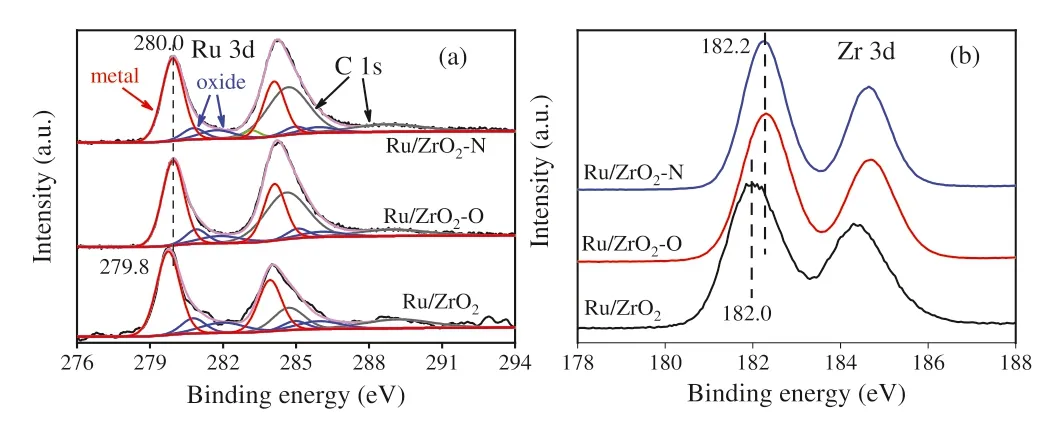
Fig.2.XPS spectra of Ru catalysts (a) Ru 3d and (b) Zr 3d.
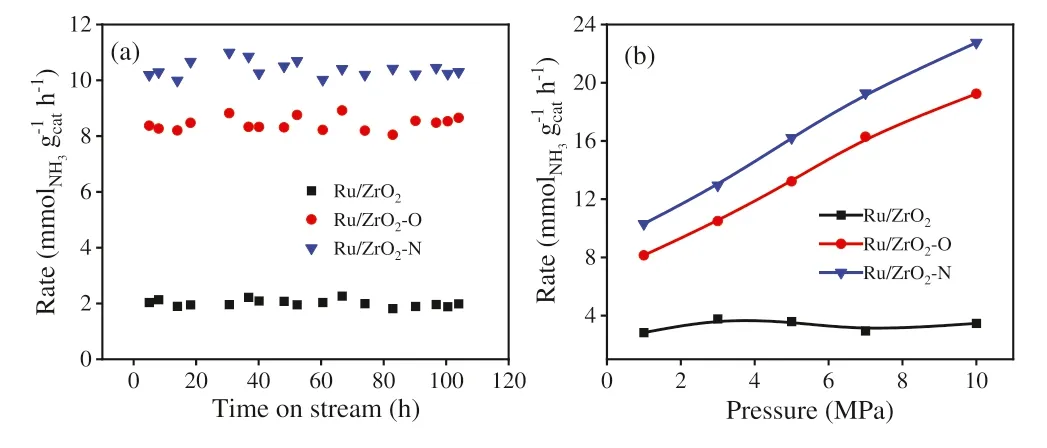
Fig.3.(a) Time dependence of ammonia synthesis rates and (b) pressure dependence of ammonia synthesis activities at 400°C,36,000 mL g-1 h-1 and 3:1 of H2/N2 ratio.
As shown in the XPS spectra of Ru catalysts (Fig.2),the ratio of C 1s to Ru 3d are 0.29,0.68 and 0.59 for Ru/ZrO2,Ru/ZrO2–O and Ru/ZrO2–N catalysts,confirming that there is a larger amount of carbon species for the samples prepared from ZrCl4.The binding energy of Ru metal appears at about 279.8 eV for Ru/ZrO2,while the values can be found at 280.0 eV for Ru/ZrO2–O and Ru/ZrO2–N.It is well known that electronic metal support interaction would affect the electronic property of metal-support interfacial sites[42,43].The lower Zr binding energy and Ru binding energy of Ru/ZrO2indicates that there is an electronic metal support interaction for ZrO2supported Ru catalysts,which is accordance with the findings on Au@TiO2-x/ZnO [42].On the other hand,the Zr 3d peak and Ru 3d peak appear at higher binding energy for the samples with carbon species,indicating that the electronic metal support interaction would be lessened by the presence of carbon species.Besides metallic Ru,two other Ru 3d5/2peaks at 280.8 and 281.7 eV,which are characteristic XPS peaks of Ru oxides (RuO2and RuOx/Ru) [29,44-48],can be found in the XPS spectra of Ru catalysts.The slight difference in the ratio of Ru0/(Ru0+Run+)(0.70,0.75 and 0.77 for Ru/ZrO2,Ru/ZrO2–O and Ru/ZrO2–N) suggests that the presence of carbon species has a negligible influence on the proportion of metallic Ru.
The catalytic performances of Ru/ZrO2,Ru/ZrO2–O and Ru/ZrO2–N are shown in Fig.3.The ammonia synthesis rates remain stable for 120 h at 400°C and 1.0 MPa,indicating that the long-term stability of all Ru catalysts.The ammonia synthesis rate of Ru/ZrO2is 2.0 mmol gcat-1h-1.The rates of Ru/ZrO2–O are 4 times higher than those of Ru/ZrO2,and Ru/ZrO2–N shows highest ammonia synthesis rates (10.3 mmol gcat-1h-1),which are comparable to other oxide- or carbon-supported Ru catalysts reported in the literatures (Table S1) [10,11,14,27–31].The rates of Ru/ZrO2are independent of reaction pressures (from 1 to 10 MPa),demonstrating that there is hydrogen poisoning for Ru/ZrO2,which is in line with the findings over Ru catalysts supported on electride,calcium amide or La0.5Ce0.5O1.75[13,49-51].By contrast,the catalytic activities increase greatly with the increase of reaction pressure from 1 MPa to 10 MPa for Ru/ZrO2–O and Ru/ZrO2–N,indicating that the ill effect of hydrogen poisoning on ammonia synthesis rates is effectively alleviated for the samples obtained from UIO-66 which might be due to the change in the hydrogen adsorption/desorption property.
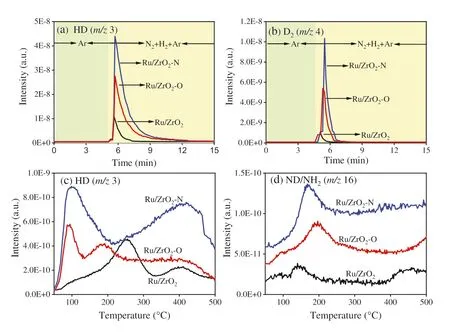
Fig.4.Mass signals of (a) HD,(b) D2 at 50°C during the D/H exchange reaction and (c) HD,(d) D2 during the TPSR study.
As shown in Fig.S6 (Supporting information),the results of H2temperature-programmed desorption show that a larger amount of hydrogen would be adsorbed on Ru catalysts using UIO-66 as precursor.Most hydrogen would desorb below 200°C for Ru/ZrO2–N,in contrast,a larger proportion of hydrogen species desorbs above 300°C for Ru/ZrO2–O.It is well known that H2molecules mainly adsorb and dissociate on metal sites for oxide-supported metal catalyst,but the resulting H atoms might migrate into the oxide support.Thus a D/H exchange reaction was performed to further study the hydrogen adsorption property of various Ru catalysts(Fig.4).After Ar purging and 3.3% N2-10% H2-Ar mixture introducing,the release of HD and D2can be found for all Ru catalysts,indicating that there is an exchange of the adsorbed deuterium species and the hydrogen species from the gaseous phase.The amount of the deuterium-containing species desorbed at 50°C decreases in the order of Ru/ZrO2–N>Ru/ZrO2–O>Ru/ZrO2.It should be noted there is no hydrogen desorption peak for Ru/ZrO2with the rise of temperature in Ar (Fig.S6),and a strong hydrogen desorption peak appears at above 320°C in the H2-TPD profile of Ru/ZrO2–O.On the contrary,strong HD signals appear at 255°C for Ru/ZrO2with the rise of temperature during the TPSR study in the N2-H2-Ar mixture over the catalyst preadsorbed with D2(Fig.4c).Moreover,a larger amount of HD species would release from Ru/ZrO2–O below 200°C during TPSR study.There results indicate that the presence of nitrogen species facilitates the desorption of hydrogen species,which is in line with previous work [23].On the other hand,besides the strongest HD signals below 200°C,there are strong HD signals in the temperature range of 220–460°C for Ru/ZrO2–N.There results suggest that the preparation of ZrO2-supported Ru catalysts from UIO-66 not only enhances the amount of the adsorbed hydrogen or deuterium species,but also facilitates the desorption of these species,which might be due to the presence of carbon species,and the formation of monoclinic ZrO2leads to the enhancement of these effects.
It is well known that there is competition between hydrogen adsorption and nitrogen adsorption on the same active sties[27,52].For a certain Ru catalyst,the presence of a larger amount of hydrogen species indicates that there are a larger number of active sites,which are responsible for nitrogen activation and ammonia synthesis reaction.In the meantime,a higher proportion of hydrogen species desorbed at low temperature leads to enhancement of active sites available for nitrogen activation and ammonia synthesis under the reaction condition.As a result,a larger amount of nitrogen deuterium species would be observed during the TPSR study (Fig.4d),as well as the higher ammonia synthesis rates for the samples obtained from UIO-66 (Fig.3).
In summary,we have successfully developed an effective ZrO2-supported Ru catalyst containing carbon species and monoclinic ZrO2from ZrCl4following the preparation route of UiO-66.The introduction of carbon species leads to lowering of the electronic metal support interaction between Ru species and ZrO2(Fig.2).Moreover,the presence of carbon not only enhances the amount of the adsorbed hydrogen species,but also facilitates the desorption of these species (Fig.4).In such a case,the ill effect of hydrogen species on the nitrogen adsorption-desorption and ammonia synthesis would be alleviated.As a result,Ru catalyst supported on the NH3-treated ZrO2obtained from ZrCl4following a synthetic route of UiO-66 shows 4 times higher ammonia synthesis activity than that prepared from zirconium nitrate.This work not only provides us an effective ZrO2-supported Ru catalyst,but also highlights a promising strategy to develop the effective oxidesupported metal catalysts used in ammonia synthesis or other involved-hydrogen reactions.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Nos.22178061,21776047,21825801,and 21978051).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.02.042.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
