Small incision lenticule extraction and femtosecondassisted laser in situ keratomileusis in patients with deep corneal opacity: case series
Zhi Fang, Xiao-Ying He, Wei Han
1Department of Eye Center, the Second Affiliated Hospital,Zhejiang University School of Medicine, Hangzhou 310009,Zhejiang Province, China
2Zhejiang Provincial Key Lab of Ophthalmology, Hangzhou 310009, Zhejiang Province, China
Abstract
● KEYWORDS: small incision lenticule extraction;femtosecond-assisted laser in situ keratomileusis; corneal opacity
INTRODUCTION
Small incision lenticule extraction (SMILE) is a flapless refractive surgery with advantages such as better postoperative corneal biomechanical stability and less dry eye[1]. SMILE has shown superior safety, efficacy,predictability, and visual quality[2-3]. Even among patients with high myopic astigmatism, corneal opacity, or thin cornea,SMILE also exhibited satisfactory performance[4-6]. Meanwhile,femtosecond-assisted laserin situkeratomileusis (FS-LASIK)was another classical and robust corneal surgery for refractive correction[7-8]. Corneal opacity, which occasionally being present in patients, may increase the risks of complications such as gas breakthrough, and flap tear when creating a flap with the femtosecond laser[9-10]. Cases in superficial corneal opacity [depth <200 μm or 1/5 central corneal thickness (CCT)]without corneal perforation, severe chemical injury, or intraocular surgery history were reported safe and effective with SMILE[6]or phototherapeutic keratectomy (PTK)[11].However, few studies reported refractive surgery in patients with deep cornea opacity, especially with corneal perforation,chemical burns, or intraocular surgery history. In particular,the assessment of long-term outcomes is lacking. We included4 myopia or myopia astigmatism patients with deep corneal opacity, treated by SMILE or FS-LASIK combined with limbal relaxing incision (LRI) according to preoperative evaluation of depth and location of corneal opacity. Long-term outcomes until 6mo after FS-LASIK and LRI were also evaluated.

Table 1 Preoperative demographics of 4 cases

Table 2 Postoperative demographics of 4 cases
SUBJECTS AND METHODS
Ethical ApprovalAll the procedures in this study were approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University (No.2022-0584). Written informed consent was given from every patient.Altogether, four patients with monocular corneal opacity lesions (3 due to mechanical injury, 1 due to firecracker wound)received refractive surgery (3 by SMILE, 1 by FS-LASIK combined with LRI). Anterior and posterior segment examination,corrected distance visual acuity (CDVA), refraction,intraocular pressure (IOP), CCT, corneal topography and IOP were evaluated before surgery. The process of laser scanning and lenticule extraction was closely monitored during the surgery. The depth of the corneal opacities and distance from central optical zone were measured by anterior segment optical coherence tomography (AS-OCT) before and after surgery.The density parameter of corneal opacity was obtained from Scheimpflug tomography system as gray values from 0 to 100,representing the range from a clear (value 0) to completely opaque (value 100). All the patients have at least 1 follow-up,range from 1wk to 6mo. Pre- and postoperative demographics were listed in Tables 1 and 2, respectively.
RESULTS
Case 1A 42-year-old woman with myopic astigmatism presented for preoperative evaluation for bilateral SMILE. She had a traumatic history in the right eye caused by a spectacle fracture about 20 years ago. Preoperative CDVA was 20/20 with a manifest refraction of -4.50/-0.75×34 in the right eye(OD) and 20/20 with a manifest refraction of -5.25/-0.50×130 in the left eye (OS). Slit-lamp photography revealed central cornea opacity (0.856 mm from the central optical zone)with endothelium iris pigmentation (Figure 1A, 1B), and AS-OCT showed endothelial cells were affected in the right eye (Figure 1C), indicating that the lesion is performative.Corneal topography was normal (Figure 1E). The slit-lamp examination also found capsule opacification of the lens in the right eye. The cornea and lens were transparent in the left eye.Fundus examination was unremarkable in both eyes (OU). IOP was 15.5 mm Hg OU. Central corneal thickness (CCT) was 563 μm OD and 561 μm OS. Specular microscopy revealed endothelial cell density of 2271 cells/mm2OD and 2535 cells/mm2OS with a uniform endothelial cell pattern without any sign of guttate or abnormalities.
SMILE, especially the separation of lenticule, was performed with great care. However, suspicious leakage of anterior aqueous humor into the sub-cap space occurred during lenticule separation, which also indicated corneal perforation history. Finally, SMILE was completed successfully.
One week postoperatively, the best CDVA was 20/20 in both eyes with a manifest refraction of +0.75 OD and +0.75/-0.50×137 OS. IOP was 11.5 mm Hg OD and 11.0 mm Hg OS. The density of cornea opacity changed from 29.5±4.1 to 29.2±5.4, the area decreased from 0.254 mm2to 0.221 mm2and the depth changed from 76-548 μm to 57-490 μm. ASOCT showed good morphology of the corneal cap (Figure 1D).Corneal topography showed no decentration following SMILE(Figure 1F).

Figure 1 The preoperative corneal opacity was noted by slit-lamp microscopy (A, B; red arrow in B), AS-OCT examination before (C) and after (D) SMILE,corneal morphology before (E) and after (F) SMILE by Scheimpflug tomography system AS-OCT: Anterior segment optical coherence tomography; SMILE: Small-incision lenticule extraction.

Figure 2 The preoperative AS-OCT examination of corneal opacity (A), slit-lamp microscopy (B, D, corneal opacity was indicated by red arrow) and AS-OCT examination (C, E) 2wk or 6wk after SMILE, respectively AS-OCT: Anterior segment optical coherence tomography; SMILE:Small-incision lenticule extraction.
Case 2A 24-year-old man with myopic astigmatism presented for preoperative evaluation for bilateral SMILE. He had a traumatic history in his left eye during childhood without a detailed medical record or any treatment. Preoperative best CDVA was 20/20 with a manifest refraction of -7.25/-1.5×1 OD and 20/25 with a manifest refraction of -3.0/-2.0×32 OS.Slit-lamp photography and AS-OCT revealed an inferior nasal corneal opacity lesion (3.155 mm from the central optical zone) involving the endothelium layer in the left eye (Figure 2A).Lens and fundus examination was unremarkable in both eyes.IOP was 18.5 mm Hg OD and 19.5 mm Hg OS. CCT was 592 μm OD and 597 μm OS. Specular microscopy revealed endothelial cell density of 2929 cells/mm2OD and 2972 cells/mm2OS with a uniform endothelial cell pattern without any sign of guttate or abnormalities except in the lesion area.SMILE was performed with careful separation of lenticule,especially in the zone around the corneal opacity lesion.Finally, an uneventful SMILE was completed.

Figure 3 The corneal morphology before (A) SMILE, 2wk (B), or 6wk (C) after SMILE by Scheimpflug tomography system SMILE: Small-incision lenticule extraction.
Two weeks postoperatively, the best CDVA was 20/20 with a manifest refraction of Plano OD and 20/22 with a manifest refraction of -0.50×83 OS. IOP was 12.0 mm Hg OD and 13.0 mm Hg OS. Slit-lamp examination found stable cornea opacity (Figure 2B, 2D). AS-OCT showed edema above the corneal opacity (Figure 2C 287 μm of inferior nasalvs139 μm of superior temporal). Six weeks postoperatively, AS-OCT showed alleviated edema (Figure 2E, 259 μm). The density of cornea opacity decreased from71.4±19.4 to 65.1±18.4,while area decreased from 0.534 mm2to 0.407 mm2and depth changed from 284-880 μm to 204-738 μm. Corneal topography showed no decentration following SMILE (Figure 3).
Case 3A 23-year-old woman with myopic astigmatism presented for preoperative evaluation for bilateral SMILE.She denied any trauma or treatment history. Preoperative best CDVA was 20/22 with a cycloplegic refraction of-4.75/ -1.25×6 OD and 20/22 with a cycloplegic refraction of -4.75/ -0.75×16 OS. Slit-lamp photography (Figure 4A,4B) and AS-OCT showed inferior linear deep corneal opacity of the left eye (Figure 4C: 1.297 mm from the central optical zone, density: 43.7±8.2, area: 0.137 mm2, depth: 65-258 μm).Corneal topography showed K1 43.0×2.6, K2 44.7×92.6 OS (Figure 4D). Anterior segment and fundus examination were unremarkable in both eyes. IOP was 20.5 mm Hg OD and 18.0 mm Hg OS. CCT was 529 μm OD and 532 μm OS.Specular microscopy revealed endothelial cell density of 2309 cells/mm2OD and 2397 cells/mm2OS with a uniform endothelial cell pattern without any sign of guttate or abnormalities.
SMILE was performed with meticulous separation of inferior lenticule due to the opacity lesion. Finally, the SMILE procedure was completed uneventfully.
In one week of postoperative follow-up, the uncorrected distance visual acuity (UDVA) was 20/20 OD and 20/25 OS.Two weeks and one month postoperatively, the UDVA was 20/20 OU. Two months postoperatively, AS-OCT and slitlamp photography of both eyes showed stable opacity. Corneal topography showed K1 38.7×2.7, K2 40.0×92.7 OD; K1 38.6×4.9, K2 40.2×94.9 OS.
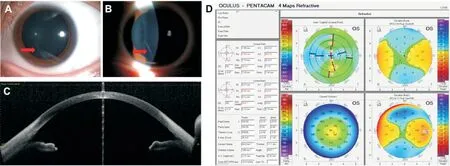
Figure 4 The preoperative slit-lamp microscopy (A, B, corneal opacity was indicated by red arrow), AS-OCT examination (C), and corneal topography (D) of corneal opacity AS-OCT: Anterior segment optical coherence tomography.
Case 4A 22-year-old man with astigmatism presented for preoperative evaluation for bilateral FS-LASIK. He had a firecracker wound history in the right eye 18 years ago and received pupil reconstruction surgery 14 years ago. Six months ago, he presented with a declined vision of the right eye with a CDVA of 20/40 and was diagnosed with a traumatic cataract in the right eye. He then received right eye phacoemulsification and posterior chamber intraocular lens (IOL) implantation successfully in our hospital. Six months after surgery, his CDVA increased to 20/20 with a manifest refraction of +1.75/-5.00×179 OD and 20/20 with a manifest refraction of-3.00×5 OS. Although his CDVA is good, astigmatism induced by cornea irregularity was rather high and degraded the visual quality significantly. Therefore, he presented for preoperative evaluation for bilateral FS-LASIK to correct high astigmatism.Slit-lamp photography showed cornea scar and deformation from 3 to 5 o’clock of the corneal limbus and irregular pupil(Figure 5A). Corneal topography showed irregular astigmatism of 4.9 D with K1 40.2×178.2 and K2 45.2×88.2. Fundus examination was unremarkable in both eyes. IOP was 20.5 mm Hg OD and 18.0 mm Hg OS. CCT was 574 μm OD and 560 μm OS. Specular microscopy revealed endothelial cell density of 2551 cells/mm2OD and 3087 cells/mm2OS with a uniform endothelial cell pattern without any sign of guttate or abnormalities except the scar lesion zone.
FS-LASIK in both eyes and combined LRI in the right eye would be the preferred option for high astigmatism caused by corneal scarring. An LRI with about 75% depth of corneal thickness was made from 4 to 5 o’clock of the corneal limbus in the right eye as shown in slit-lamp photography at 1mo(Figure 5B) and 5mo (Figure 5C) postoperatively.
One week postoperatively, his CDVA was 20/33 with a manifest refraction of -1.00×142 OD and 20/20 with a manifest refraction of -0.25/-0.50×170 OS. Three months postoperatively, his CDVA increased to 20/25 with a manifest refraction of -1.00×140 OD and 20/20 with a manifest refraction of Plano OS. Six months postoperatively, his CDVA was 20/20 with a manifest refraction of +1.25/-1.25×156 OD and 20/20 with a manifest refraction of -0.50×145 OS. ASOCT showed corneal scarring, and irregular pupil without flap edema 5mo postoperatively (Figure 5D). Corneal topography showed irregular astigmatism decreased significantly from 1mo postoperatively and stay stable until 6mo with K1 40.9×143.9 and K2 42.5×53.9 in the right eye (Figure 6).
DISCUSSION
The corneal opacity may decrease the femtosecond laser energy and scanning quality, thus resulting in higher risks of complications[9]. Normally, lamellar corneal refractive surgery is not of priority for myopia patients with corneal opacity.However, previous studies reported good visual outcomes after SMILE among patients with superficial corneal opacity[6]. In this work, we reported the attempt-to treat myopia patients with traumatic deep corneal opacitiesvialamellar corneal refractive surgery of SMILE or FS-LASIK. The present study showed that patients with traumatic deep corneal opacities can be successfully treated by the SMILE or FS-LASIK based on rigorous preoperative evaluation and careful surgical maneuvers. Patients combined with high astigmatism resulting from traumatic irregular corneal topography can effectively be treated by FS-LASIK and LRI. The short and mid-term outcome is good and stable for SMILE, while the long-term outcome until 6mo of FS-LASIK and LRI is also satisfactory in the case with chemical burn and intraocular surgery history.In cases 1 and 4, the eye with corneal opacity showed lower corneal endothelial cell density compared with the fellow eye. We speculate that the traumatic history by spectacles of case 1 or severe firecracker wound and subsequent intraocular surgeries of case 4 contributed to preoperative endothelial cell loss (ECL). ECL can result from inherited, inflammatory,traumatic, immunological, intraocular manipulation, and infectious etiologies[12-14]. Mechanical injury in infants caused significant ECL with a higher annual decrease compared to the fellow eye[15]. The ocular chemical burn was observed with corneal endothelial insufficiency and corneal endothelial decompensation may develop, which needs corneal endothelial keratoplasty[16]. Of note, although SMILE reported no adverse effects on corneal endothelial cells[17], careful evaluation by specular microscope preoperatively, or even long-term followup postoperatively, is necessary for traumatic patients to ensure the safety of surgery.

Figure 5 The preoperative AS-OCT examination of corneal opacity Slit-lamp microscopy (A: preoperative, corneal opacity was indicated by the red arrow; B: 1mo after surgery, C: 5mo after surgery, LRI was indicated by white arrow) and AS-OCT examination (D) 5mo after FS-LASIK and LRI of the right eye. AS-OCT: Anterior segment optical coherence tomography; LASIK: Laser in situ keratomileusis; LRI: Limbal relaxing incision.

Figure 6 The corneal topography before (A), 1mo (B), 3mo (C), and 6mo (D) after FS-LASIK combined with LRI in the right eye FS-LASIK:Femtosecond laser in situ keratomileusis; LRI: Limbal relaxing incision.
AS-OCT is an effective approach to detect morphological changes such as corneal atrophy or corneal edema according to the optical density[18-19]. Corneal transparency and grades of corneal opacities can be quantified using AS-OCT[20]. In this work, AS-OCT was applied to confirm the depth, area, and density of corneal opacity to assist the surgery design. Cap or flap thickness and diameter were adjusted accordingly to avoid opacity: in case 1, the optical zone was 6.6 mm; in case 3, cap thickness was 120 μm with diameter 7.3 mm; in case 4, flap thickness was 100 μm with diameter 8.2 mm, the optical zone was 6.5 mm. Corneal topography can give detailed cornea surface data, especially the preoperative irregular morphology.The relevant data can guide the surgical design like LRI and help the prediction of a postoperative visual quality outcome.The rate of corneal edema after SMILE was 0.09%[21]. Obvious edema right above the corneal opacity lesion was observed in case 2, indicating a more severe inflammatory response. Close follow-up and appropriate medication are important for quick dissipation of edema and recovery of visual quality. Although the inflammation alleviated soon and uneventfully, our case indicated the risk of corneal edema after SMILE with thick and deep corneal opacity located in the optical zone. Skillful and careful maneuver during operation, especially the dissection and separation of corneal flap or cap around the corneal lesions, is essential for the success of the surgery.
Two previous studies reported outcomes after SMILE or FSLASIK for patients with corneal opacity. However, corneal opacity in these studies was located in the superficial corneal stroma or lacked quantitative analysis[6,22]. The cases in this work included deep or full-thickness corneal opacity within the optical zone caused by diverse damage factors. Interestingly,two cases even achieved better CDVA after surgery (case 2 and case 3). The improved visual quality may be due to the decrease in thickness, area, and density of corneal opacity and amelioration of irregularity corneal topography after surgery(Tables 1 and 2, Figure 2).
The limitation of this study is that the sample size is small and may only provide information based on our practical experience. A study with more cases, prospective design and multicenter data will be more sufficient to validate the safety,efficacy, and accuracy of SMILE or FS-LASIK in patients with preoperative corneal opacity. Although, as previously described, the corneal opacity is the potential factor which may prevent the success of lamellar surgeries, especially SMILE,our results showed satisfactory outcome of treatment in these special cases. The present work is a useful attempt to treat such special cases and hence is contributory to enrich the relevant experience of SMILE or FS-LASIK.
ACKNOWLEDGEMENTS
Foundations:Supported by the Science and Technology Program of Zhejiang Province (No.2019C03046); the Natural Science Foundation of Zhejiang Province under Grant (No.LQ20H120007).
Authors’ contributions:Fang Z was responsible for the acquisition of the clinical information and writing the manuscript. He XY was responsible for the data analysis and interpretation of data. Han W was responsible for the critical revision of the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest:Fang Z,None;He XY,None;Han W,None.
 International Journal of Ophthalmology2023年2期
International Journal of Ophthalmology2023年2期
- International Journal of Ophthalmology的其它文章
- Comment on: Amniotic membrane for covering high myopic macular hole associated with retinal detachment following failed primary surgery
- Trend of glaucoma internal filtration surgeries in a tertiary hospital in China
- Effects of slanted bilateral lateral recession vs conventional bilateral lateral recession on convergence insufficiency intermittent exotropia: a prospective study
- Optical coherence tomography enhanced depth imaging of chorioretinal folds in patients with orbital tumors
- Trends in operating room-based glaucoma procedures at a single eye center from 2016-2020
- Comparison of biological behavior of lacrimal gland adenoid cystic carcinoma with high-grade transformation cells
