Effect of palmitoylethanolamide on degeneration of a human-derived retinal pigment epithelial cell induced by all-trans retinal
Yun Han, Kun-Huan Yang, Dan-Xue He, Chao-Feng Yu, Lei Tao, Chun-Yan Liao,Bin-Xiang Cai, Zu-Guo Liu, Yan Qiu, Ya-Lin Wu,4
1Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Engineering and Research Center of Eye Regenerative Medicine, School of Medicine, Xiamen University, Xiamen 361102, Fujian Province, China
2Department of Ophthalmology, Xiang’an Hospital of Xiamen University, Xiamen 361102, Fujian Province, China
3Xiamen University Affiliated Xiamen Eye Center, Xiamen 361102, Fujian Province, China
4Shenzhen Research Institute of Xiamen University, Shenzhen 518000, Guangdong Province, China
Abstract
● KEYWORDS: palmitoethanolamide; ARPE-19; fundus;all-trans retinal; apoptosis
INTRODUCTION
Age-related macular degeneration (AMD), is the main reason leading to irreversible and severe vision damage over 60 years old all over the world[1]. AMD is a multivariate fundus disease affecting the maculae, photoreceptors and retinal pigment epithelium (RPE) deprive the function owing to the late-onset progressive neurodegeneration. Many factors are relevant to AMD pathogenesis, including immunity,metabolic disorders, oxidative stress, inflammation, and so on.AMD divides two types including dry AMD and wet AMD[2].The more general form is dry AMD about 88% in AMD patients.
In recent years, people used the aggressive and combined therapy methods, such as laser coagulation, vascular endothelial growth factor (VEGF) receptors, anti-oxidants,gene therapy,etc.However, there is no effective cure to treat dry AMD, the blindness rate continue rises[3-5]. Stargardt’s disease (STGD) is an inherited eye disease of adolescent macular dystrophy[6]. STGD caused byAbca4gene mutation,which is called STGD1[7]. STGD1 children may be blindness when they are adults, however there is no effective treatment.So, it’s necessary to find new therapeutic agents with less toxicity. To study its molecular mechanisms to cure dry AMD and STGD1.
Palmitoylethanolamide (PEA), an endocannabinoid mimetic amide, is used in the anti-inflammatory, analgesic characteristic specifically in humans[8-9]. PEA has been demonstrated safety and tolerability[9-11]. PEA is indicated hopefully to use in many therapeutic fields both in preclinical and clinical studies as an endogenous cell protective lipid, such as eczema, pain,and neurodegeneration[12-17]. In the 70s of the 20thcentury,PEA was evaluated as a preventive and therapeutic agent for the treatment of influenza and colds[18-19]. Besides its antiinflammatory and analgesic characteristic, PEA is a natural retinoprotectant[20-25]. PEA has been evaluated for glaucoma,diabetic retinopathy, and uveitis, pathological states based on chronic inflammation, respiratory disorders, and various pain syndromes in a number of clinical trials[26]. But by now,the role of PEA on all-trans retinal (atRAL) induced retinal denaturation has not been reported.
In the current study, we investigated effects of PEA on atRAL caused retinal denaturation. We used a mouse model ofAbca4-/-Rdh8-/-, an animal model with photoreceptor loss and RPE dystrophy[27-29]. PEA improved the retinal functional,prohibited both RPE and photoreceptor from death, ameliorates light-induced fundus impairment inAbca4-/-Rdh8-/-mice. PEA inhibits RPE c-Jun N-terminal kinase (JNK), phosphorylated JNK (p-JNK), c-Jun, phosphorylated c-Jun (p-c-Jun), Bak,cleaved caspase-3, the transcription factor C/EBP homologous protein (CHOP) and binding (Bip) protein levelsin vitro(induced by atRAL)andin vivo(Abca4-/-Rdh8-/-mice)experiments. PEA may favor for RPE cell apoptosis therapy in the presence of retinopathies caused by atRAL accumulation.It has potential as a therapeutic strategy for dry AMD and STGD1. The molecular mechanism may affect reactive oxygen species (ROS)-JNK-CHOP signaling.
MATERIALS AND METHODS
Ethical ApprovalThe Animal Care and Utilization Committee of School Medicine Xiamen University approved all the animal experiments. All animal operations strictly according to Chinese Association for Research in Vision and Ophthalmology (CARVO). The Institutional Review Board approved the study is “The occurrence and mechanism of photoreceptor iron death in dry age-related macular degeneration” and the approval number is XMULAC 20200072.
ReagentsHoechst 33342, atRAL bought in Sigma-Aldrich(St. Louis, MO, USA). The 2’,7’-dichlorodihydrofluoresce in diacetate (H2DCFDA) reagent was bought in Thermo Fisher Scientific (Eugene, OR). Antibodies including cleaved caspase-3 (9664S and 9661S), p-JNK (9255S), JNK (9252S),p-c-Jun (9261S), c-Jun (9165S), Bak (12105S), CHOP(D46F1; 5554S), BiP (C50B12; 3177) and GAPDH (5174S)were purchased in Cell Signaling Technology (Danvers, MA).p-JNK (4821) was bought in Abcam. Horseradish peroxidaseconjugated goat anti-rabbit IgG (H L; 31460) and donkey antirabbit IgG (H L; A21207) secondary antibodies were provided by Thermo Fisher Scientific (Rockford, IL).
AnimalsIn this experiment, we usedAbca4-/-Rdh8-/-mice modelwhich had been described before[29]. Four weeks old ofAbca4-/-Rdh8-/-mice, being 48h dark-adapted, 1% tropicamide dilated the pupils, then exposed to10 000 lx light emitting diode (LED) light with 450-460 wavelength range for 1h.The control mice ofAbca4-/-Rdh8-/-, feed at normal darkness with 5d without 10 000 lx LED exposed. Additionally, after 48h dark adaptation, 4 weeks old mice ofAbca4-/-Rdh8-/-were intraperitoneally injected with 4 mg/kg body weight PEA or vehicle dimethyl sulfoxide (DMSO). An hour later,1% tropicamide dilated the mice pupils and then PEA or vehicle DMSO-treated mice layed bare in 10 000 lx LED 1h.PEA or vehicle DMSO intraperitoneally injected the controlAbca4-/-Rdh8-/-mice which were without light exposure. After treatment PEA or vehicle DMSO for 4d once-daily, the mice were euthanized and the eyeballs collected for follow-up study at 5d after illumination.
As previously mentioned[30], dissolved PEA in solution: DMSO with 0.01%, Tween 80 with 10% (SigmaAldrich, St. Louis,MO, USA), 20% polyethylene glycol 400, 69.9% physiological saline. To study PEA roles onAbca4-/-Rdh8-/-mice RPE, PEA(30 mg/kg body weight) was injected intraperitoneally inAbca4-/-Rdh8-/-mice.
Fundus ImagingThe mice fundus images were performed as what mentioned before[29].All mice were induced sufficient pupil dilation by 1% tropicamide and were anesthetized deeply during testing. The mice placed on the lifting platform, applied carbomer to the cornea. A fundus imaging system (OptoProbe;OPIMG-L, UK) used to collect data. The position of the eye was adjusted to ensure the fundus image was clear. After data collection, normal saline and levofloxacin eye drops were administered to prevent infection.
Optical Coherence TomographyBefore optical coherence tomography (OCT) imaging was performed, each animal was anesthetized and the pupils were dilated with a drop of 1% tropicamide. Then, a drop of 0.2% carbomer was applied to each cornea to keep the tissue moist. An adjustable custom-made platform was used for maintaining appropriate positioning of the mice. After altering the position and angle of the mice, the retinal OCT images of each eye were acquired with the optic nerve head centered on the corresponding box by using a Small Animal Retinal Imaging System (OptoProbe,OPIMG, UK). The neural retinal thickness and thickness of the outer nuclear layer (ONL) were analyzed with using OCT Image Analysis software (Version 2.0, Optoprobe, UK).
ElectroretinographyBefore the electroretinogram (ERG)recordings, mice were dark-adapted at least 12h, and all operation processes were performed under dim red-light conditions.The pupils were dilated with a drop of 1%tropicamide, next, animals were anesthetized with inhalation(0.6 L/min) of 2% isoflurane for induction phase and then 1.5% isoflurane for maintenance phase. The gold electrode was placed at the center of the cornea. Scotopic ERG recordings were obtained at the following increasing light intensities:0.01, 0.1, 1, 10 cd·s/m2. Flash ERGs responses were recorded using the Diagnosys Espion E2 ERG system (Diagnosys, LLC,Lowell, USA) and acquired data were analyzed using Espion software. Immediately after ERG recording, imaging of the fundus was performed as previously described above.
Cell CultureHuman-derived retinal epithelial cells (ARPE-19)bought in Fudan IBS Cell Center (Shanghai, China) and cultured as what mentioned before[28].
Treat with Palmitoylethanolamide and All-trans RetinalThe1.5×104cells per well of ARPE-19 cells inoculated in 96-well plates or 3×105cells per well cells in 6-well plates,cultured them overnight. PEA (2.5, 5, 10, and 20 µmol/L)pretreated ARPE-19 cells for 2h. Then, atRAL (2.5, 5, 15, and 20 µmol/L) or blank control treated ARPE-19 cells for 6h.
Cell ViabilityAs mentioned earlier, the MTS method was used to evaluate cytotoxicity[29].
Measurement of Intracellular Reactive Oxygen SpeciesatRAL with 15 μmol/L treated ARPE-19 cells for 6h. After incubation with 10 μmol/L H2DCFDA or 5 μmol/L MitoSOX Red at 37°C for 10min, Hochest 33342 labeled the nuclei.After PBS washed, used confocal microscope to examine the cells.
Western BlotCell and tissue extraction manipulate by Western blot analysis as previously described[29].
Statistical AnalysisThe software of GraphPad Prism software(Version 5.0; La Jolla, CA, USA) analyzed all data. The data with three separate tests were averaged from to compute mean and standard deviation (mean±SD). Single-factor or two-factor analysis of variance (ANOVA) was used for statistical analysis,using Tukey’s test as shown in legends. Significance marked byP<0.05.
RESULTS
Palmitoylethanolamide Ameliorates ARPE-19 Cells Apoptosis Induced by All-trans RetinalTo examined the health ARPE-19 cells viability which layed bare to atRAL 6h. ARPE-19 cells viability decreased in a concentration dependent way causing by atRAL (Figure 1A). atRAL cultured with ARPE-19 cells, its IC50 value was 18 µmol/L for 6h.Besides, ARPE-19 cells culturing under (2.5, 5, 15, and 20 µmol/L)atRAL for 6h, reduced cell survival rate significantly about 10%, 28%, 45%, 65%, respectively. According to the cell viability tests results, 15 µmol/L atRAL induced ARPE-19 cells with following tests. MTS results indicated that PEA protected ARPE-19 cells from apoptosis at 2.5, 5, 10, and 20 µmol/L concentration dependent and effectively induced by 15 µmol/L atRAL (Figure 1B). When exposed to atRAL for 6h at concentrations 15 μmol/L, ARPE-19 cells morphology changed significantly assuming roundness, contraction,and cytoplasmic rupture. PEA ameliorates ARPE-19 cells morphology caused by atRAL (Figure 1C).
Immunoblot analysis indicated that PEA diminished proapoptotic protein Bak level ARPE-19 cells induced by atRAL(Figure 1D). ARPE-19 cells were treated by 15 µmol/L atRAL or 15 μmol/L atRAL 6h with 10 μmol/L PEA or DMSO serving as a blank control for Bak immunoblot analysis. The expression of Bak decreased dramatically (Figure 1E) in atRAL with PEA-loaded ARPE-19 cells, which showed PEA suppressed Bak protein.
PEA Suppresses Reactive Oxygen Species Yield Cause by atRAL in ARPE-19 CellsROS leads to apoptosis, intracellular ROS production was detected by fluorescence microscopy ARPE-19 cells being atRAL-loaded which cultured in H2DCFDA. The intracellular ROS generation significantly increased in ARPE-19 cells cultured with 15 µmol/L atRAL for 6h, about 26 times as many as the control cells (Figure 2A,2B). ARPE-19 cells ROS production effectively lessen, when treated 10 µmol/L PEA exposing 15 µmol/L atRAL after 6h(Figure 2A, 2B). Immunoblot analysis showed that 10 µmol/L PEA markedly reduced CHOP protein level in ARPE-19 cells caused by atRAL accumulation (Figure 2C, 2D).
PEA Ameliorates ARPE-19 Cell Apoptosis by Affecting JNK Signaling Induced by atRALARPE-19 cells treated by 15 µmol/L atRAL for 6h, Western blotting analyses displayed that p-JNK protein levels were significantly upregulated. It demonstrated that atRAL actived ARPE-19 cells JNK signaling.

Figure 1 PEA ameliorates ARPE-19 cell apoptosis induced by atRAL A: MTS method tested ARPE-19 cell viability when ARPE-19 cells exposure to atRAL (2.5, 5, 15, and 20 µmol/L) for 6h. B: MTS assay measured ARPE-19 cell viability, incubated in 15 µmol/L atRAL with PEA (2.5, 5, 10,and 20 µmol/L) 6h. C: Leica DMi8 inverted microscope imaged cellular morphology. The scale bars, 100 µm. D: Bak immunoblot analysis ARPE-19 cells lysates with 15 µmol/L atRAL or 15 µmol/L atRAL at 10 µmol/L PEA or vehicle. E: Bak protein levels, showed folding changes relative to DMSO control. aP<0.05; bP<0.01; cP<0.001. PEA: Palmitoylethanolamide; atRAL: All-trans retinal; ARPE: human-derived retinal epithelial cells;DMSO: Dimethyl sulfoxide; MTS: CellTiter 96® Aqueous One Solution Cell Proliferation Assay.
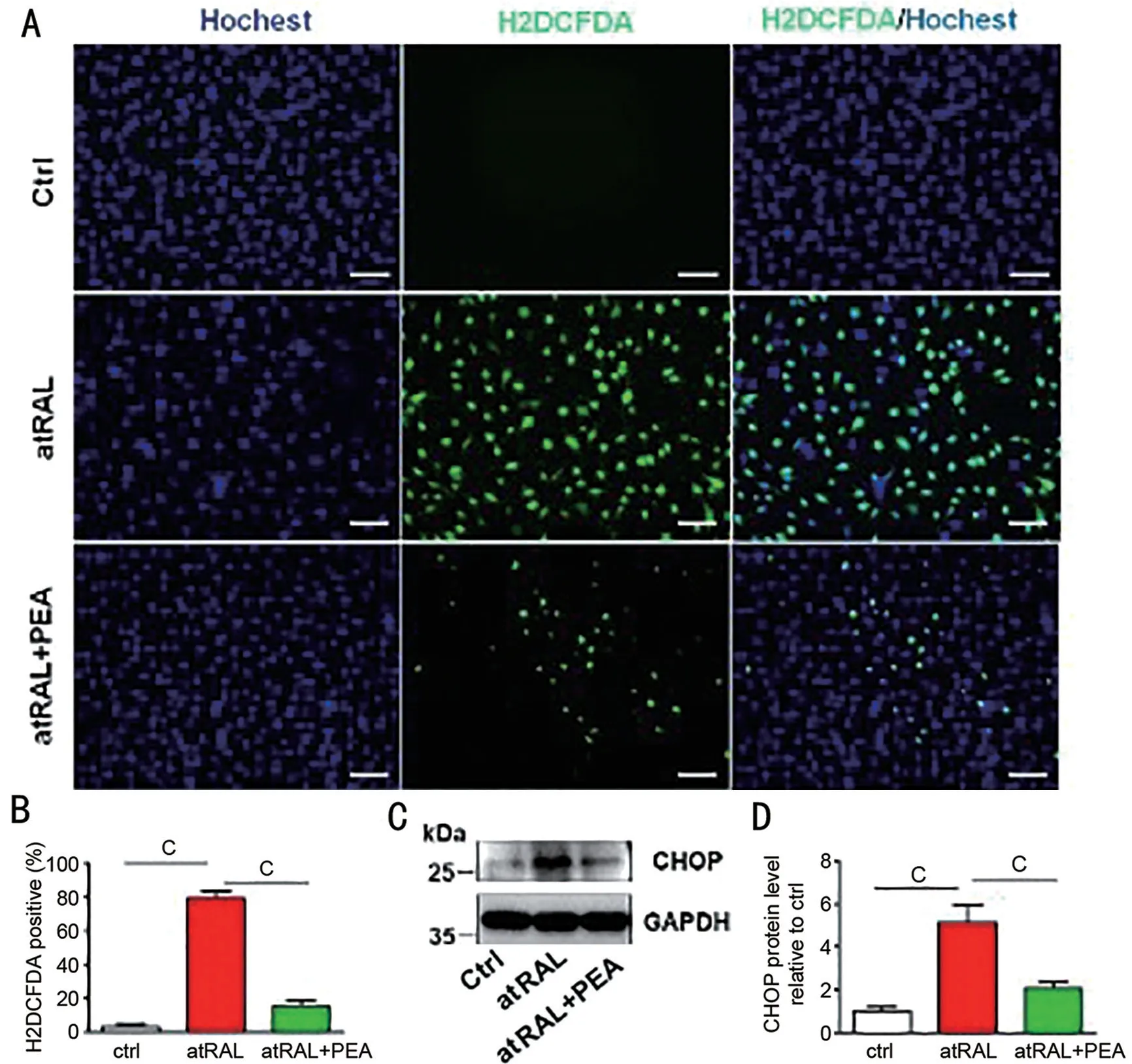
Figure 2 PEA suppresses ROS (including mitochondrial ROS) generation in ARPE-19 cells caused by atRAL A: Intracellular ROS production,H2DCFDA staining tested by fluorescence microscopy. Scale bars, 50 µm. B: Ratio of H2DCFDA positive cells by fluorescence microscopy. C:Immunoblot analysis of CHOP in ARPE-19 cells cultured to atRAL 15 µmol/L or atRAL 15 µmol/L with 10 µmol/L PEA or DMSO 6h. D: Expression levels of CHOP protein relative to control. cP<0.001. PEA 10 µmol/L pretreated the cells for 2h. PEA: Palmitoylethanolamide; atRAL: All-trans retina; ROS: Reactive oxygen species; H2DCFDA: 2’,7’-dichlorodihydrofluorescein diacetate; CHOP: C/EBP homologous protein.
However, results showed that PEA down-regulated ARPE-19 cells p-JNK protein levels which treated by 15 µmol/L atRAL for 6h (Figure 3A, 3B). Since phosphorylation of C-Jun, the JNK direct substrate, which is the JNK activation indicator,we use Western blotting to test ARPE-19 cells c-Jun activated state induced by atRAL. ARPE-19 cells treatment with atRAL, protein level of p-c-Jun, which is JNK downstream transcription factor, was significantly increased. However, PEA down-regulated ARPE-19 cells p-c-Jun protein levels treated by atRAL15 µmol/L 6h (Figure 3C, 3D). Results showed PEA suppressed ARPE-19 cells apoptosis induced by atRAL through inhibition JNK signaling. Western blotting analysis indicated that 10 μmol/L PEA obviously decreased p-JNK, p-c-Jun, cleaved caspase-3 protein levels in ARPE-19 cells caused by atRAL (Figure 3E, 3F). Collectively, these findings disclose that PEA diminishes ARPE-19 apoptosis evoked by atRAL partly by affecting JNK signaling pathway.
PEA Ameliorates Photoreceptor and RPE Degeneration Effectively in Light-exposedAbca4-/-Rdh8-/- MicePEA(4 mg/kg body weight) treatedAbca4-/-Rdh8-/-mice by intraperitoneal injection.PEA effectively relieved photoreceptor atrophy, prevented the ONL and the whole neural retina thickness reduction from light-exposed ofAbca4-/-Rdh8-/-mice using histological assessment with OCT(Figure 4A-4B). In addition,in vivo, fundus retinal imaging showed that intraperitoneal injection of PEA eliminated RPE degeneration inAbca4-/-Rdh8-/-mice on day 5 after light exposure (Figure 4C). The ultimate goal of retinal degeneration is to preserve visual function. Full-field electroretinal imaging(ERG) was used to measure the response of rod photoreceptors to light stimulation under dark patch conditions (Figure 4D).The retinal function of DMSO-treated and the absence of light exposure groups showed the same strong scotopic ERG curve as the normal control group, while the retinal function ERG curve ofAbca4-/-Rdh8-/-mice upon light exposure group significantly decreased, indicating retinal damage. Fortunately,PEA treatment effectively prevented light-induced drop in aand b-waves under scotopic conditions, reflecting improved retinal function after PEA treatment. The quantification of a- and b-wave amplitudes under scotopic condition further clearly demonstrated PEA improved the retinal functional(Figure 4E, 4F). Together, these datas suggest that PEA has a strong protective effect on light-induced photoreceptor and RPE atrophy inAbca4-/-Rdh8-/-mice, suggesting its promising therapeutic strategy.
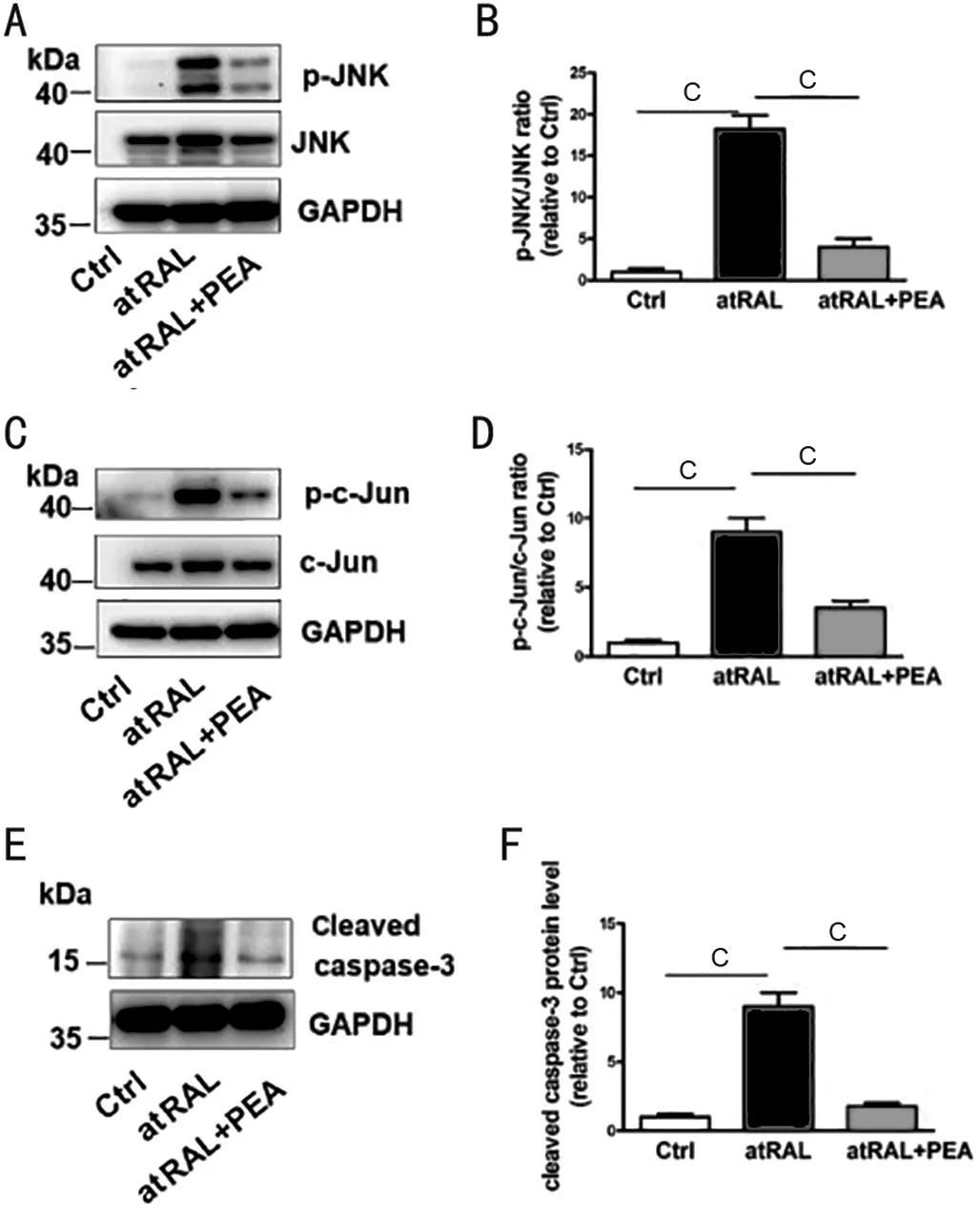
Figure 3 PEA ameliorates RPE cell apoptosis via affecting JNK signaling induced by atRAL A: Western blotting to analyze JNK and p-JNK; B: p-JNK/JNK protein level ratios; C: Immunoblot to analyze c-Jun and p-c-Jun protein level; D: c-Jun and p-c-Jun protein level ratios. E: Western blotting to analyse cleaved caspase-3. F: Cleaved caspase-3 protein level ratios. cP<0.001. PEA: Palmitoylethanolamide;atRAL: All-trans retina; JNK: c-Jun N-terminal kinase; p-JNK:Phosphorylated JNK; p-c-Jun: Phosphorylated c-Jun; RPE: Retinal pigment epithelium.
PEA Ameliorates RPE Cell Apoptosis by Affecting ROSJNK-CHOP Signaling in Light-inducedAbca4-/-Rdh8-/-MiceIntraperitoneally injection of PEA amelioratedAbca4-/-Rdh8-/-mice RPE degeneration after day 5 irradiation imaging byin vivoretinal fundus (Figure 4). To verify PEA suppressed JNK signaling pathway and its relative proteins in RPE with illuminationofAbca4-/-Rdh8-/-mice, p-c-Jun, p-c-Jun, Bak, CHOP, and Bip protein levels were used to detect by immunoblot. Western blotting analysis displayed p-JNK protein levels significantly increased in RPE illumination ofAbca4-/-Rdh8-/-mice (Figure 5A, 5B). Additionally, p-c-Jun,Bak, CHOP, and Bip protein levels, which function relative to the JNK signaling, markably increased inAbca4-/-Rdh8-/-mice neural retina with illumination(Figure 5C-5J). The study showed that PEA administration obviously reduced p-c-Jun,Bak, CHOP, and Bip protein levels inAbca4-/-Rdh8-/-mice RPE with light exposure (Figure 5C-5J).
DISCUSSION

Figure 4 Effects of PEA on photoreceptor and RPE degeneration in light-exposed Abca4-/-Rdh8-/- mice A: Examination of mouse retina morphology by using OCT. PEA or vehicle (DMSO; 4 mg/kg body weight) were intraperitoneally injected into 48h dark-adapted Abca4-/-Rdh8-/- mice at 4 weeks of age. One hour later, the mice were irradiated by 10 000 lx LED light for 1h after their pupils were dilated with 1%tropicamide, followed by once-daily administration of PEA or vehicle for 4d. Control Abca4-/-Rdh8-/- mice were intraperitoneally injected with PEA or vehicle in the absence of light exposure. Representative OCT images taken at day 5 upon light exposure. B: Thickness of ONL, and whole retina was quantified by OCT image analysis software. Data are presented as mean±SD (n=6). Statistical analyses were conducted by one-way ANOVA with Tukey’s multiple comparison test. cP<0.001. C: Typical fundus image. D: Representative dark-adapted ERG wave forms at 10 cd·s/m2 in mice. E: Implicit times for scotopic a-wave were calculated at flash intensities of 0.01, 0.1, 1, and 10 cd·s/m2. F: Implicit times for scotopic b-wave were calculated at flash intensities of 0.01, 0.1, 1, and 10 cd·s/m2. Data are expressed as mean±SEM (n=6). Statistical analyses were performed with one-way ANOVA with Tukey’s multiple comparison test. cP<0.001, (DMSO+light) vs (PEA+light), cP<0.001, DMSO vs (DMSO+light). PEA:Palmitoylethanolamide; atRAL: All-trans retina; OCT: Optical coherence tomography; DMSO: Dimethyl sulfoxide; ONL: Outer nuclear layer; RPE:Retinal pigment epithelium.
There are some Food and Drug Administration (FDA)approved therapies to treat wet AMD. However, there is no approved therapies to treat dry AMD. AMD pathogenesis is very complex and isn’t understood yet. Different therapeutic approaches to treat retinal damage are varied and debated in dry AMD[31-32], including visual cycle modulation, gene therapy, complement inhibition, neuroprotection, antiinflammatory therapy, cell-based treatments, prosthetic devices, photobiomodulation. None of the methods described is without shortcomings. Such as, the concerns with stem cell-based therapies include immune rejection, differentiation into undesired cell types, damage to surrounding tissues,and tumor formation. Visual cycle modulators with oral route of administration are appealing to patients, but the downside of these treatments is that dark adaptation and low-light vision can be adversely affected by modulating the visual cycle. Fortunately, PEA is abioactive endogenous acyl ethanolamine lipid. Different from the secretion of other endogenous signaling molecules, PEA is synthesized and released by membrane phospholipids “on demand” only when the cell membrane is stimulated by external stimuli, and exerts physiological and pharmacological effects. PEA does not accumulate in the body after it has taken effect. It will be re-absorbed by the corresponding transporter to retrieve intracellular hydrolytic inactivation. It has been demonstrated with the high safety and tolerability. PEA is indicated hopefully to use in many therapeutic fields both in preclinical and clinical studies, such as eczema, pain, and neurodegeneration. PEA might be a natural retinoprotectant.
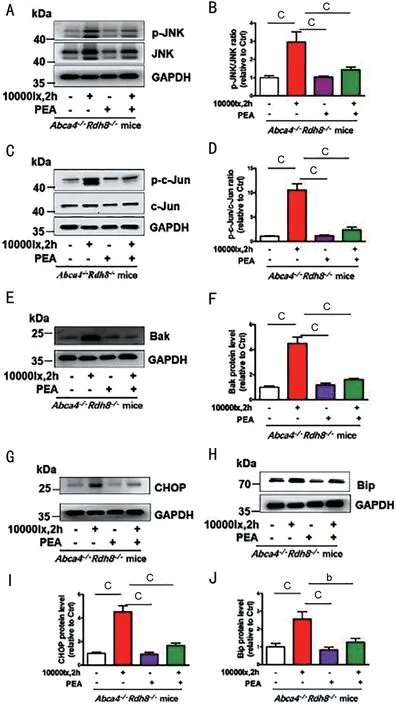
Figure 5 PEA ameliorates RPE cell apoptosis by affecting ROS-JNKCHOP signaling in Light-induced Abca4-/-Rdh8-/- mice A: p-JNK and JNK Western blotting analysis. B: p-JNK/JNK protein level ratios.C: Immunoblot analysis p-c-Jun/c-Jun protein level. D: p-c-Jun/c-Jun ratio. E: Western blotting analysis of Bak protein level. F: Bak protein level ratios. G: Western blotting analysis of CHOP protein level. I: CHOP protein level ratios. H: Western blotting analysis of Bip protein level. J: Bip protein level ratios. bP<0.01; cP<0.001. PEA:Palmitoylethanolamide; atRAL: All-trans retina; ROS: Reactive oxygen species; JNK: c-Jun N-terminal kinase; p-JNK: Phosphorylated JNK;p-c-Jun: Phosphorylated c-Jun; RPE: Retinal pigment epithelium;CHOP: C/EBP homologous protein; Bip: Binding protein.
Central visual damaged in AMD patients partly caused by RPE cells degeneration[33], which becomes a major public health issue[34-35]. STGD1 and dry AMD patients had typical manifestations of retinal dystrophy which caused photoreceptors and the RPE atRAL accumulation[27-29]. So,vision sustaining is important to clear the released atRAL timely[2,36]. Evidence demonstrate that transient amassing of atRAL by delayed clearance from the retina is one of the key mechanisms in light-induced retinal degeneration[27,37-38]. In the visual (retinoid) cycle, ABCA4 and RDH8’s responsibility are to clear up the retina atRAL[39-40]. And theAbca4-/-Rdh8-/-mice model exhibit the photoreceptor loss and RPE dystrophy,which are STGD1 and dry AMD primary features[27,37-38].Previous studies findings demonstrate photoreceptor/RPE dystrophy was related to atRAL toxicity[29,31].
The stress stimuli, growth factors, and inflammatory cytokines may activate c-Jun N-terminal kinases[41-47]. JNK signaling activation linked to apoptosis[46], some degenerative illness,such as, Parkinson’s and Alzheimer’s diseases involves the development[48-49]. Our earlier research suggested atRAL promotes ARPE-19 cells apoptosis by mitochondrial damage[50-51], nucleotide oligomerization domain-like receptor thermal protein domain associated protein 3 (NLRP3)inflammasome activated lead to ARPE-19 cells deathviapromote caspase-3/GSDME-mediated pyroptosis caused by atRAL[28]. Recently, studies found RPE cells apoptosis relative to JNK activation caused to caspase-3/DNA injure dependent by atRAL[31].
PEA concentration is lower than normal subjects in glaucoma patients ciliary body[52]. PEA levels are lower in retina of AMD and diabetic retinopathy than healthy[24]. PEA remarkablely decreased splanchnic artery occlusion (SAO) shock cell death[53]. Nitric oxide (NO) and ROS levels decreased by PEA regulation the main cytokines in the COVID-19 infected induced by lipopolysaccharide (LPS) injure[54].
In this study, we explored PEA effects in retinal degeneration induced by atRAL.In vitrocell tests, studies indicated that 15 µmol/L atRAL induced ARPE19 cells apoptosis significantlyviaupregulating JNK signaling. Meanwhile,PEA downregulated JNK signaling which induced by atRAL. Previous studies showed stress and ROS production caused by atRAL in ARPE-19 cells[38,50-51], activation of JNK induced RPE cells apoptosis. As expected, PEA suppressed ARPE-19 ROS (including mitochondrial ROS) generation induced by atRAL in our study. Bak plays a main role in apoptotic cell death[55]. Currently, study reviewed that NLRP3 inflammasome was activated by atRAL to induce ARPE-19 cell death and promotes caspase-3/Gasdermin domaincontaining protein (GSDME)-mediated pyrodeath[28]. Bak and cleaved caspase-3 proteins expression reduced, which revealed that PEA repressed atRALR caused RPE cell apoptosis.Researches indicated stress of ER marker proteins such as Bip upregulation related to CHOP expression upregulation[56].CHOP is downstream of JNK[57]. Our study showed in ARPE-19 cells, PEA remarkablely reduced Bip and CHOP protein levels in JNK signaling pathway by atRAL accumulation.
Abca4-/-Rdh8-/-mice after light exposure revealed dry AMD and STGD1 characteristics with photoreceptor/RPE degeneration caused by rapid elevation of atRAL levels in the retina[32]. In the retina,Abca4-/-Rdh8-/-mice were exposed to fluorescent light at 10 000 lx resulting in rapid accumulation of atRAL[58],and promoted photoreceptor cell death and retinal damage[59].Similar data were found inin vivostudies, studies showed thatAbca4-/-Rdh8-/-mice which irradiated bylight activated JNK signaling in the neuro-retina[29]. More importantly,intraperitoneal injection of PEA effectively amelioratedAbca4-/-Rdh8-/-mice RPE degeneration and apoptosis after light irradiation. PEA effectively relieved photoreceptor atrophy and prevented the reduction in the thickness of ONL and whole neural retina from light-exposedAbca4-/-Rdh8-/-mice. PEA treatment effectively prevented light-induced drop in a- and b-waves under scotopic conditions, reflecting an improvement in retinal function by PEA administration.
PEA also inhibits JNK, p-JNK, c-Jun, p-c-Jun, Bak, cleaved caspase-3, CHOP, and Bip protein levels in RPE partly. Thus,PEA may be favor in the therapy of RPE cell death of retinal degeneration caused by atRAL accumulation.
Our results indicated that PEA may be a therapeutic to treat dry AMD and STGD1. However, the mechanism of PEA action is not clear yet. In the future, we will continue to study the mechanism of PEA in dry AMD and STGD1.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.82171064; No.81870671;No.82274162); Natural Science Foundation of Fujian Province (No.2020J01013); Guangdong Basic and Applied Basic Research Foundation (No.2022A1515012514;No.2021A1515011391).
Conflicts of Interest: Han Y,None;Yang KH,None;He DX,None;Yu CF,None;Tao L,None;Liao CY,None;Cai BX,
None;Liu ZG,None;Qiu Y,None;Wu YL,None.
 International Journal of Ophthalmology2023年2期
International Journal of Ophthalmology2023年2期
- International Journal of Ophthalmology的其它文章
- Perspectives and clinical practices of optometrists in Saudi Arabia concerning myopia in children
- Progression of myopia among undergraduate students in central China
- Flipped classroom approach to global outreach: crosscultural teaching of horizontal strabismus to Chinese ophthalmology residents
- Topical ketotifen treatment for allergic conjunctivitis: a systematic review and Meta-analysis
- Pseudomembranous conjunctivitis in a patient with DRESS syndrome
- Two cases of persistent shallow anterior chamber after cataract surgery combined with goniosynechialysis
