Emerging multifunctional iron-based nanomaterials as polysulfides adsorbent and sulfur species catalyst for lithium-sulfur batteries—A mini-review
Xinxing Sun,Shuangke Liu,Weiwei Sun,Chunman Zheng
College of Aerospace Science and Engineering,National University of Defense Technology,Changsha 410073,China
Keywords:Iron-based nanomaterials Polysulfides Chemical anchoring Electrocatalyst Lithium-sulfur batteries
ABSTRACT Lithium-sulfur (Li-S) battery has been considered as one of the most promising next generation energy storage technologies for its overwhelming merits of high theoretical specific capacity (1673 mAh/g),high energy density (2500 Wh/kg),low cost,and environmentally friendliness of sulfur.However,critical drawbacks,including inherent low conductivity of sulfur and Li2S,large volume changes of sulfur cathodes,undesirable shuttling and sluggish redox kinetics of polysulfides,seriously deteriorate the energy density,cycle life and rate capability of Li-S battery,and thus limit its practical applications.Herein,we reviewed the recent developments addressing these problems through iron-based nanomaterials for effective synergistic immobilization as well as conversion reaction kinetics acceleration for polysulfides.The mechanist configurations between different iron-based nanomaterials and polysulfides for entrapment and conversion acceleration were summarized at first.Then we concluded the recent progresses on utilizing various iron-based nanomaterials in Li-S battery as sulfur hosts,separators and cathode interlayers.Finally,we discussed the challenges and perspectives for designing high sulfur loading cathode architectures along with outstanding chemisorption capability and catalytic activity.
1.Introduction
As a dominant type of secondary batteries in the energy storage market,lithium-ion battery (LIB),based on lithium intercalation electrochemistry,has experienced a prosperous development and growth over the last few decades,from hand-held electronic devices to all-electric vehicles and even micro/mini smart grids,almost covering all the aspects concerning to our lives.However,Li-ion battery will eventually meet its density limits (merely 400 Wh/kg under ideal conditions) with the advancement of energy storage demand within next 10 years [1,2].Therefore,a battery chemistry with a higher energy storage capability at a reasonable cost is expected.Out of the state-of-the-art energy storage technologies,lithium-sulfur (Li-S) battery,of which the constitute parts are displayed in Fig.1a has been considered as one of the most promising candidates to fulfill the forthcoming energy requirements for its overwhelming merits,including ultrahigh theoretical capacity (1672 mAh/g) and theoretical energy density(2500 Wh/kg) [3],which is almost an order of magnitude higher than that of commercial LIBs (Fig.1b) [4],and cost-effectiveness as well as natural abundance of sulfur species.
Different from LIBs,the reaction mechanism of Li-S batteries involves a multiple-electron transfer process,which can be divided into three stages approximately as shown in Fig.1c [5]:(1) S8+2e-+2Li+→Li2S8; (2) Li2S8+2/3e-+2/3Li+→4/3Li2S6;Li2S6+e-+ Li+→3/2Li2S4; and (3) Li2S4+2e-+2Li+→2Li2S2;Li2S2+2e-+2Li+→2Li2S.Notably,the reaction rate constants of processes from long-chain Li2S8to short-chain Li2S4and to Li2S2are 0.368 h-1and 0.276 h-1,respectively.In comparison,the reaction rate constant of the process from Li2S2to Li2S reaches only 0.061 h-1,which can be ascribed to the intrinsic nature of the solid-solid conversion reaction,indicating that the reaction rate of sulfur conversion is predominantly controlled by the last solidsolid process [6].Unfortunately,to achieve the practical application of Li-S battery,there is still a long way to go,which is mainly plagued by several obstacles [7,8].One is the insulting nature of sulfur and lithium sulfides,which limits the continuous conduction of cathode,thus leading to sluggish dynamics and insuffi-cient performance of active materials.Second,the intermediate lithium polysulfides (LiPSs) are highly soluble in the electrolyte,which shuttle back and forth between the cathode and anode (socalled “shuttle effect”) and react with lithium metal to produce irreversible products which deposited on the anode surface,resulting in an irreversible loss of active substrates and increasing internal resistance which led to sluggish kinetics of LiPSs conversion reaction consequently.Third,the undesirable volume fluctuation of sulfur during charge and discharge process since the grant difference in density between S8(2.07 g/cm3) and Li2S (1.66 g/cm3).Last but not least,the uncontrollable lithium dendrites grow on the anode surface owing to the repeated dissolution of lithium (stripping process) and its heterogeneous deposition on the anode (platting process).
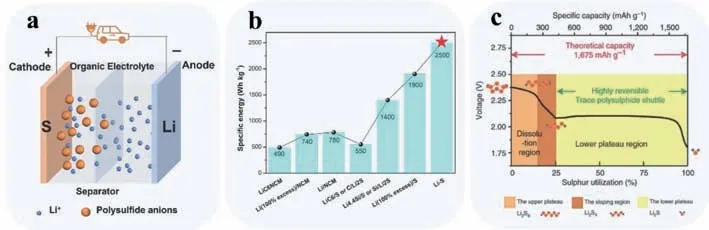
Fig.1.(a) A schematic diagram of a Li-S battery.(b) Specific energies of alternative technologies for LIBs.Reproduced with permission [4].Copyright 2021,The Royal Society of Chemistry.(c) The discharge curve correlated to reaction mechanism of Li-S battery.Reproduced with permission [5].Copyright 2013,Nature Publishing Group.
To address the above-mentioned critical issues,enormous efforts have been dedicated towards different components of Li-S battery to promote the electrochemical performances,including multifunctional cathodes [9],modified separators [10],intermediate interlayers [11] and additive electrolytes [12],etc.Among these sundry optimization methods,the most studied and effective measurement is to construct a multifunctional cathode.With the developing understanding about Li-S battery system,the construction strategy of cathode can be divided into three stages.
At the first stage,various carbon-based materials with elaborately designed porous structures and interconnected networks such as graphene sheets,porous carbon,carbon spheres or nanotubes,are designed and adopted to accommodate sulfur for enhanced battery performances.For instance,these carbon matrix materials,usually with good compatibility for sulfur and excellent conductivity of electrons and ions,are designed to provide abundant sulfur loading space and physical confinement for polysulfides.
However,the weak interaction between the non-polar carbonaceous matrix and polar polysulfides cannot suppress the shuttle effect effectively over long-term cycling,leading to severe capacity degradation irresistibly.In order to enhance the chemisorption ability of carbon host,heteroatoms [4] and intrinsic defect [13] are introduced in carbon matrix to offer active sites with polar bonds for the second stage.The doped atoms contain metal atoms (Fe,Co and Ni,etc.) and non-metal atoms (N,P,S and B,etc.),which exhibit strengthened capability to immobilize polysulfides to an extent.Nevertheless,their electrochemical performances are still insufficient to afford large-scale utilization because of the finiteness of the adsorption sites and the sluggish reaction kinetics.
For the third stage,researchers found sulfur host materials that feature both chemical adsorption and electrocatalytic capabilities for LiPS species more effectively to solve the above problems of sulfur cathode [14].The polar metals (Pt,Ag,etc.) and transition metal compounds (oxides,nitrides,sulfides,phosphides,borides,hydroxides,etc.) catalysts could accelerate the conversion reaction of anchored LiPSs as well as suppress the shuttling effect for strong chemical adsorptions which is favorable for developing practically useable Li-S battery [15].Avaraet al.[16,17] first studied the electrocatalytic effects of noble metal Pt towards the polysulfides redox reactions in the early days and found that the Pt nanocrystals can promote the conversion of Li2S2or Li2S to long-chain polysulfides effectively,and mitigate the agglomeration of active substances on the electrode during cycling.In order to explore more scalable metal catalysts,numerous transition metals and their compounds have been introduced into Li-S battery system for catalyzing the redox reactions of LiPSs,as well as supplying with stronger chemical bonding forces towards polar polysulfides,such as Ti-based[18],Fe-based [19],Co-based [20],Ni-based [21].
Among these common metal elements,Fe-,Co- and Ni-based compounds [14,22] attracted most attentions because they belong to the VIII family in the periodic table,and have excellent catalytic properties due to the unfilled d orbital in valence electrons.In addition,due to the natural abundance,low cost,good biocompatibility,non-toxicity and catalytic activity of iron,Fe-based electrocatalysts have attracted researchers’interests in many fields such as conversion of CO2to CO [23],oxygen reduction reaction [24],as well as energy storage systems like sodium-ion battery [25],potassium-ion battery [26],lithium-ion battery [27] and lithiumsulfur battery [28].The uniqueness of electronic structure and low field splitting energy of iron atoms which benefit the formation of various iron-based compounds effortlessly have granted the prosperous development of iron-based materials.Besides,the characteristics of rich raw materials,low cost and environmental friendliness of iron-based electrocatalysts favors their large-scale application compared with other transitional metal catalysts,such as Coand Ni-based compounds [20,29,30].
In recent years,a massive number of iron-based nanomaterials with extraordinary chemisorption abilities and catalytic effects towards polysulfides have been incorporated with carbon matrices as sulfur hosts,separator modifications or cathode interlayers for Li-S battery.The research and development of iron-based nanomaterials towards Li-S battery generally focus on the following three aspects: First,modulating iron-based compound types to explore the catalytic and chemisorption properties of different iron compounds on polysulfides conversion such as iron oxides,iron carbides,iron nitrides,iron sulfides,iron-based heterostructures,Secondly,hybridizing iron-based nanomaterials with carbon substrates with different morphologies for increasing the conductivity of iron-based compounds and improving their catalytic features through various regulation strategies,including defect engineering,heterostructure construction,etc.Thirdly,regulating the adsorption abilities and catalytic effects for polysulfides conversion by adjusting the orbital energy level,energy band structure and charge distribution of iron-based nanomaterials.
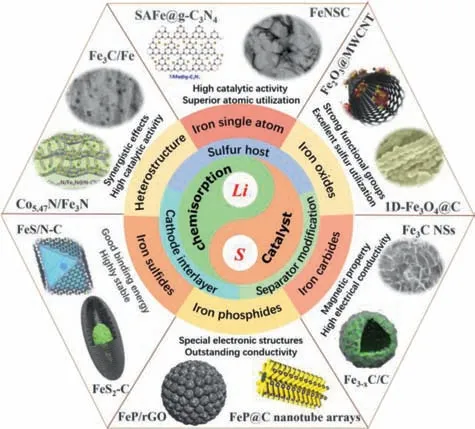
Fig.2.Schematic illustration of the current publications on iron-based nanomaterials in Li-S battery according to material types and their advantages.
Although iron-based nanomaterials have demonstrated outstanding electrochemical performances in Li-S battery,a comprehensive summarization about their application in Li-S battery and corresponding mechanist insights for the electrochemical performance enhancement has not been reported yet.Therefore,it is essential to give a timely review on the employment of iron-based nanomaterials in Li-S battery to clarify and highlight the origin of internal activities.Fig.2 summarizes the current publications on iron-based nanomaterials in Li-S battery according to material types.The introduction of iron-based nanomaterials into the highly complicated Li-S battery system can subsequently favor the effective entrapment for LiPSs,the LiPSs redox reaction kinetics acceleration and electronic conductivity enhancement,thus promoting the capacity and reversibility performances of Li-S battery.
In this review,for the first time,we systematically discuss the latest advances about popular iron-based nanomaterials applied in Li-S battery.On the basis of the recent progress,we elucidate the chemisorption and catalysis mechanisms of iron-based nanomaterials for the electrochemical reactions of Li-S battery comprehensively,and a perspective on their future research and development is also provided at last.
2.Fundamental understanding of iron-based nanomaterials by theoretical calculations
As a growing family of transitional metal compounds,ironbased nanomaterials have become one of the most suitable materials for grid-scale energy storage-conversion systems since their superior natural abundance,low cost,high safety and non-toxicity[31].For Li-S battery,with the increasing studies of iron-based materials,researches have focused on the mechanistic insights into the chemisorption interaction and catalytic effects for polysulfides conversion theoretically.Density functional theory (DFT) calculation has been gradually applied to investigate their theoretic potential in performance improvement from an atomic level,providing an effective guideline to the rational design of iron-based chemical adsorbents and electrocatalysts.
In general,depending on synthesis methods,different types of iron nanomaterials (FexOy,FexSy,SA-Fe,FexNy,FexCy,FexSy,etc.) are obtained.The ionic bonds play a dominant role in the chemical interaction with polysulfides.For instance,Yanget al.[32] studied the adsorption mechanism of iron oxides towards polysulfides through the synthesis of three-dimensional (3D) hierarchical porous graphene macrostructure coupled with uniformly distributedαFe2O3nano-particles (denoted as Fe-PGM) by implementing LiPSs static adsorption tests and DFT calculations,as shown in Fig.3a(i).TheαFe2O3displayed a strong affinity towards LiPSs,which was mainly ascribed to the forceful bonding between Li (LiPSs)-O (αFe2O3) and Fe (αFe2O3)-S (LiPSs) ions according to the DFT calculation results (Fig.3a(ii)).Moreover,with the sulfur chains shortening,the binding strength of Li-O and Fe-S both increased,correlated to the upward charge transferred from S-containing clusters to the substrate ofαFe2O3.Consequently,iron oxides can restrain the shuttling of soluble polysulfides evidentlyviathe powerful chemical interaction with LiPSs.Similar conclusions were promoted by Zhonget al.[33].Combining X-ray photoelectron spectroscopy (XPS) results as shown in Fig.3a(iii) and DFT calculations as we mentioned above,the authors found that the iron oxides can effectively immobilize LiPSs intermediates through the formation of Li-O and Fe-S bonds,while the Li-O bonds dominated in these two.On the other hand,XPS analysis was also utilized to confirm the bonding of Fe-S and Li-O as Fanet al.[34] and Liet al.[35] elucidated.
As with the iron oxides,iron sulfides also interact with polysulfides effectively through two atomic binding configurations from theoretical perspective in previous reports,namely S-binding and Li-binding (Figs.3b(i) and (ii)),where the S-binding means Fe-S bond (S is from extrinsic adsorbates such as Li2S),and Li-binding means Li-S bond where Li is from Li2S.Chenet al.[36] demonstrated that for iron sulfides,S-binding was preferable to Libinding.This was primarily concluded theoretically from the difference of the Mulliken charge change between the Li and S in Li2S (denoted as Liδand Sδ,respectively,whereδrefers to Li2S).The Mulliken charge of Sδchanged from -1.780 to -0.630 after adsorbed by FeS,which was more obvious than that of Liδ(from 0.890 to 1.030).Furthermore,it was revealed that the chemical S-binding formation is induced by electrons transferring form Sδin absorbed Li2S to Fe in FeS,of which the transferred electrons occupied the d-orbitals of Fe.Projected density of states(PDOS) analysis was also applied to reflect the obvious electron transfer between the 3d-orbitals of Fe and 3p-orbitals of Sδas displayed in Fig.3b(iii).Beyond that,another important surface property of iron sulfides was also judged according to the relative energy of lithium-ion diffusion barrier,which can influence the intercalation-deintercalation process of lithium and the reaction between lithium and sulfur significantly.As displayed in Fig.3b(iv),there is a metastable point existing between two stable points in the bimodal curve of the lithium-ion diffusion pathway of iron sulfides,of which the relative energy (0.2 eV) is much lower than that of graphene materials (0.31 eV).The bonding configurations were further confirmed by Boyjooet al.[37],which designed and fabricated the structure of highly dispersed pyrrhotite Fe1-xS nanoparticles embedded in hierarchically porous nitrogen-doped carbon spheres (Fe1-xS-NC).To investigate the chemisorption capability theoretically,DFT simulations were conducted on various surface models with different d-orbital structures,including Fe7S8,FeS2and Fe3S4,which exhibited distinct adsorption energies for polysulfides theoretically and experimentally subsequently.In this work,Li2S8was absorbed on iron sulfides through the bonding of both Li-S and S-Fe,while for other LiPSs,like Li2S2,Li2S4,Li2S6,showed the most stable adsorption structures with only Li oriented to the surface.
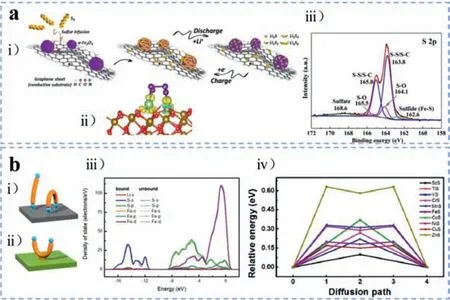
Fig.3.(a) Fe-PGM: (i) Schematic of the conversion process of sulfur on the surface of Fe-PGM.(ii) Calculated differential charge density of the Li2S4 adsorption on αFe2O3.Green,purple,yellow,red and gray balls stand for the Li,S,Fe,O and C atoms,respectively.(iii) XPS curve of S 2p of Fe-PGM-S.Reproduced with permission [32].Copyright 2017,Elsevier.(b) FeS: (i) Schematic illustration of Li-binding and (ii) S-binding.(iii) PDOS analysis of FeS.Solid line refers to iron sulfides bound with Li2S,and dotted line,unbound with Li2S.(iv) Energy profiles for diffusion processes of Li ion on various transition metal sulfides.Reproduced with permission [36].Copyright 2017,American Chemical Society.
Beyond that,the electrocatalysts coupled with Fe-N active sites are the most studied iron-based compounds for accelerating polysulfides conversion,including single-atom (SA)-Fe and iron nitrides.The SA-Fe,usually incorporated in nitrogen-doped carbon(NG) matrix with plane-symmetric Fe-4 N coordination,whereas,the iron nitrides,for example,Fe2N usually with triangular pyramidal Fe-3 N coordination (Fig.4a(i)).According the DFT calculation results promoted by Maet al.[38],the Li-N bonds and Fe-S bonds both emerged for their rationally synthesized SA-Fe/Fe2N@NG (Fig.4a(ii)),where N atoms were from the nitrogen-doped carbon and the Fe-S bonds were induced by the interaction between the Fe from SA-Fe (or Fe2N) and the S from Li2S.Interestingly,the SAFe and Fe2N displayed different electronic distribution which can be attributed to their dissimilarity of coordination structure with N atoms.The SA-Fe allowed only one-site adsorption mechanism,while for Fe2N,preferable to bind to the bridge site of two adjacent Fe sites on the surface as shown in Fig.4a(iii).The synergistic effect of Fe and N dopants has been proved to be efficacious in boosting the anchoring ability of carbon materials towards polysulfides by Liet al.[39] and Zhanget al.[40].What is more,Zenget al.[41] elaborated how the Fe-N active sites improved the performance of Li-S battery by simulating the anchoring mechanism of single-atom Fe and N co-doped graphene with different number of N coordination (FexN (x=1,2,3,4)).It was revealed that the chemisorption capability of polysulfides on FexN was evidently enhanced than on pure graphene,which can be mainly credited to the strong orbital hybridization of Fe and S atoms,resulting in the formation of strong chemical Fe-S bonds.Li-N bonds were also demonstrated to be effective to contribute to improve the electrochemical performance and strengthen the stability of co-doped FexN.
To further investigate the origin of catalytic activity of transitional metal based catalysts,Qianet al.[42] combined theoretical simulations with experimental measurements and unveiled that the d-band centers of metal atoms and the p-band centers of anions from metal compounds influenced their catalytic effects towards polysulfides reduction and oxidation significantly.The smaller energy band gap,the better polysulfides redox reaction reversibility.This theory provided a vital guidance in designing the iron-based catalysts.Yuet al.[43] fabricated carbon cloth with grown FeP@C nanotube arrays which exhibited a stronger affinity towards polysulfide species than that of Fe3O4owing to the Li-P binding and Fe-S binding (Figs.4b(i) and (ii)).It was notable that the superiority of chemisorption ability of FeP can be ascribed to the shift of the p band in FeP,which could accelerate the interfacial electronics transfer dynamics.The corresponding density of sates analysis as presented in Figs.4b(iii) and (iv) revealed that the shift triggered a significant decrease of the energy gap between the energy centers of the Fe 3d and P 2p bands.Hence,the reduction of the energy gap between bonding and antibonding orbitals led to the P atoms more easily to combine with or detach from other atoms,bringing the promotion of electron transfer and LiPSs conversion dynamics.Other iron-based phosphide compounds were also exploredviaDFT calculations [44].The chemical interactions were substantially originated from two configurations,the Li-P binding and Fe-S binding.The coexistence of these two binding endowed FeP material with remarkable capability to restrain LiPSs shuttle effect.
The polysulfide anchoring and converting activity of iron carbides were also investigated through DFT simulations.As elucidated by Zhouet al.[45],the structure of necklace-like Fe3C/Ndoped carbon nanoboxes (Fe3C/NC) connected by N-doped carbon(NC) nanofibers was utilized as multifunctional sulfur hosts.The DFT calculations revealed that the N modified carbon formed Li-N bonds as displayed in Fig.4c,while Fe3C formed Li-C bonds and Fe-S bonds,respectively,enhancing the binding properties of polysulfide species effectively.
Concluding this section,theoretical studies based on DFT calculating or DOS analyzing underlined the origin of the superior adsorption capability and catalytic activity of iron-based compounds towards polysulfides.In general,the coexistence of two binding mechanisms as we mentioned above,which are Li-X binding (X represents heteroatoms such as O,N,P and S) and S-binding (S represents sulfur atoms from polysulfides),allows for powerful affinity through chemical interaction.Furthermore,a low lithiumion diffusion barrier of polysulfides on the surface of iron-based compounds benefits the growth and deposition of insoluble Li2S,leading to the promotion of polysulfide redox reaction kinetics.On the other hand,the electrochemical performance of Li-S battery system with iron-based nanomaterials as anchors or electrocatalysts was greatly enhanced due to their strong affinity and catalytic effects.The calculated theory about the energy centers of d/p bands elucidated to some extent why the electrochemical performance of different types of iron-based compounds varied.
3.Application of iron-based nanomaterials in Li-S batteries
3.1.Iron single-atom material
Single-atom composites (SACs),which consist of isolated metal atoms dispersed or anchored on matrices,have been regarded as rising stars in the field of catalysis recently [46].With the merits of maximum atomic utilization,uniformly distributed active sites and splendid catalytic activity and selectivity,SACs have been widely employed in numerous electrochemical reactions,for instance,oxygen reduction reactions [47],hydrogen evolution reactions [48] and advanced energy systems [49–51].
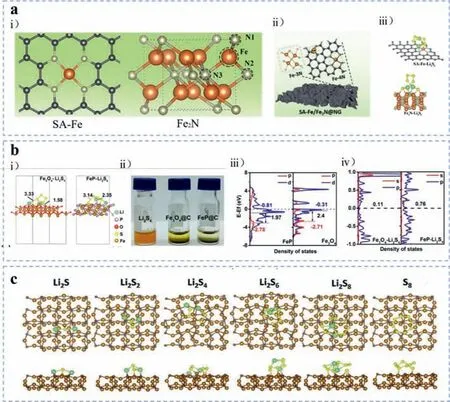
Fig.4.(a) SA-Fe/Fe2N@NG: (i) Simulated structural models of SA-Fe and Fe2N.The black,gray,and orange balls refer to C,N and Fe,respectively.(ii) Schematic nanoarchitecture of SA-Fe/Fe2N@NG.(iii) Optimized binding configurations of Li2S6 on SA-Fe and Fe2N,respectively.Reproduced with permission [38].Copyright 2021,Wiley-VCH GmbH.(b) FeP@C: (i) Simulated structural models of Fe3O4(111) and FeP(211) after Li2S4 absorption.(ii) Visual static adsorption tests of CF/Fe3O4 @C and CF/FeP@C with Li2S4.(iii) Density of states analysis (DOS) of p bands of anions and d bands of Fe in Fe3O4 and FeP.(iv) DOS of Li2S4 on Fe3O4(111)-Li2S4 and FeP(211).Reproduced with permission [43].Copyright 2019,American Chemical Society.(c) Binding structures of LiPSs and S8 on the Fe3C(220) surfaces.Reproduced with permission [45].Copyright 2021,Wiley-VCH GmbH.
Among these,the latest researches revealed that the nonprecious iron-based SACs,with stable and explicit active centers,can demonstrate the same or even better electrochemical performances than other traditional precious metal catalysts.In particular,iron-based SACs coordinated with nitrogen-doped carbon matrix have achieved much attention and great progresses as effi-cacious anchors and catalysts [52–54],especially in Li-S batteries [55–57].For example,one of the early studies using ironbased SACs as sulfur hosts was reported by Liu and co-workers in 2018 [58].The researchers fabricated the sulfur host material of single iron active sites in porous nitrogen-doped carbon (Fe-PNC,as shown in Fig.5a(i)) through polymerizing and carbonizing diphenylamine in the presence of iron phthalocyanine and a hard template.With a sulfur loading of 1.3 mg/cm2,the as-prepared Fe-PNC/S cathode displayed an initial specific capacity of 1138.6 mAh/g at 0.1 C and retained 427.1 mAh/g after 300 cycles (Fig.5a(ii)).The morphology of Li2S in both Fe-PNC/S and PNC/S after the first and second cycles at 0.1 C were also observed to clarify the comparison of phase nucleation overpotential for the formation of nanoscale Li2S between Fe-PNC and PNC.It was notable that Li2S nanospheres deposited on Fe-PNC/S cathode surface were smaller and held the morphology better in Fig.5a(iii).Encouraged by the attractive surface chemistry of single iron atoms,Jianget al.[59] developed novel self-supporting carbon nanofibers with hierarchical porous structure and Fe/N adsorption/nucleation centers (Fe/N-HPCNF) as high-performance sulfur hostsviaa facile co-spinning method.Benefiting from the porous carbon fiber structure,electrolyte infiltration,conductivity,Li ion transportation rate and sulfur redox kinetics were all improved even with high sulfur loading.The evenly distributed Fe/N heteroatoms served as restraints on polysulfide diffusion through strong chemisorption and as regulators for homogeneous sulfur nucleation.Consequently,S@Fe/N-HPCNF cathodes presented a high initial specific capacity of 1273 mAh/g for over 500 cycles at 0.5 C (the sulfur areal density equals 3.5 mg/cm2).There has been an increasing interest on utilizing iron-based SACs for Li-S battery systems as sulfur cathodes [60–63],separators [38,64,65] or cathode interlayers [66] to improve the polysulfide immobilization or sulfur species conversion.For instance,Wanget al.[60] fabricated a holey Fe,N codoped graphene (HFeNG)viaa one-step scalable calcination process.The corresponding sulfur cathodes delivered a high-rate capacity of 1154 mAh/g at 0.5 C initially and cycled stably with a slight capacity decay of 0.083% per cycle as displayed in Fig.5b(ii).The electrochemical performance advancement can be attributed to the unique holey structures and the strong adsorption forces of Fe-N2at the edges which are represented in Fig.5b(i).Considering the utilization efficiency of SACs,Zhanget al.[64] proposed a multifunctional commercial polypropylene separator coated by graphene foam impregnated with Fe SACs catalysts.As a result,the cycling stability improved hugely,with an initial specific capacity of 891.6 mAh/g and 83.7% retention after 750 cycles at 0.5 C.
Given the performance advancement arising from iron-based materials,many researches have been focused on the mechanist insights about the chemisorption capability enhancement and catalytic effect by theoretical analysis.Furthermore,Zhanget al.[67] investigated the anchoring mechanism of Fe-N4/graphene to LiPSs species by means of comprehensive DFT computations.The calculation results of binding energy suggested that the long-chain polysulfides can bind with Fe-N4/graphene stably,with two interaction configurations of Fe-S bond and Li-N bond.The computed binding energies of S-containing clusters on various metal-N4/graphene as shown in Fig.5c revealed that Cr-,Mn-,Fe-,Coand Cu-nitrogen/graphene exhibited optimal interaction with soluble lithium polysulfides due to the synergistic effects between the metal and N atoms,which not only effectively trapped the soluble lithium polysulfides to suppress the shuttle effect,but also well kept their cyclic structures.Notably,the Fe-N4/graphene displayed an appropriate binding energy among them,which facilitates a smoother trapping-diffusion-conversion process of LiPSs[68,69].The researchers also compared the electronic band structures of Co-N/graphene before and after polysulfides adsorption and found that there was a small band gap of less than 0.50 eV,which indicated the obvious intrinsic semiconducting nature of Co-N4/graphene even after polysulfides adsorption.This theory also provides some experiences for Fe-N4/graphene.However,the relationship between these two binding mechanisms still remained mysterious.Lianget al.[70] explored that there is a competitive relationship between Li-bonding and S-bonding.Moreover,the S-bonding configuration usually has better performance for both chemisorption and catalysis than Li-bonding.Similar results were obtained by Linet al.[71] and Zhanget al.[40].
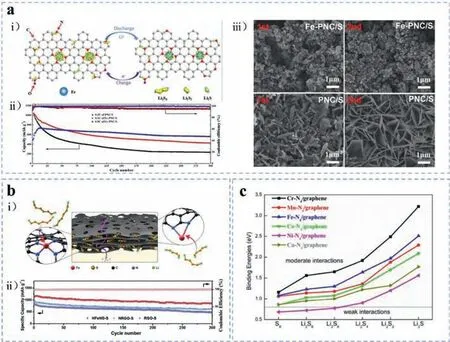
Fig.5.(a) Fe-PNC: (i) Schematic diagram of the redox reaction of polysulfides catalyzed by single iron atoms on the surface of Fe-PNC.(ii) Long-term cycling stability of PNC/S and Fe-PNC/S electrodes at 0.1 C and 0.5 C,respectively.(iii) Micro morphologies of PNC/S and Fe-PNC/S electrodes after cycling at 0.1 C for the first and second cycle,respectively.Reproduced with permission [58].Copyright 2018,American Chemical Society.(b) HFeNG: (i) Schematic elucidation of entrapment of sulfur species in the layered nanostructure and the additional lithium-ion diffusion pathways (purple arrow line) through the holey structure (the black sheets).(ii) The long cycling performance and relative Coulombic efficiency of RGO-S,NRGO-S,and HFeNG-S electrodes at 0.5 C.Reproduced with permission [60].Copyright 2018,WILEY-VCH GmbH.(c) The computed binding energies of S-containing clusters on various metal-N4/graphene.Reproduced with permission [67].Copyright 2018,Elsevier.
3.2.Iron oxides
Owing to the low cost,easy availability and strong functional groups on the surface,iron oxides,including FeO,Fe2O3,Fe3O4,etc.have been extensively applied in lithium-ion battery and sodiumion battery since early stages [72–74].However,the poor conductivity and uncontrollable size seriously retard the electrochemical performance of iron oxides.Hence,to overcome these limitations,hybridizing iron oxides with various carbon-based materials has been an effective way.
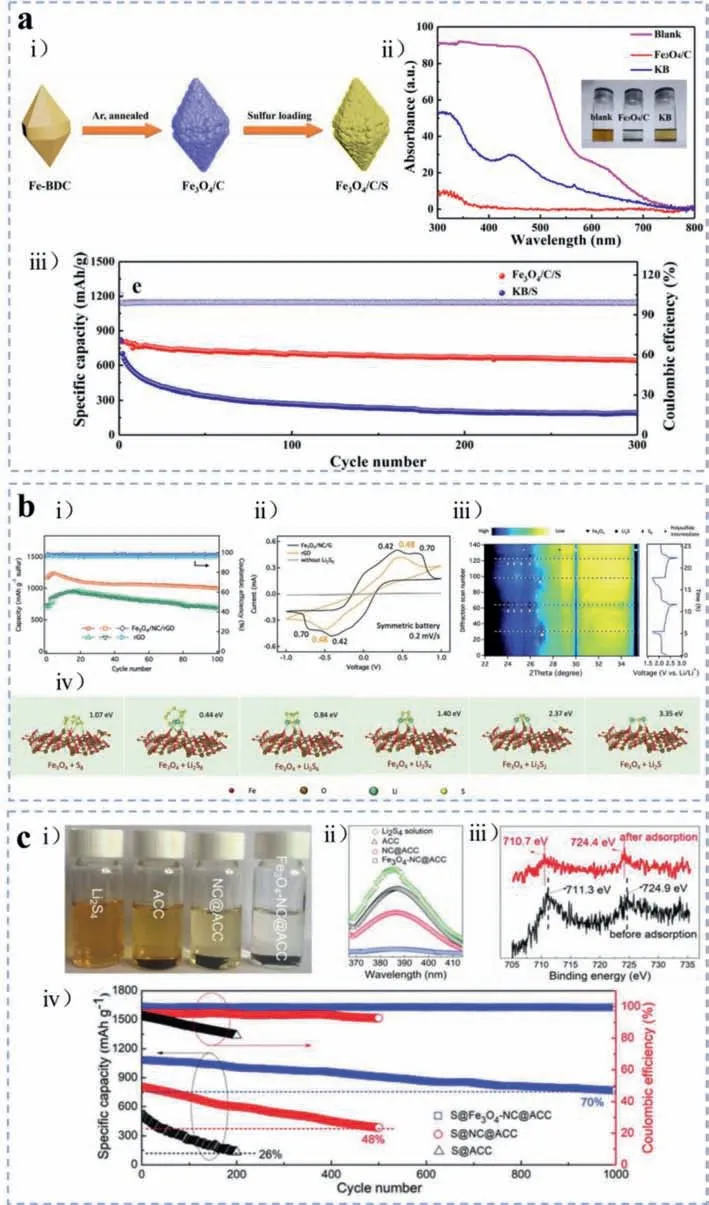
Fig.6.(a) Fe3O4/C: (i) Schematic illustration of synthesis method of Fe3O4/C.(ii) UV–vis adsorption spectrum of the polysulfide solution after absorbed by Fe3O4/C and KB,and pristine polysulfide solution.(iii) Long-term cycling stability of KB/S and Fe3O4/C/S electrodes at 1 C.Reproduced with permission [34].Copyright 2019,Elsevier.(b) Fe3O4/NC/G: (i) Cycling stability and relative coulombic efficiency of Fe3O4/NC/G and rGO aerogels with the Li2S6 catholyte at the current density of 0.1 C.(ii) CV curves of corresponding electrodes scanning at 0.2 mV/s.(iii) The cycling profiles of Li-S batteries with Fe3O4/NC/G/S cathodes within the first 2 cycles at 0.1 C and the correlative in-situ XRD patterns presented in a contour diagram.(iv) DFT simulated molecular nanostructures and adsorbent energies of sulfur and Li2Sn on the surface of Fe3O4.Reproduced with permission [75].Copyright 2019,The Royal Society of Chemistry.(c) Fe3O4-NC@ACC: (i) Visual polysulfides adsorption tests of ACC,NC@ACC,and Fe3O4-NC@ACC,with 5 mm Li2S4 solution immersed for 30 min.(ii) UV–vis adsorption spectra of corresponding solution after adsorption.(iii) XPS analysis of Fe 2p from Fe3O4-NC@ACC before and after polysulfides immobilization.(iv) Cyclic stability and Coulombic efficiency of corresponding electrodes as shown in the chart at 0.2 C.Reproduced with permission [77].Copyright 2018,WILEY-VCH GmbH.
Iron oxides were used in lithium sulfur battery until 2017 by Yanget al.[32] as we mentioned above.Considering the large surface area and abundant pore structures which benefit sulfur utilization,iron-based metal-organic frameworks (MOFs) have been widely applied as precursors to synthesize iron oxides/carbon composite materials [34,75,76].For example,Fanet al.[34] prepared a jujube pit like Fe3O4/C composite (Fe3O4/C) calcinated from Fe-MOFs (MIL-53),of which the schematic diagram is presented in Fig.6a(i).After a facile melt diffusion technique,sulfur was loaded into the internal hollow space of Fe3O4/C.The static polysulfides adsorption tests and relative UV–vis adsorption spectrum proved the promotional adsorption capability of Fe3O4/C as shown in Fig.6a(ii).Consequently,such Fe3O4/C/S cathodes displayed a high specific capacity of 819 mAh/g at 1 C,with 642 mAh/g retained after 300 cycles (Fig.6a(iii)).Then Dinget al.[75] proposed an ordered microchannel graphene scaffold with incorporated catalytic MOF-derived Fe3O4nanocrystals and porous carbon (denoted as Fe3O4/NC/G) as a multifunctional sulfur host,which exhibited a reversible specific capacity of 1007 mAh/g at 0.1 C,with a small capacity decay rate of less than 0.01% after 100 cycles as shown in Fig.6b(i).Cyclic voltammetry tests of symmetric cells were performed to verify the catalytic effects of Fe3O4/NC/G towards polysulfide reaction kinetics and stability in Fig.6b(ii).The higher current density and stronger redox peaks demonstrated that polar Fe3O4nanocrystals accelerated the electrochemical reactions and expeditious conversion with catalytic effects.Furthermore,the conversion mechanism of Fe3O4/NC/G for Li-S battery was studied throughin-situX-Ray Diffraction (XRD) during cycling for the first 2 cycles at 0.1 C as elucidated in Fig.6b(iii).During the discharge process,the signals of polysulfides intermediates almost disappeared,suggesting that the diffusion of long-chain LiPSs to the Li anode was greatly reduced and then the undesired anode corrosion can be effectively suppressed.DFT calculations were also carried out to analyze the chemical interaction between Fe3O4and polysulfides.The inferior bonding energy of Fe3O4towards LiPSs(0.44–3.35 eV) was much higher than that of pristine carbon (0.10–0.52 eV),which can be credited to the strong polar-polar interaction between Fe3O4and polysulfides.The accurate calculation results of binding energies of polysulfides on the surface of Fe3O4were displayed in Fig.6b(iv).Besides these Fe-MOFs derivatives,various iron oxide/carbon nanomaterials emerged through diverse synthesis methods.Luet al.[77] reported a new approach to fabricate strongly coupled Fe3O4and N-doped carbon which were grown on self-standing activated carbon fiber cloth,namely Fe3O4-NC@ACC.This unique nanostructure was obtainedviapyrolysis of molecular mixtures of Fe(CN)64-and pyrrole which were coated on ACC.During the oxidation polymerization of pyrrole prompted by Fe(CN)64-,the reduced Fe(CN)64-compounds would be incorporated in the PPy (polypyrrole) framework due to the chemical interaction between the C≡N ligands and pyrrole,which means,the Fe3O4nanocrystals would be embedded in the carbon framework with rigid carbon layers covering the surface.In the LiPSs adsorption evaluation,the Li2S4solution immersed with Fe3O4-NC@ACC exhibited nearly complete LiPSs adsorption within only 30 min as displayed in Fig.6c(i),which can be ascribed to the chemical binding of strongly coupled Fe3O4for LiPSs.The following UV–vis adsorption spectra and XPS analysis of Fe3O4-NC@ACC composite before and after LiPSs adsorption further proved that in Figs.6c(ii) and (iii).Beyond that,the discharge capacity of S@Fe3O4-NC@ACC cathodes with varied crystallinity and Fe/N loading levels were analyzed in correlation with the reaction stages.By controlling the pyrolyzing temperatures and the amount of pyrrole,the discharge capacity occurred in stage III (from Li2S4to Li2S2and Li2S) differentiated,suggesting the strongly coupled Fe3O4and N-carbon played a key role in the improvement of short-chain polysulfide conversion.With these merits,as shown in Fig.6c(iv) the S@Fe3O4-NC@ACC cathodes presented a high specific capacity of 1316 mAh/g at 0.1 C,and a retention of around 70% after 1000 cycles at 0.2 C under a high sulfur areal density of 4.7 mg/cm2.Similar synthesis mechanism was utilized by Zhanget al.[78],but with different morphologies.Herein,novel hierarchical mesoporous nitrogen-rich carbon nanospheres comprising 1D carbon nanotubes encapsulating Fe3O4nanoparticles (Fe3O4@CNTs nanospheres) were manufactured as sulfur hosts.Supported by the theoretical simulations,the soluble polysulfides interacts with iron oxides stronger than with carbonaceous material,thus alleviating the shuttling effect of LiPSs effectively.Benefiting from the synergistic effect of chemical adsorption and redox reaction catalyzing,the S/Fe3O4@CNTs cathodes displayed the specific discharge capacity change from 937.6 mAh/g initially to 538.5 mAh/g after 1800 cycles at 1 C.Iron oxides can also be obtained through the pyrolysis of iron oleate according to Heet al.s’report [79].The researchers assembled core-shell structured Fe3O4@C nanodots with 5 nm diameter as a valid sulfur host,of which the specific surface area reached 216.2 m2/g owing to the nanosized structures of Fe3O4particles.Due to the porous structures and loose surface of Fe3O4@C,the sulfur content of S/Fe3O4@C host material was calculated to be 66%.And the resultant S/Fe3O4@C cathode showed a high initial capacity of 1089 mAh/g at 0.2 C and 816 mAh/g at 1 C.
3.3.Iron carbides
Iron carbides have attracted intensive attention because of their potential utilization in the field of catalysis and energy storage systems,which can be obtained through versatile synthesis methods,such as sol-gel method,high temperature organic phase method,physical vapor deposition [80].Notably,the unique electronic,catalytic and magnetic properties of iron carbides originated from their intrinsic structures favor their usage in Li-S batteries [81–83].
For instance,inspired by the work of introducing ferroelectric BaTiO3(BTO) into the sulfur cathode to control the “shuttle effect” of polysulfides by the “spontaneous polarization” of BTO[84],Gaoet al.[85] introduced ferromagnetic iron/iron carbide(Fe/Fe3C) nanoparticles with a graphene shell (Fe/Fe3C/graphene)onto a flexible activated cotton textile (ACT) fiber to prepare the ACT@Fe/Fe3C/graphene sulfur host.The innovation spot was that the path of the dissolved negative polysulfide ions can be alteredviaLorenz force since the built-in magnetic field brought by ferromagnetic iron carbides,namely the “shielding effect”viaferromagnetic nanoparticles.In order to study the polysulfide trapping mechanism of magnetic Fe/Fe3C nanoparticles,custom-design liquid cells of ACT@Fe/Fe3C/S were performed in comparison with that of ACT/S.The strikingly different polysulfide diffusion behavior shown in Figs.7a(i) and (ii) unveiled that the magnetic field can alter the diffusion path of LiPSs effectively through Lorenz force.In the following schematic illustration of Fig.7a(iii),it can be found that owing to the instinctive magnetization of Fe/Fe3C NPs,the generated magnetic field manipulated the motion of polysulfide ions through attracting them to the cathode side,thus resulting in a darker brown region of electrolyte around.Weiet al.[86] fabricated ordered mesoporous graphitic carbon/iron carbide nanocomposites (GC/Fe3C) as sulfur host materialsviaan evaporation-induced self-assembly (EISA) procedure combined with Teflon-assisted solid-state decomposition.Herein,the Fe3C nanocrystals contributed to catalyzing the graphitization of amorphous carbon,increasing its pore size.The large quantity of micropores and small-size mesopores enabled the hybrid framework a large specific surface area of 3105 m2/g and pore volume of 3.32 cm3/g,which guaranteed a high sulfur loading (85%) and alleviated the volume expansion to some extent.The as-prepared cathode displayed excellent capacity and cycling stability,with an initial discharge capacity of 1203 mAh/g at 0.2 C and a retention of 91.4% after 500 cycles.Wanget al.[87] also designed and synthesized a unique porous and conductive nanocomposite for which Fe3C nanoparticles are embedded in nitrogen-doped porous carbon sheets (Fe3C@NPCS).The porous carbonaceous nanostructures can provide more accommodation space for sulfur species and Fe3C nanoparticles can enhance the electronic conductivity of the composite (Fig.7b(i)).According to DFT simulations presented in Fig.7b(ii),Fe3C exhibited strong affinity towards polysulfides through Fe-S bonds.Benefiting from the outstanding conductivity of Fe3C nanocrystals,the Fe3C@NPCS-S cathode showed a reduced polarization and enhanced reversibility compared with NPCS-S in cyclic voltammogram (CV) measurements as demonstrated in Fig.7b(iii).More importantly,as exhibited in Fig.7b(iv),the Fe3C@NPCS-S electrodes delivered greatly improved specific discharge capacities of 1251,1127,1020,907,802,731 and 647 mAh/g at 0.2 C,0.3 C,0.5 C,1 C,2 C,3 C and 5 C,respectively.The well-performed rate capability further proved that the introduction of Fe3C nanocrystals facilitated the electrochemical kinetics,internal resistance and charge transfer as previous research reported.Apart from the increasing utilizations in sulfur hosts [88–91],iron carbides have also represented their advantages of superior conductivity in the application of interlayers for separators [92,93] or electrodes [94–96].For example,as Songet al.[92] proposed,the MOF-derived Fe3C nanocrystals compounded with the nitrogen-doped graphene-like carbon nanosheet (Fe3C/NG) were coated onto separators given the catalytic and conductive properties of Fe3C.The Li-S cell with a lightweight Fe3C/NG-coated separator demonstrated splendid rate performances and cycling stability.Specifically,the as-prepared cell delivered an initial discharge specific capacity of 954.5 mAh/g even at a high current density of 6 C and maintained 439.9 mAh/g after 500 stable cycles with a sulfur loading of 1 mg/cm2.Zhanget al.[94] fabricated freestanding graphitized carbon interlayers decorated with Fe/Fe3C nano-catalysts acquired by thermal treatment of cellulose paper with adsorbed ferric nitrate at 1000 degrees.Thein-situgrown Fe nanoparticles can promote the graphitization of carbon,enhancing the conductivity of interlayers.Thanks to the rationally designed nanoarchitecture and the efficacious acceleration of Fe/Fe3C nanocrystals towards polysulfide conversion,the Li-S cell with an optimal carbon/Fe/Fe3C interlayer displayed an initial specific capacity of 1556 mAh/g at 0.1 C and stabled at around 772 mAh/g at 1 C after 200 cycles.
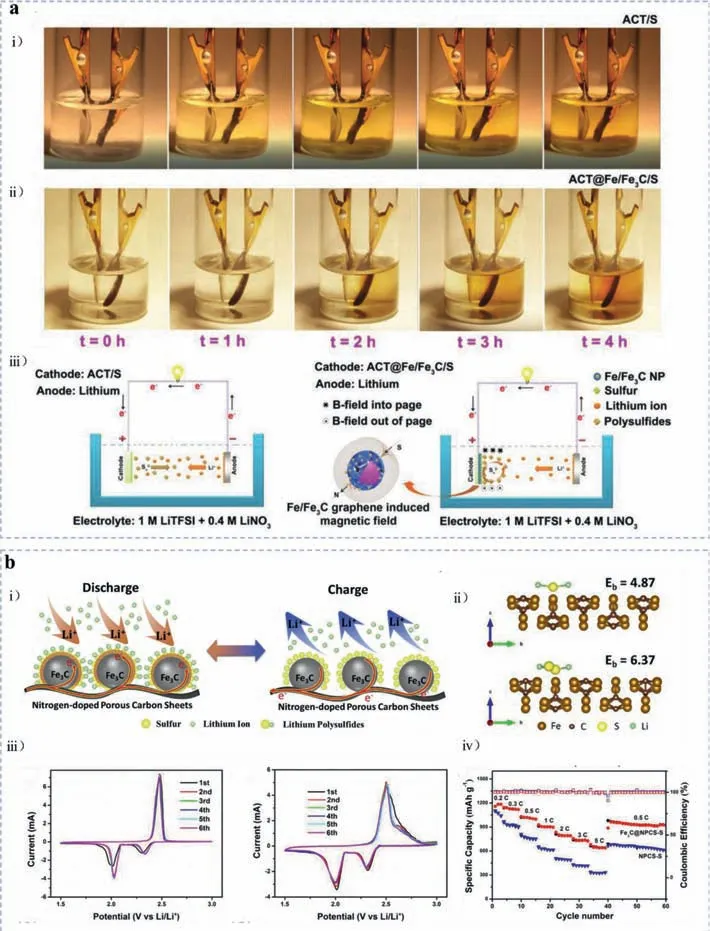
Fig.7.(a) ACT@Fe/Fe3C/S: Digital images of (i) ACT/S and (ii) ACT@Fe/Fe3C/S electrodes during the first cycle in a liquid cell under a current density of 0.1 mA/cm2.(iii)Schematic illustration of the polysulfide entrapment mechanism through Fe/Fe3C NPs in the ACT@Fe/Fe3C/S cathode.Reproduced with permission [85].Copyright 2018,WILEY-VCH GmbH.(b) Fe3C@NPCS: (i) Schematic diagram of the influence of as-proposed Fe3C@NPCS in Li-S battery system,with porous nanostructures accommodating more sulfur and polysulfides,enhanced conductivity supported by Fe3C NPs.(ii) DFT calculated binding energy (Eb) of Li2S and Li2S2,respectively on the Fe3C(100) surface.(iii) CV curves of Fe3C@NPCS-S electrodes (right) and NPCS-S electrodes (left) at a scan rate of 0.1 mV/s.(iv) Rate performance with relative Coulombic efficiency of Fe3C@NPCS-S and NPCS-S electrodes at different current densities from 0.2 C to 5 C.Reproduced with permission [87].Copyright 2018,Elsevier.
3.4.Iron phosphides
Originated from the multi-electron orbitals of phosphorus,the superior chemical properties of iron phosphides have prompted their thriving development in catalytic applications in hydrogen evolution reaction (HER),and oxygen reduction reaction (ORR),lithium-ion batteries (LIBs),sodium-ion batteries (SIBs) and supercapacitors [97,98].As reported in ORR catalysis,the lone pair electrons in the 3p orbital of phosphorus can result in a correlated local concentrated charge density,and the empty 3d orbital can supply with accommodation for the lone pair electrons from the p orbital of oxygen molecule,thus promoting the activity of phosphide catalysts [99],besides,the low cost,high conductivity and special electronic structures boost the application of iron phosphides in catalysis and energy storage systems.
Until recently,iron phosphides have demonstrated their superiorities in Li-S batteries [100–104].Chenet al.[105] manufactured a phosphorized rGO/CNTs hybrid aerogel with embedded FeP nanocubes (p-HA/FeP) through the instant freezing and phosphorization of Fe-MOF precursors as shown in Fig.8a(i).With the strong affinity of FeP NPs towards polysulfide immobilization,and the sufficient interfaces for charge transformation offered by the conductive and microporous carbon scaffold,as well as the potential catalytic effects of FeP NPs for accelerating polysulfide redox reaction,the corresponding p-HA/FeP/S cathode delivered a high specific capacity of 1312.3 mAh/g at 0.2 C and maintained a lifespan of over 500 cycle at 1 C with only 0.037% capacity decay rate per cycle (Figs.8a(ii) and (iii)).Liet al.[106] reported a facile approach to synthesize hierarchically structured composite of Fe2P@nitrogen,phosphorus co-doped carbon (Fe2P@NPC).Herein,Fe2P nanoparticles were producedviathe direct biological recycling of iron metal from electroplating sludge using bacteria,resulting in the uniform distribution of nanosized Fe2P.According to DFT calculations,Fe2P can not only suppress the polysulfide shuttling effect through strong chemical adhesion ability,but also facilitate the decomposition of insoluble Li2S during the delithiation process.Meanwhile,with the merits of the multiple channels for ions transportation and adequate space for volume expansion,the Fe2P@NPC/S cathode exhibited an obviously improved initial specific capacity of 1555.7 mAh/g at 0.1 C and prolonged cycling for 500 cycles with a reversible specific capacity of 761.9 mAh/g at 1 C.
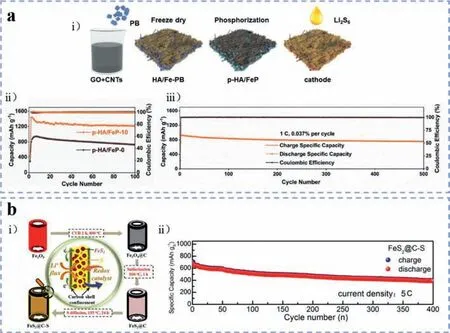
Fig.8.(a) p-HA/FeP (phosphorized GO/CNTs hybrid aerogel embedding FeP nanoparticles): (i) Schematic illustration of synthesis process of the p-HA/FeP/S cathode.(ii)Cycling performance and Coulombic efficiency of p-HA/FeP/S electrode at 0.2 C.(iii) Long-term cycling stability with relative Coulombic efficiency of p-HA/FeP/S electrode at 1 C.Reproduced with permission [105].Copyright 2020,Elsevier.(b) FeS2@C: (i) Schemed synthesis process of FeS2@C composite as well as the promotion mechanism of Li ions transportation and sulfur species redox reactions.(ii) Prolonged cycling stability of FeS2@C-S cathode at a high current density of 5 C.Reproduced with permission[111].Copyright 2020,American Chemical Society.
3.5.Iron sulfides
Considering that the d orbital electron layer of the metal cation in transitional metal sulfides is more easily to obtain or lose electrons,which facilitates their utilizations in electrochemical catalysis,iron sulfides have been expected as cost-efficient and efficient alternatives for noble Pt-based catalysts.Additionally,iron sulfides usually possess high conductivity and appropriate chemical bond intensity for catalyzing conversion reactions [68,107].However,the irresistible volume change during cycling and difficult synthesis methods hinders the practical applications of iron sulfides.Hence,in the field of Li-S battery,an increasing number of efforts have been devoted to explore and develop iron sulfides with different morphologies and hybridized matrices to overcome these obstacles[108–110].
After systematical investigation of the immobilization effects and mechanisms of transitional metal sulfides (Fe,Co,Ni,Cu,Zn,etc.) by Chenet al.[36],diverse iron sulfides have been dedicated to sulfur cathodes.For instance,Zenget al.[111] fabricated sulfur cathode composites with FeS2nanoparticles encapsulated in hollow carbon shells through a facile way (Fig.8b(i)).In addition to the enhanced adsorption capability towards polysulfides arising from FeS2which was confirmed by FTIR,Raman,and XPS analysis,more importantly,FeS2also demonstrated remarkable electrocatalytic effects in Li-S battery.In the CV measurements of FeS2@CS and MC/S cathodes,the cathodic and anodic peaks of FeS2@C-S cathode were sharper than those of MC/S cathodes (mesoporous carbon/S),as well as the higher current densities,suggesting the improved redox reaction kinetics attributed to FeS2.The FeS2@C-S electrode exhibited a reversible specific discharge capacity of 800 mAh/g after cycling for 200 rounds at 0.2 C,while for the MC/S electrode,the specific discharge capacity faded to 200 mAh/g after only 50 cycles at 0.2 C.Even at a current density of 5 C,the FeS2@C-S electrode still delivered a reversible discharge specific capacity of ~400 mAh/g after cycling for 400 times as shown in Fig.8b(ii).Similarly,Liet al.[112] reported a rationally designed nanoarchitecture of N-doped carbon-coated iron sulfide (abbreviated as FeS/N-C) as sulfur hosting material fabricated through a simple pyrolysis method.With the virtues of abundant sulfiphilic sites for immobilizing polysulfides and catalyzing polysulfide conversion,the FeS/N-C based sulfur cathode showed a significant cycling stability promotion,with a stable discharge specific capacity of ~729 mAh/g retained after 500 cycles at 0.5 C.
3.6.Others
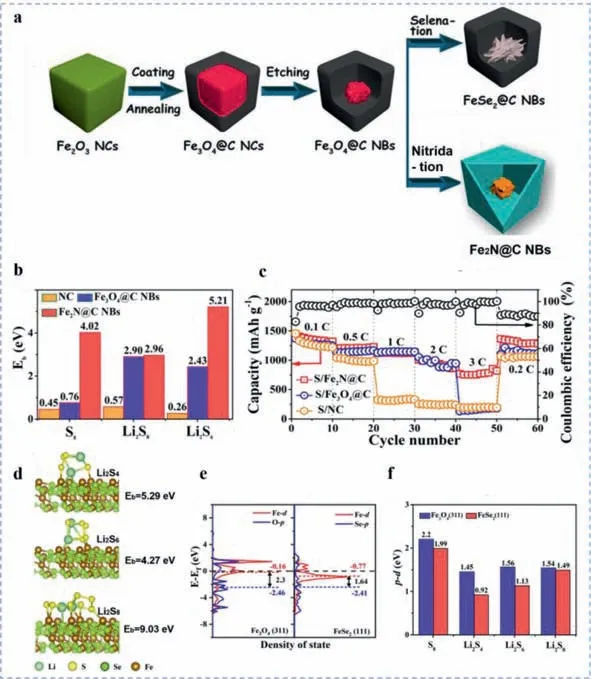
Fig.9.(a) Schematic illustration of the fabrication process of Fe2N@C NBs and FeSe2@C NBs.(b) DFT calculated binding energy of relative sulfur species on NC,Fe3O4 and Fe2N.(c) Rate performance of corresponding electrodes at current densities from 0.1 C to 3 C.(d) DFT calculated binding energy of Li2Sn (n=4,6,8) on the FeSe2(111)surface.(e) DOS patterns of p bands of anions and d bands of Fe in the correlative Fe3O4(311) and FeSe2(111).(f) Calculated energy gap values of FeSe2 and Fe3O4 after absorbed by S8,Li2S4,Li2S6,and Li2S8.(a-c) Reproduced with permission [121].Copyright 2019,American Chemical Society.(a,d-f) Reproduced with permission [123].Copyright 2021,Elsevier.
In addition to the simple inorganic compounds,there have been increasing attention in other binary inorganic or organic iron-based compounds or heterostructures for ameliorating the electrochemical performance of Li-S battery,such as iron-based MOFs [113–115],multi-metallic alloy [116–119] and other iron-based compounds or heterostructures [120].
Taken the marvelous porosity and crystallinity of pristine MOFs in account,most studies in the past decades focused on the modification of MOFs derivatives due to the poor conductivity of MOFs,however,the application of pristine MOFs as cathode materials in Li-S battery attracts researchers’attention until currently.For example,Benítez group [113] synthesized MIL-88A based on iron fumarate,which possessed a central prismatic portion and pyramidal terminal portions,resulting in a dual micro-mesoporous MOF system.The relative MIL-88A@S composite cathode displayed an initial discharge specific capacity of around 400 mAh/g at 0.1 C.Meanwhile,iron-based multi-metallic alloys exhibited great potential as catalysts for Li-S battery since their simpler and more suitable synthesis methods for practical utilizations.According to Chenet al.s’report [116],during the thermal treatment at a high temperature,Fe3+and Ni2+can be transformed into FeNi3as well as catalyze the graphitization of carbon materials,thus upgrading the electronic conductivity of carbon-based sulfur hosting materials.Confirmed by DFT calculations and experimental results,the bimetallic alloy FeNi3played a vital role in impeding polysulfide shutting effect through its chemical adsorption ability and catalyzing polysulfide conversion reaction.
Recently,in the case of metal catalysts,transition-metal-based alloys or intermetals have gained promising applications in catalyzing gas involved electrochemistry due to their high activity and stability arising from their good electronic and chemical properties.Considering the similar chemical property of oxygen and sulfur,researchers explored the functional mechanism of intermetallic catalysts in Li-S electrochemistry.For example,Manthiram group[117] reported a cost effective hexagonal close-packed (hcp)-phase Fe-Ni alloy serve as an efficient electrocatalyst to promote the LiPS conversion reaction in Li-S batteries.The electrocatalysis mechanisms of Fe-Ni toward LiPS conversion is considered that both the pristine nanosized Fe-Ni phase and the thin layer coated on the Fe-Ni alloy consists of various sulfurized phases provide high catalytic activity,thus the Fe-Ni alloy delivers a long lifespan over 800 cycles and high areal capacity of 6.1 mAh/cm2under lean electrolyte conditions with high sulfur loading of 6.4 mg/cm2.Yanget al.[118] reported Ni3Fe as electrocatalyst to enhance efficient polysulfide-involving surface reactions.They claimed the incorporation of iron into nickel phase induce strong electronic interaction and lattice distortion and promotes the redox kinetics of the multiphase conversion in Li-S electrochemistry.The 70 wt% sulfur cathodes with Ni3Fe-modified separator delivers initial capacities of 1310.3 mAh/g at 0.1 C and 598 mAh/g at 4 C as well as stable long cycle life of 1000 cycles with low-capacity fading rate of~0.034% per cycle.
Considering the benefits of iron-based compounds,our group has explored the promotion effects of iron nitrides [121] towards Li-S battery system.The yolk-shelled Fe2N@C nanoboxes were designed and fabricated through hard-templating method using Fe2O3as precursor and etching core for the conversion into Fe2N (Fig.9a).The as-prepared yolk-shelled Fe2N@C nanoboxes can provide strong chemical affinities,efficacious catalytic effects and electronic conductivity arising from polar Fe2N cores,and sufficient loading space for sulfur due to the large internal void.With the support of Fe2N cores,the binding energy of LiPSs on the surface of the as-proposed cathode composites were further confirmed to be greatly advanced through DFT calculations as shown in Fig.9b.And the advancement can be attributed to the chemical Fe-S and Li-N binding configurations.In the relative rate performance as shown in Fig.9c,the S/Fe2N@C NBs and S/Fe3O4@C NBs electrodes delivered similar electrochemical performance from 0.1 C to 2 C,however,when the current density raised to 3 C,the S/Fe2N@C NBs electrode still exhibited a specific capacity of 778 mAh/g while the S/Fe3O4@C NBs electrode reached only 161 mAh/g,demonstrating the superior rate capability of S/Fe2N@C NBs electrode under higher current densities,which can be ascribed to the faster charge transfer dynamics and accelerated redox kinetics prompted by Fe2N dominantly.
Benefiting from the considerable electrical conductivity,vigorous catalytic effect and cost effectiveness,iron-based chalcogenide compounds including iron-sulfides,iron-selenides have been widely employed in Li-S battery and displayed extraordinary electrochemical performance.Notably,iron-based selenides have displayed their advantages in ORR [122],which enlightens their potential utilizations for high-performance Li-S batteries since the similar redox mechanism between oxygen and sulfur electrochemistry.In this work,our group encapsulated FeSe2nanoparticles inside the hollow carbon nanocubes,promoting the electrochemical performance of Li-S cell through strong polysulfide immobilization and catalytic effect [123].The binding energy of Li2Sn(n=4,6,8)on the FeSe2(111) surface were simulated through DFT calculations as shown in Fig.9d,which ranged from 4.27 eV to 9.03 eV,much higher than those on Fe3O4(311) surface.Furthermore,in the longterm cycling stability test,the S/FeSe2@C electrode still retained 684 mAh/g discharge specific capacity at 1 C after 700 cycles,with an ultralow decay rate of 0.04% per cycle.In order to investigate the nature of the remarkable enhancement of electrochemical performance triggered by FeSe2NBs,DOS analysis of pristine FeSe2and Fe3O4were performed in comparison as shown in Fig.9e.Combined with the corresponding calculated energy gap of FeSe2and Fe3O4after absorbed by S8,Li2S4,Li2S6,and Li2S8,respectively(Fig.9f),the patterns revealed that the energy gap values of original FeSe2and FeSe2after adsorbed polysulfides were all smaller than those of Fe3O4,which enable Se ions more easily to interact with polysulfides.
To achieve multiple functions of individual component,heterostructured materials attracted increasing attention due to their extraordinary strengths towards effective adsorption and catalytic conversion of LiPSs.The rational design and construction of multiple solid-state materials into heterostructures which take advantageous synergistic effects provide a common strategy to address the longstanding intrinsic issues of Li-S batteries.Leeet al.[124] fabricated conductive Co5.47N/Fe3N heterostructures wrapped with 3D nitrogen-doped CNTs and graphene framework(3D Co5.47N/Fe3N@N-CNT-G) to improve the electron and lithiumion transport.The synergistic effect of high electrocatalytic activity of polar Co5.47N/Fe3N heterostructure can provide strong LiPS adsorption,accelerate the LiPS conversion reaction,control the kinetic behaviors of dissolved LiPS,and promote the Li2S nucleation.The sulfur loaded 3D Co5.47N/Fe3N@N-CNT-G/S cathode delivered a capacity of ~1293 mAh/g at 0.1 C and a low degradation capacity of 0.019% per cycle for 500 cycles at 1 C rate.
4.Summary and future perspectives
In general,in order to promote the electrochemical performance of Li-S battery through trapping soluble polysulfide shuttling effect and boosting the electrochemical kinetics,it has been proven to be an effective strategy to induce transitional-metal compounds as chemical anchors and electrocatalysts.Among the various transitional metals,iron has exhibited superiority in Li-S battery owing to its low cost,environmental friendliness,similar characteristics of rich raw materials and high catalytic activity.
In this review,we concluded the paramount findings and the recent progress in iron-based nanomaterials for rechargeable Li-S battery.We summarized the mechanist insights towards the anchoring and catalytic effect of diverse iron-based nanomaterials for polysulfides entrapment and conversion.Through the discussion on the electrochemical performance of different iron-based materials,including iron single-atom material,iron oxides,iron carbides,iron phosphides,iron sulfides,iron-based heterostructure and so on,the intrinsic natures of various iron-based nanomaterials for realizing high performance Li-S battery have been elucidated.Table 1 condenses the electrochemical performance of some typical recently proposed iron-based nanomaterials which were utilized in Li-S battery system.
With the aim of advancing electrochemical performance and practical utilization of Li-S battery,balancing the polysulfides trapping and diffusive capability as well as conductivity of cathodes appears to be predominant.Overall,several predominant concerns and opportunities in future development are proposed as follows:
(1) Optimizing nanostructures with enhanced electrical conductivity and mechanical flexibility,such as 2D ultrathin materials and 3D hierarchical porous materials,are pivotal to the suffi-cient utilization of sulfur active species.Faster electron transportation can diminish the overpotential at the cathode interface.Other than introducing different carbonaceous substrates into cathodes,interface engineering on the electrode surface or separators has also been applied as an effective method to modify the conductivity or mechanical flexibility of battery system.On the other hand,to obtain higher energy density of electrodes,the sulfur loading should be as further improved with abundant accommodation space of rationally designed nanostructures.
(2) Engineering the structures and compound elements of ironbased nanomaterials as chemical anchors and electrocatalysts in Li-S battery enables superior specific capacity and excellent rate performance,which can be credited to the special electric structures of iron-based nanomaterials.However,the type and the content of multifunctional iron-based materials influences the electrochemical performance of batteries obviously,which is mainly due to the diverse electron structures,resulting in different chemical immobilization ability and catalytic effect.Therefore,the iron-based electrocatalysts should be well-characterized using XRD,SEM,TEM,XPS,and XAS and other measurements during the interval of Li-S measurement,to monitor the local structure and the variation of composition.
(3) Although the application of iron-based nanomaterials in Li-S battery is in its preliminary stage of exploration,there are still substantial space for further development.Apart from modulating the morphology,composition,and surface electronic structure of iron-based nanomaterials for Li-S battery,more essentially,clearer fundamental understanding of the chemical entrapment and catalytic mechanism towards polysulfides from an atomic level is required.Until now,the real active site and the detailed catalytic mechanism of iron-based catalysts are still lack of in-depth research.A deep understanding of the catalytic mechanism and the precise role of iron is important to clarify design guideline for the high-efficiency electrocatalysts in Li-S chemistry.Morein-situtechniques should be developed to monitor the intermediates and the conversion process of sulfur species in Li-S batteries.
To achieve high-performance Li-S battery,with splendid electrochemical performances,the safety and economic benefits are also needed to be considered.As a rising star,iron-based nanomaterial exhibits remarkable performance in Li-S battery.There is still a long way to go,yet,with the virtue of inherent advantages,more exciting achievements related to the mechanism and performance will be gained in the coming future.
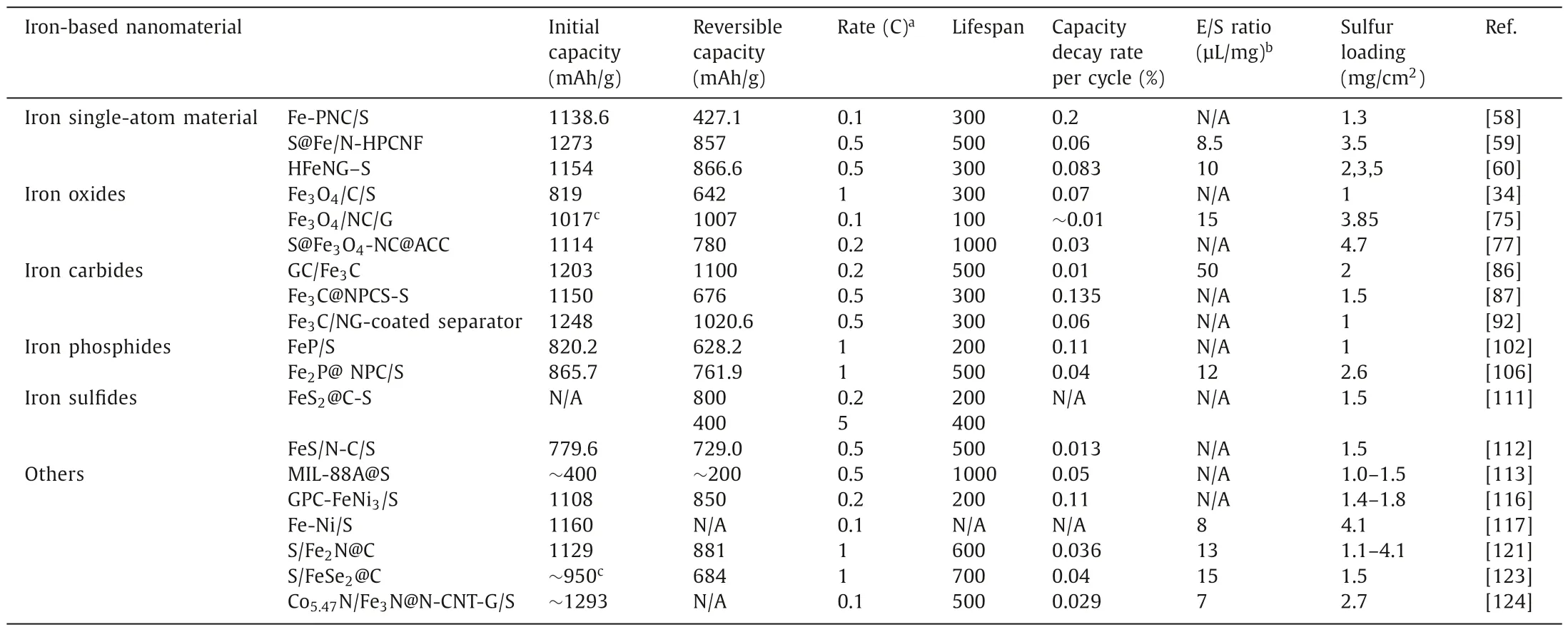
Table 1 Summary of electrochemical performance parameters for multifunctional iron-based nanomaterials in Li-S battery system.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Nos.51702362 and 21875282),Natural Science Foundation of Hunan Province (Nos.2022JJ30663,2022JJ40551) Scientific Research Project of National University of Defense Technology (No.ZK19–27),and Significant Independent Research Projects for Young Talents of College of Aerospace Science and Engineering,National University of Defense Technology.
 Chinese Chemical Letters2023年1期
Chinese Chemical Letters2023年1期
- Chinese Chemical Letters的其它文章
- Diabetic wound healing activated by supramolecular cascade reaction
- MBenes: Two-dimensional transition-metal borides with ordered metal vacancies
- Wet-adhesive materials of oral and maxillofacial region: From design to application
- Diverse catalytic systems for nitrogen-heterocycle formation from O-acyl ketoximes
- Fluorine-containing drugs approved by the FDA in 2021
- The development and application of dual-comb spectroscopy in analytical chemistry
