Pseudomembranous conjunctivitis in a patient with DRESS syndrome
Young Joo Lee, Youngsub Eom,,3, Seo Yeon Park,4, Jung Jin Shin, Young Choi,6, Jong Suk Song,Hyo Myung Kim
1Department of Ophthalmology, Korea University Ansan Hospital, Gyeonggi-do 15535, Republic of Korea
2Department of Ophthalmology, Korea University College of Medicine, Seoul 02841, Republic of Korea
3Department of Ophthalmology, Emory University School of Medicine, Atlanta, GA 30322, USA
4BGN Jamsil Lotte Tower Eye Clinic, Seoul 05551, Republic of Korea
5Department of Dermatology, Korea University Ansan Hospital, Gyeonggi-do 15355, Republic of Korea
6Shin’s Eye Clinic, Chungcheongnam-do 31513 , Republic of Korea
Dear Editor,
Drug rash with eosinophilia and systemic symptoms(DRESS) syndrome is a drug reaction featuring high fever, eosinophilia, skin rashes, and multi-organ involvement that may be fatal. Ocular manifestations, including cicatrizing conjunctivitis, corneal infiltration, and anterior uveitis, are rarely reported[1-6]but may cause serious complications if untreated. Therefore, it is important to diagnose DRESS syndrome in patients with ocular features promptly with appropriate treatment. This article reports a case diagnosed with DRESS syndrome associated with pseudomembranous conjunctivitis secondary to carfilzomib.
A 52-year-old man visited our ophthalmology department complaining of ocular pain, foreign body sensation, and blurred vision in both eyes that had begun two days prior.Ocular symptoms were manifested with a fever over 38℃.Two days after the ocular manifestation, a skin rash started to spread over his whole body (Figure 1). Laboratory findings showed eosinophilia (eosinophils of 12.5%) and elevated liver enzyme levels [aspartate transaminase (AST) 41 IU/L, alkaline phosphatase (ALP) 165 IU/L, and gamma-glutamyl transferase(GGT) 332 IU/L]. Two years prior, he was diagnosed with multiple myeloma and was treated with peripheral blood stem cell transplantation. Recently, the patient suffered a relapse of multiple myeloma and began chemotherapy with carfilzomib three weeks before visiting our clinic. The written informed consent was obtained from the patient to publish a caser report.All research and data collection adhered to the tenets of the Declaration of Helsinki.
The best corrected visual acuity (BCVA) was 20/50 for each eye. Under the slit lamp examination, we noted thick pseudomembrane in both the upper and lower palpebral conjunctiva and sloughing in the bulbar conjunctiva (Figure 2).We did not find hemorrhagic follicles or discharge, which are common in epidemic keratoconjunctivitis. The cornea was edematous and had Descemet membrane folds. We initially instilled topical 0.5% moxifloxacin (Vigamox; Alcon, Fort Worth, TX, USA) and 5% sodium chloride hypertonicity ophthalmic solution (Muro 128, Bausch+Lomb, Bridgewater,NJ, USA). After two days, cornea epithelial defects developed and the thick pseudomembranes in the palpebral conjunctiva were static. Pseudomembranes were removed once daily for one week, and therapeutic bandage contact lenses were applied to both eyes to protect the corneas from the friction caused by the thick conjunctival membranes and to alleviate pain.We added topical 0.1% tacrolimus (Alymus; Taejoon Pharm,Republic of Korea) to improve ocular inflammation. After the corneal epithelial defects healed, a topical corticosteroid was added to improve ocular inflammation. A skin biopsy was done and the result showed lymphocytic exocytosis, consistent with skin eruption. We diagnosed the patient with DRESS syndrome.

Figure 1 Pechial skin rash spread over trunk and upper and lower extremities.
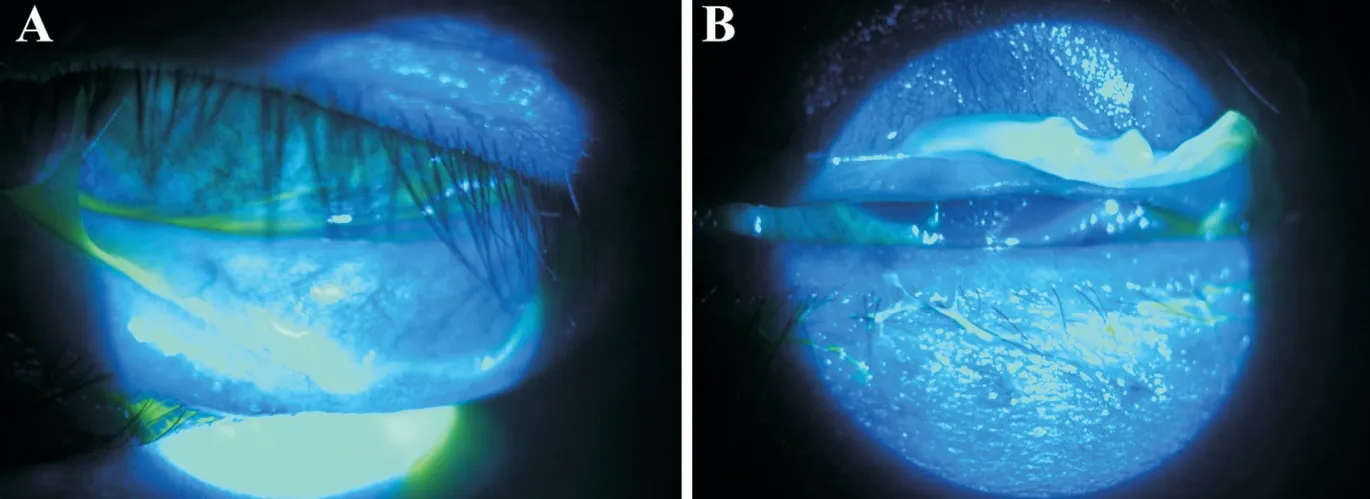
Figure 2 Thick pseudomembrane on upper and lower palpebral conjunctiva A: Right eye; B: Left eye.
After the diagnosis of DRESS syndrome, intravenous dexamethasone (6 mg/d) was administered for six days, and the patient’s general condition improved gradually. Cutaneous rashes subsided as his health improved. Thus, intravenous dexamethasone was changed to oral methylprednisolone(16 mg/d) and then tapered as a discharge medication for two weeks. After his hospital discharge, the patient’s ocular symptoms were observed through outpatient follow-up. Four weeks after the first visit to the ophthalmology department for ocular symptoms, pseudomembranous conjunctivitis was improved and a chronic cicatricial change in palpebral conjunctiva remained. Five months after the onset of DRESS syndrome, the patient’s BCVA improved to 20/20 in both eyes. The patient’s conjunctival scar changes were partially improved with continued use of topical 0.1% tacrolimus, but some conjunctival scars remained and the patient developed dry eye syndrome.
DRESS syndrome is a hypersensitivity-related drug reaction marked by high fever, high eosinophil counts, skin eruption,or severe multi-organ involvement. The onset is usually 2-8wk after the initiation of the causative drug[5-6]. According to the Registry of Severe Cutaneous Adverse Reaction (RegiSCAR),patients meeting more than three of these criteria can be diagnosed with DRESS syndrome: 1) hospitalization, 2)acute rash, 3) drug-related suspect reaction, 4) high fever,5) lymphadenopathy, 6) involvement of more than one internal organ, or 7) abnormal lymphocyte count[2]. Our patient met four of these criteria. Pseudomembrane may complicate distinguishing DRESS syndrome from epidemic keratoconjunctivitis, with the major difference being patient discharge[7-8]. Drugs that may cause DRESS syndrome include anticonvulsants and antibiotics, such as carbamazpine,phenytoin, phenobarbital, and minocycline[2]. Carfilzomib treats relapsed multiple myeloma, and hematologic toxicity has been reported after its administration[9].
Ocular features of DRESS syndrome, including conjunctivitis,corneal limbal stem cell destruction, symblepharon, corneal infiltration, anterior uveitis, angle-closure, and serous retinal detachment[1-3], are rarely reported, but once manifested may downgrade the patient’s condition. In previous studies, the ocular symptoms associated with DRESS syndrome were treated with a combination of topical and systemic corticosteroids[1-3].However, we applied topical 0.1% tacrolimus (Alymus), a calcineurin inhibitor that blocks T lymphocyte activity that is typically used for allergic conjunctivitis and systemic corticosteroids. Topical tacrolimus reportedly has 10-100 times stronger immunosuppressive effects than topical cyclosporine A[10]. By inhibiting the release of inflammatory cytokines,topical tacrolimus may alleviate inflammation[11]. Topical steroids can improve inflammation but cannot be used with corneal abrasion because they may increase susceptibility to infection and time-to-healing[12]. Therefore, in ocular manifestations accompanied with corneal abrasion, topical tacrolimus may be a good treatment choice.
In conclusion, systemic corticosteroids should be used to treat systemic symptoms of DRESS syndrome[13]and may also be effective in treating ocular signs and symptoms[1-3].Eye drops and ocular management are also needed[1-3]. Ocular manifestations may leave chronic and long-term sequelae after acute inflammation. Ophthalmologists should be aware of DRESS syndrome in patients with ocular features and promptly suggest appropriate treatment.
ACKNOWLEDGEMENTS
Foundations:Supported by the SNUBH Research Fund(No.13-2020-007); TRC Research Grant of the Korea University Medicine and Korea Institute of Science and Technology, by Korea University Ansan Hospital grant, by Korea University grants (No.K1625491; No.K1722121;No.K1811051; No.K1913161; No.K2010921); the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety)(No.1711174253, RS-2020-KD000296); Korea Environment Industry & Technology Institute (KEITI) through Technology Development Project for Safety Management of Household Chemical Products, funded by Korea Ministry of Environment(MOE) (No.2020002960007, NTIS-1485018521); the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021R1F1A1062017); the Technology Development Program (S3127902; S3305836)funded by the Ministry of SMEs and Startups (MSS, Korea).
Conflicts of Interest: Lee YJ,None;Eom Y,None;Park SY,None;Shin JJ,None;Choi Y,None;Song JS,None;Kim HM,None.
 International Journal of Ophthalmology2023年2期
International Journal of Ophthalmology2023年2期
- International Journal of Ophthalmology的其它文章
- Perspectives and clinical practices of optometrists in Saudi Arabia concerning myopia in children
- Progression of myopia among undergraduate students in central China
- Flipped classroom approach to global outreach: crosscultural teaching of horizontal strabismus to Chinese ophthalmology residents
- Topical ketotifen treatment for allergic conjunctivitis: a systematic review and Meta-analysis
- Two cases of persistent shallow anterior chamber after cataract surgery combined with goniosynechialysis
- Bedside anterior segment optical coherence tomographyassisted reattachment of severe hemorrhagic Descemet’s membrane detachment after ab externo 360-degree suture trabeculotomy combined with trabeculectomy
