Research progress on electrolytes for fast-charging lithium-ion batteries
Dn Zhng,Le Li,Weizhuo Zhng,Minghui Co,Hengwei Qiu,Xiohui Ji,*
a Shaanxi Key Laboratory of Catalysis,School of Chemistry and Environment Science,Shaanxi University of Technology,Hanzhong 723001,China
b Shaanxi Key Laboratory of Industrial Automation,School of Mechanical Engineering,Shaanxi University of Technology,Hanzhong 723001,China
c School of Electronic and Information Engineering,Qingdao University,Qingdao 266071,China
d Department of Chemistry,Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology,Tsinghua University,Beijing 100084,China
Keywords:Fast charging Lithium-ion battery Liquid electrolytes Solid-state electrolytes Salts Additive Solvent
ABSTRACT Fast-charging is considered to be a key factor in the successful expansion and use of electric vehicles.Current lithium-ion batteries (LIBs) exhibit high energy density,enabling them to be used in electric vehicles (EVs) over long distances,but they take too long to charge.In addition to modifying the electrode and battery structure,the composition of the electrolyte also affects the fast-charging capability of LIBs.This review provides a comprehensive and in-depth overview of the research progress,basic mechanism,scientific challenges and design strategies of the new fast-charging solution system,focusing on the influences that the compositions of liquid and solid electrolytes have on the fast-charging performance of LIBs.Finally,new insights,promising directions and potential solutions for the electrolytes of fast-charging systems are proposed to stimulate further research on revolutionary next-generation fastcharging LIB chemistry.
1.Introduction
Since their initial development,lithium-ion batteries (LIBs)have improved significantly in terms of their efficiency and energy density,making them the go-to choice of the automotive industry in the production of pure electric vehicles (EVs).However,the charging time of EVs remains their main limitation.While the fast-charging of EVs would boost sales and thus increase their market share,it would also eliminate mileage anxiety (the fear of being stranded in a location due to not being able to fuel/charge a vehicle) for consumers who cannot use home or office charging equipment as their main charging method [1,2].The technical limitations associated with extremely fast-charging,including lithium electroplating,accelerated aging,thermal inefficiency and electrolyte degradation,have been widely documented in recent literature [3–10].Colclasureet al.recently developed a continuum model to rank the factors that limit the rapid charging performance of the electrodes and found that the main limitations are poor electrolyte transport that leads to salt depletion at the anode and lithium plating on the graphite/diaphragm interface [1,11].As a conclusion of this study,reducing the bending inside the electrode and/or operating at high temperatures was recommended,but it was also stated that the limitations of the electrolyte must be overcome to achieve meaningful fast-charging of batteries.
LIBs currently used in EVs mainly comprise a graphite anode,a lithium transition metal oxide cathode,and a LiPF6-carbonatebased electrolyte.However,LIBs with this structure cannot be charged quickly without affecting their performance and lifespan.In many cases,fast-charging results in the structural degradation of the graphite anode,lithium plating and cathode layered cathode materials [12–19].Carbonate solvents are thermodynamically unstable with plated lithium metals and active oxygen intermediates are produced from degraded cathode materials in high state-of-charges [20].Parasitic reaction of the electrolyte with electroplating lithium and release of oxygen not only results in the electrolyte solvent being and in the recyclable lithium ions being exhausted.Fast-charging also promotes the electrochemical reduction of the electrolyte solvent on the graphite anode [21–24].Additionally,the solid and gaseous by-products produced in these reactions lead to increased battery impedance and volume expansion [25],which reduce the Coulombic efficiency of the battery and accelerate the degradation of its performance.Therefore,there is an urgent need to develop advanced electrolytes to alleviate the problems related to the rapid charging of LIBs.
In this review,an overview of the research progress on fastcharging electrolytes for LIBs is presented,highlighting their design principles.The chemical reaction mechanism of the electrode and the electrolyte is discussed,as well as the relationship between the electrochemical performance and the interface phase,focusing on the influence that the compositions of liquid and solid electrolytes have on the fast-charging performance of LIBs.
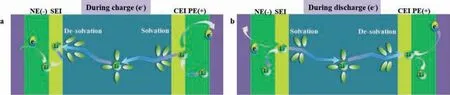
Fig.1.Schematic diagram of lithium-ion transfer during (a) charge and (b) discharge.
2.Effect of the electrolyte on the fast-charging of LIBs
LIBs should ideally have a long life,high energy and power density; be able to operate over a long period on a single charge;and be quick to charge under any weather conditions.Unfortunately,the physical properties of these requirements require various trade-offs [26]; for example,the thicker electrodes required to meet the high energy density suffered more acutely from the concentration and potential gradients resulting from fast charging[12,27,28].As shown in Fig.1,when a LIB is charged,ions move from the cathode to the anodeviathe electrolyte.The key mechanisms that affect this process are ion transport (1)viathe solid electrode,(2)viathe electrode/electrolyte interface of the anode and cathode,and (3)viathe electrolyte,including Li+ion solvation and desolvation [12].
The process of passing through a solid electrode includes five steps (Fig.2): (1) the migration of solvated Li+from the electrolyte to the electrode,(2) desolvation of the solvated Li+ions at the electrolyte-solid electrolyte interphase (SEI) interface,(3) transport of the desolvated Li+ions across the SEI,(4) charge-transfer of graphite at the SEI-graphite interface,and (5) the diffusion of Li+ions into the bulk of electrode intercalation compounds [25].In this process,the electrolyte only affects the first three steps,with the last two steps being mainly related to the electrode materials [25].The diffusion coefficient of lithium ions in liquid electrolytes is several orders of magnitude higher than that of solid electrodes.The transport of Li+ions in the electrolyte between and within the electrodes,namely the ionic conductivity of the electrolyte,is unlikely to be the rate-limiting step in fast-charging unless the electrode loadings,namely the thickness,are extremely high and lengthen the Li+ion diffusion path.The desolubilisation of solvated Li+ions at the electrolyte–SEI interface (and the solvation of Li+ions at the cathode),as well as the transport of desolubilised Li+ions in the SEI,is an important factor that determines the rechargeability of LIBs.
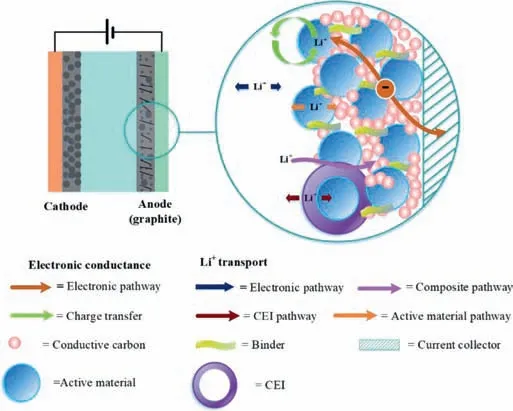
Fig.2.Anode of a LIB including the kinetic processes and Li+ pathways.
From the above analysis,it can be seen that the electrolyte characteristics have an important impact on the fast-charging performance of LIBs.Thus far,strategies used to modify LIBs and improve their fast-charging performance have included the application of electrolyte additives,the development of new solvent compositions and the development of ultra-concentrated electrolytes.Overall,an improvement in the fast-charging performance can be mainly attributed to high electrolyte conductivity and enhanced interface dynamics.
3.Electrolytes for fast charging LIBs
The electrolytes used in LIBs typically include a solution of lithium salt dissolved in a solvent mixture,with small variations in salt concentration,solvent ratio and electrolyte additive [25].The mixture of solvent and lithium salt components determines the ion migration kinetics in the electrolyte and the electrode/electrolyte interface layer [29,30].The ionic conductivity of the electrolyte does not play the main role in limiting the kinetics of lithium ion diffusion.The SEI layer is composed mainly of electrolytes,meaning that it has a significant effect on the fast-charging performance of the electrolyte.Moreover,side reactions that occur as a result of fast-charging can also lower the stability of the electrolyte.To make matters worse,the generation of internal heat or the growth of lithium dendrites will deteriorate the conductivity of the electrolyte,triggering an exothermic reaction [31–33].At a high current density,a high Li-ion concentration gradient is established in the electrolyte,which in extreme cases may limit the usable capacity of the battery.Therefore,it is necessary to develop fast-charging and safe electrolytes.
3.1.Liquid electrolytes
Among the different electrolyte systems that have been considered for LIBs to date,liquid solvent-based electrolytes offer the best combination of cost and performance due to their safety,greenness and low cost [9,34].However,the energy density and output voltage of these electrolytes are limited by their inconvenient potential window [35].Moreover,the activity of free water in an aqueous electrolyte affects its stable electrochemical window,which mainly determines the working potential and transfer capacity of the electrode [35].Overcoming the thermodynamic limitations of aqueous electrolytes has always been a longstanding scientific challenge.
3.1.1.Salts
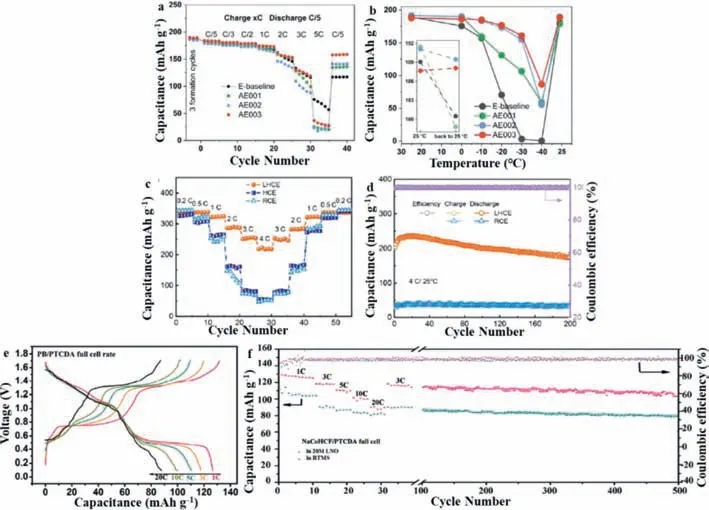
Fig.3.(a) Battery performances of different electrolytes in graphite||NMC811 coin cells of varying charge rates (xC) with the same discharge rate at C/5.(b) Battery performances of different electrolytes in Graphite||NMC811 coin cells of low-temperature discharge performance at C/5 discharge rate.Reproduced with permission [37].Copyright 2020,Wiley.(c) Rate capability for lithiation of graphite.(d) Cycling performance and Coulombic efficiency with different electrolytes at 4 C,Reproduced with permission[38].Copyright 2021,Wiley.(e) The full cell model charging and discharging curves at different rate.(f) The rate and long cycle performance of full cell at 3 C.Reproduced with permission [39].Copyright 2021,Wiley.
Realizing the fast-charging capability of LIBs would further expand their practical application.However,the consumption of lithium ions in the electrolyte limits the fast-charging of LIBs,therefore making it essential to improve the transport of lithium ions from the cathode to the anode.Conventional carbonate electrolytes used in LIBs do not exhibit satisfactory fast-charging performance; thus,there is an urgent need to develop new and efficient fast-charging electrolyte for use in LIBs.In 2019,Duet al.compared the fast-charging performance of high energy density(NMC811/graphite) LIBs when different lithium salts were added to the electrolyte [36].They found that compared with the traditional LiPF6salt,lithium bisfluorosulfonimide (LFSI) exhibited a higher conductivity and higher lithium-ion transference number.During fast-charging over 12 min,a battery containing LiPF6electrolyte reached the cut-off voltage in 4.2 min,much earlier than the 7.4 min of a battery containing LFSI.During the first 12-min charge cycle,the LFSI electrolyte showed a 13% increase in capacity,with higher capacity retention of at 87.7% after 500 cycles and less lithium plating compared with LiPF6.Zhanget al.developed an advanced electrolyte with a high oxidation potential of over 4.9 V and used it in NMC811-based LIBs to achieve excellent cycling stability across a voltage range of 2.5–4.4 V at room temperature and 60°C,good rate capabilities under fast-charging and discharging up to a rate of 3 C and good low-temperature discharge performance down to -30°C with a capacity retention of 85.6%at a rate of C/5 (Figs.3a and b) [37].These excellent properties are mainly attributable to ethylene carbonate forming an effective cathode-electrolyte interface (CEI) on the NMC811 cathode,which improves the high voltage stability of the electrolyte,and a thin but strong conductive layer,rich in inorganic components,which protects the graphite anode.Additionally,the enhanced CEI layer can effectively protect the nickel-rich NMC811 cathode from corrosion by the electrolyte and inhibit the occurrence of cracking during cycling.The enhancement of the stability of the cathode and anode interface allows LIBs to continue long-term cycling at both room and high temperature,and the increase in electrode overpotential is negligible,thereby allowing a high energy density to be maintained during operation.The results also indicate that it is not the electrolyte conductivity and viscosity but rather the interfacial layers on both the anode and cathode that govern the LIB performance during fast charging/ discharging and low-temperature discharge.Jianget al.found that a localized high-concentration electrolyte consisting of 1.5 mol/L lithium bis(fluorosulfonyl)imide in dimethoxyethane with bis(2,2,2-trifluoroethyl) ether as the diluent enables the fast-charging of working LIBs [38].A uniform and robust SEI can be achieved on graphite surface through the preferential decomposition of anions.The established SEI can significantly inhibit the co-intercalation of ether-based solvent into graphite,allowing highly reversible Li+intercalation/de-intercalation to be achieved.Graphite/Li cells exhibited fast-charging potential of 340 mAh/g at 0.2 C and 220 mAh/g at 4 C,excellent cycling stability(85.5% initial capacity retention over 200 cycles at 4 C) and impressive low-temperature performance (90 mAh/g at rate of 0.1 C at -20 °C) (Figs.3c and d).Wanget al.proposed an economical room temperature inorganic hydrated molten salt (RTMS) electrolyte with a wide electrochemical stability window of 3.1 V [39].Compared with organic fluorinated molten salt,RTMS was made up of lithium hydroxide and sodium nitrate and was much lower in cost.Based on the RTMS electrolyte,a lithium/sodium ion hybrid battery was assembled using cobalt hexacyanoferrate (NaCo-HCF) as the positive electrode and perylene-3,4,9,10-tetracarboxylic acid dihydride (PTCDA) as the anode.The full-cell with the RTMS electrolyte exhibits excellent performance,exhibiting a high capacity of 139 mAh/g at 1 C,90 mAh/g at 20 C and capacity retention of 94.7% over 500 cycles at 3 C (Figs.3e and f).This excellent performances can be attributed to the unique properties of RTMS,such as its wide electrochemical window,being solvated water-free,and its high Li+ion mobility,which promotes the excellent Li-ions insertion and extraction capacity of NaCoHCF.Zouet al.proposed a new carbonate-based high-voltage electrolyte using a mixture of ethyl methyl carbonate (EMC) and methyl acetate (MA) [40].At a normal concentration of 1.2 mol/L LiPF6,the electrolyte exhibited high stability at high voltage (4.5 Vvs.Li/Li+),lithium-dendrite-free features upon fast-charging (162 mAh/g at 3 C) and superior longterm battery performance at low temperature.More importantly,a pioneering interface model related to the Li+solvation structure was proposed for the cathode and anode,revealing the molecularscale interactions of Li+solvent anions on the electrode surface and the role they play in battery performance.
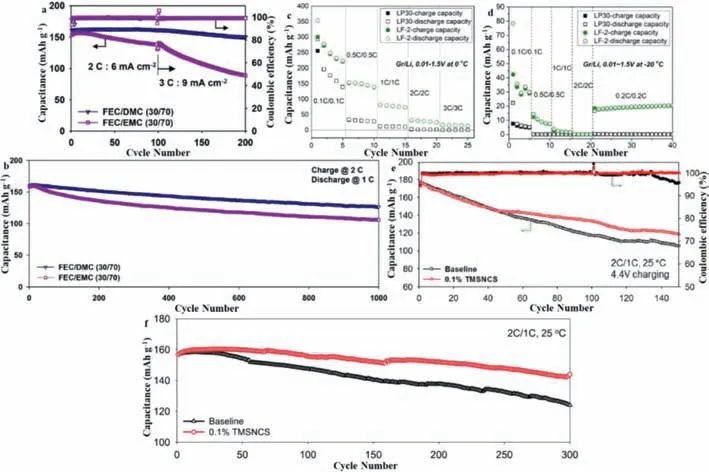
Fig.4.(a) Fast charging performance and Coulombic efficiency of NCM/graphite full cells at 2 C and 3 C.(b) Cycle performance of NCM/graphite full cells at a 2 C charge rate and 1 C discharge rate during 1000 cycles at 30°C.Reproduced with permission [43].Copyright 2018,Elsevier.(c) Rate capacity of graphite/Li coin cells with LP30 and LF-2 at 0°C.(d) Rate capacity of graphite/Li coin cells with LP30 and LF-2 at -20°C,Reproduced with permission [34].Copyright 2019,Elsevier.(e) Cycle performance of the NCM622/graphite full cells with baseline electrolyte and 0.1 wt% TMSNCS-containing electrolyte with 4.4 V charge cutoff voltage at 25 °C,a 2 C charge rate and a 1 C discharge rate during 150 cycles.(f) Cycling performance of the NCM622/graphite full cells with baseline and 0.1 wt% TMSNCS containing electrolyte at a 2 C charge rate and a 1 C discharge rate at 25°C during 300 cycles.Reproduced with permission [44].Copyright 2020,Elsevier.
There has been heavy development of new systems,such as high-concentrated,local high-concentration and perfluorinated electrolytes.The improvement in battery performance when using these new electrolytes can be mainly attributed to the formation of a strong CEI film,which helps inhibit electrolyte decomposition.However,the molecular-scale interactions of Li+ions,anions and solvents at the electrolyte-electrode interface are not yet fully understood; and the influence that they have on Li+ion transport,the electrochemical stability of the electrolyte and electrode performance is also unclear.
3.1.2.Additive
When LIBs are rapidly charged,various polarizations,such as the resistance between the electrode/electrolyte,limit the utilization of active materials and increase the growth of lithium dendrites [13].Therefore,it is necessary to design a chemically stable ion conductive passivation layer to prevent further electrolyte decomposition of the electrode [41].A good way of forming a stable passivation layer is to add a small amount of foreign molecules(also known as additives) to the bulk electrolyte [42].Therefore,in the development of fast-charging LIBs,it is particularly important to develop efficient electrolyte additives.
In 2018,Sonet al.used the reducing compounds ethylene carbonate,fluoroethylene carbonate and ethylene carbonate to conduct a comparative study to evaluate the fast-charging of a LiNi0.6Co0.2Mn0.2O2(NCM)/graphite full battery ability [43].This investigation revealed that the combination of fluoroethylene carbonate and dimethyl carbonate in the electrolyte leads to fastcharging NCM/graphite full cells that show the excellent capacity retention of 79% after 1000 cycles at a high charging current density of 6 mA/cm2,corresponding to 2 C and a discharge rate of 1 C without the occurrence of Li plating on the graphite anode(Figs.4a and b).This excellent fast-charging performance can be mainly attributed to the formation of a low-resistance interface layer on the graphite anode and the effective dissolution of lithium ions at the NCM cathode.Shiet al.used fluorosulfonyl isocyanate(FI) as an electrolyte additive to create a graphite anode SEI [33].Due to its high reduction potential of>2.8 Vvs.Li+/Li,FI is reduced before the carbonate-based electrolyte,leading to the formation of a conductive SEI on the surface of the graphite.This SEI serves as a thick and protective inorganic inner layer that prevents the growth of an outer organic layer and has the effect of greatly reducing the resistance of the graphite/electrolyte interface.Therefore,compared with the reference liquid electrolyte (LP30),Li/graphite cells with 2 wt% FI exhibit excellent rate performance at room (20 °C)and low (0 °C and -20 °C) temperature (Figs.4c and d).Hanet al.reported an electrolyte additive,(trimethylsilyl)isothiocyanate (TMSNCS) based on aminosilane,which has good electron-donating ability and can scavenge HF and PF5 [44].TMSNCS effectively suppresses the formation of the interfacial layer on the anode and cathode in LiPF6-based electrolyte,leading to the interfacial layer exhibiting long-term stability.After 300 cycles at a charge rate of 2 C and a discharge rate of 1 C,NCM622/graphite full-cell with 0.1%TMSNCS delivered a superior discharge capacity of 144 mAh/g,exhibiting excellent capacity retention of 91.8% (Figs.4e and f).Hekmatfaret al.reported the effects that the electrolyte additives fluoroethylene carbonate,ethylene carbonate and propane sulfone have on a LiNi0.5Mn0.3Co0.2O2(NMC532) electrode and lithium(half-cell) and graphite (full-cell) battery,and investigated the effect on performance when the electrodes were combined [45].Using X-ray photoelectron spectroscopy and scanning electron microscopy,the CEI layer that formed on the NMC532 electrode upon cycling at 4.5 V was studied.In this way,the electrochemical performance of the electrode was found to be related to the chemical composition,thickness and morphology of the CEI.All the electrolyte additives studied showed beneficial effects in both half- and full-cell systems,confirming the formation of an effective passivation layer and the protection of the electrolyte from further decomposition under high voltage.It was found that the thickness of the CEI layer formed on the NMC532 electrode is different in the half- and full-cell configurations.Studies have shown that ethylene carbonate and propane sulfone have a positive effect on the formation of the CEI and are the best additives to use to improve battery performance.The SEI that formed on the graphite electrode was also investigated and compared with the CEI layer formed on the cathode.The detection of typical SEI reduction products on the surface of the cathode confirmed the occurrence of cross-talking between the two electrodes.Zhaoet al.reported a simple approach by which to prepare a lithium difluorophosphate(LiPO2F2) solution as an effective film-forming additiveviathe direct adding of Li2CO3into a solution of LiPF6at 45 °C [46].Benefitting from the significantly reduced interface resistance (RSEI)and charge transfer impedance (Rct) of both the cathode and anode upon the addition of the prepared LiPO2F2solution into a baseline electrolyte,the cycling performance of the graphite||NMC532 pouch cell was remarkably improved under all of the tested condition (665 mAh/g after 350 cycles,representing 59% of its initial capacity at a rate of 0.2 C rate at -25 °C,and 1127 mAh/g after 350 cycles,repersenting 69.3% of its initial capacity at 45 °C).Chenget al.added lithium bis(oxalate)borate (LiBOB) and dopamine (DA),which have the highest-occupied molecular orbital energy levels,as functional additives to the traditional carbonated electrolyte[47].The results showed that LiBOB and DA form a strong N,B,O-rich inorganic/polymer CEI on the LiNi0.8Co0.1Mn0.1O2electrode.The CEI film not only eliminates the possibility of adverse reactions occurring at the CEI and prevents the electrolyte from penetrating into the grain boundary,but also prevents the formation of inactive rock salt phases on the surface of the material.More importantly,the N,B,O-rich interface layer enables a fast Li+diffusion kinetics process to ensure the high-rate performance of the cathode.Benefitting from the synergistic effect of dual additives containing LiBOB and DA,the cell exhibits high capacity retention of over 92% after 200 cycles at a rate of 1 C and also exhibits a high specific capacity of 118 mAh/g at a high rate of 20 C.
The above studies show that the introduction of additives into the electrolyte can lead to thein situformation of a CEI,thereby reducing or even inhibiting the electrolyte reactions that take place at the electrode interface under high pressure.However,successful electrolyte design requires a detailed study of the performance of the SEI and CEI under different cycling conditions,especially the upper cut-off potential.
3.1.3.Solvent
In standard LIBs,organic carbonate-based non-aqueous aprotic electrolytes are used.Although being the most advanced electrolytes,organic carbonates also have some disadvantages,such as their moderate ionic conductivity and low flashpoint [42,48–50].To further improve the level of battery electrolytes,many solvent classes have been comprehensively investigated to replace organic carbonates [42,51–56].Among them,nitriles and other cyano compounds exhibit high ionic conductivity and low-temperature cycling performance [57–64] and are thus expected to play an important role in the field of fast charging of LIBs.Hilbiget al.adjusted the electrolyte formula of nitrile: ethylene carbonate:fluoroethylene carbonate (BN:EC:FEC) solvent/cosolvent/additives and obtained the electrolyte formula of 1 mol/L LiPF6in nitrile:ethylene carbonate (9:1)+3% fluoroethylene carbonate,which was evaluated in terms of its ionic conductivity,electrochemical stability window,and constant current cycling performance in NMC/graphite batteries [65].At low temperatures of around 0°C,the conductivity of the nitrile:ethylene carbonate-based electrolytes was at least 32% higher (7.69 mS/cm) compared to the nitrile:ethylene carbonate-based and the organic carbonate-based electrolyte (5.83 mS/cm) electrolytes.A LIBs assembled using a nitrile-based electrolyte exhibited an average specific discharge capacity at 5 C of 103 mAh/g,which is nearly twice the capacity of the cell assembled using the reference electrolyte (66 mAh/g).Furthermore,a C-rate evaluation was performed up to 20 C.
In summary,the use of low-viscosity co-solvents,highconcentration electrolytes and additives that can achieve ideal SEI performance for fast-charging is an effective strategy to develop LIBs with a high charging rate.It should be emphasised that the rate,capacity and life of electrolytes require careful evaluation.Meanwhile,the rate capabilities of modified materials or preparation methods,including those of electrodes and electrolytes,have been mainly explored on the laboratory scale,which may not always translate into commercial batteries that show the corresponding improvements.Therefore,multidisciplinary efforts are indispensable in realizing the rapid charging of LIBs.
3.2.Solid-state electrolytes
The performance of solid electrolytes during fast charging is very different from that of liquid electrolytes [9].Since typical polymer electrolytes exhibit relatively low lithium-ion conductivity,it is difficult to use them in fast charging applications [30].Generally,due to the rigidity of the anionic polyhedral framework,inorganic solid electrolytes exhibit single-ion conductivity[66].Therefore,assuming that the charge transport is negligible,the lithium transfers number is almost equal to one unit [67].Although the ionic conductivity of most inorganic solid electrolytes is lower than the total ionic conductivity of liquid electrolytes (10 mS/cm) [54],their single ionic conductivity makes them competitive.
In 2017,Leeet al.prepared a self-crosslinking polyvinylidene fluoride (PVDF) graft copolymer using the acid group of Meldrum(MA) as the reaction siteviaatom transfer radical polymerization and utilized it as a polymer matrix for a gel polymer electrolyte(GPE) [67].Compared with pure PVDF-based GPEs,the addition of MA groups to PVDF chains simultaneously brings about crosslinking properties and high polarity,thereby improving the thermal stability,mechanical strength and polymer matrix-electrolyte affinity of the corresponding GPEs.The prepared GPE exhibits an ionic conductivity of around 1.48 mS/cm,which is higher than the value of 0.98 mS/cm measured on a commercial Celgard 2325® separator.Moreover,good LIB performance includes a high discharge capacity (136 mAh/g at 0.2 C) and high capacity retention at high C-rates (66% at 10 C and 50% at 15 C) (Figs.5a and b).Liuet al.used a simple coaxial electric spin method to prepare a core-shell structured nanofiber PHP@PHL membrane featuring a 1-methyl-1-propylpiperidinium chloride (PPCl) ionic liquid plasticizer as the core material and inorganic Li2SiO3nanoparticles as the shell material [68].The electrolyte uptake,porosity,ionic conductivity and lithium ion transference number of the PHP@PHL nanofiber membrane were around 597%,74.0%,4.05 mS/cm and 0.62,respectively.Combining an inorganic filler and ionic liquid plasticizer,a core–shell structured nanofibrous membrane was developed,and incorporating it with a carbonate-based electrolyte,a GPE was obtained that exhibits high conductivity and an outstanding Li+transference number.Notably,a Li/electrolyte/LiNi0.6Co0.2Mn0.2O2(NCM622) half-cell featuring this composite electrolyte delivers a reversible capacity of 65 mAh/g at 20 C,which is 13 times higher than the performance of a half-cell with a Celgard 2325 membrane(Figs.5c-e).It also shows enhanced long-term cycling stability at both 3 C and 5 C for suppression of lithium dendrites.
The development of a stable solid electrolyte that exhibits high ionic conductivity is one of the key steps to achieve practical fastcharging.Solid electrolytes can be producedvia in situreactions orex situcoating on lithium metal or other anodes.When the battery is charged at a high current density,the distribution and volume changes of Li+ions must be carefully considered.Further research on the characteristics of the interface between solid electrolytes and various electrodes is urgently required.
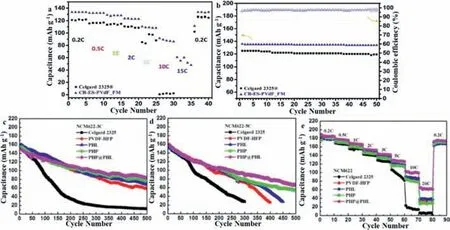
Fig.5.(a) Rate capability profile of Celgard 2325® and CR-ES-PVdF-FM at ambient temperature.(b) Capacity and coulombic efficiency of Celgard 2325® and CR-ESPVdF-FM at 0.2 C and ambient temperature.Reproduced with permission [67].Copyright 2017,Elsevier.Long-term cycling stability of the NCM622/Li cells with different membranes(Celgard 2325,PVDF-HFP,PHL,PHP,and PHP@PHL) at (c) 3 C rate,(d) 5 C rate and (e) their rate capability.Reproduced with permission [68].Copyright 2019,Frontiers.
4.Summary and outlook
Since the commercialization of ethylene carbonate-based electrolytes in the 1990s,due to the ideal coupling of ethylene-based electrolytes and graphite anodes,LIBs have been at the forefront in the field of energy storage for portable electronic products for almost 30 years and in the field of EVs for 10 years.However,the electrolytes used in traditional LIBs cannot support fast-charging.The reason for this is that the solvation and de-solvation activation energies of Li+are high,and the inter-electrode phases (SEI and CEI) are not stable enough to prevent electrolyte decomposition and structural degradation of the electrode materials.Using different solvents,lithium salts and electrolyte additives to change the structure and stability of the Li+solvation shell,advanced fast-charging electrolytes can be developed.This review provides comprehensive and in-depth progress on the research,basic mechanism,scientific challenges and design strategies for preparing new fast-charging solution systems,focusing on the influence that the compositions of liquid and solid electrolytes have on the fast-charging performance of LIBs.However,the research on electrolytes for fast-charging LIBs is still in its infancy,with many challenges that still need to be overcome.The practical application of electrolytes for fast-charging LIBs requires further efforts in terms of the following aspects:
(1) The SEI layer exhibits good ionic conductivity and stability,which are prerequisites for fast-charging,and its performance is mainly determined by the composition of the electrolyte.Therefore,it is necessary to develop electrolyte additives that form a stable interface layer that exhibits a compact shape and good ion conductivity.Moreover,solid electrolytes have potential advantages in terms of their ionic conductivity and flammability,but special structural modifications are required to solve issues surrounding solid-solid contact and interface stability.
(2) The development of stable solid electrolytes that exhibit high ionic conductivity is one of the key steps in the development of LIBs that exhibit practical fast-charging.Solid electrolytes can be producedvia in situreactions orex situcoating on lithium metal or other anodes.When batteries are charged at a high current density,the distribution and volume changes of Li+must be carefully considered.Further research on the characteristics of the interfaces between solid electrolytes and various electrodes is urgently required.
(3) To study the reactions that occur at the interface between the electrode and the electrolyte,and to determine the effects that they have on the electrochemical performance of the halfand full-cell,autopsy,on-site analysis and operational analysis are required.Additionally,an electrolyte-electrode interaction model with strong predictive ability should be established to deepen the understanding of the interphase and electrochemistry and guide the exploration of new fast-charging electrolytes.
With the amazing progress made in cutting-edge characterization technology,the development of novel theoretical calculation tools and the preparation of impressive new fast-charging electrolyte systems,it is thought that all of the above issues and challenges will eventually be resolved.The significant improvement in the electrochemical performance of fast-charging electrolytes combined with the urgent cost-driven development of LIBs will enable these promising electrolytes to be used in viable next-generation high energy LIBs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.62101296),the Natural Science Foundation of Shaanxi Province (Nos.2021JQ-760 and 2021JQ-756),the Shaanxi Province University Student Innovation and Entrepreneurship Training Program Project (No.S202110720084),and the School-level project of Shaanxi university of Technology (No.SLGRC02).
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
