L-and T-type Ca2+ channels dichotomously contribute to retinal ganglion cell injury in experimental glaucoma
Hong-Ning Wang ,Wen-Jing Qian ,Guo-Li Zhao ,Fang Li ,Yan-Ying Miao ,Bo Lei ,Xing-Huai Sun,Zhong-Feng Wang,
Abstract Retinal ganglion cell apoptotic death is the main pathological characteristic of glaucoma,which is the leading cause of irreversible blindness.Disruption of Ca2+ homeostasis plays an important role in glaucoma.Voltage-gated Ca2+ channel blockers have been shown to improve vision in patients with glaucoma.However,whether and how voltage-gated Ca2+ channels are involved in retinal ganglion cell apoptotic death are largely unknown.In this study,we found that total Ca2+ current densities in retinal ganglion cells were reduced in a rat model of chronic ocular hypertension experimental glaucoma,as determined by whole-cell patch-clamp electrophysiological recordings.Further analysis showed that L-type Ca2+ currents were downregulated while T-type Ca2+ currents were upregulated at the later stage of glaucoma.Western blot assay and immunofluorescence experiments confirmed that expression of the CaV1.2 subunit of L-type Ca2+ channels was reduced and expression of the CaV3.3 subunit of T-type Ca2+ channels was increased in retinas of the chronic ocular hypertension model.Soluble tumor necrosis factor-α,an important inflammatory factor,inhibited the L-type Ca2+ current of isolated retinal ganglion cells from control rats and enhanced the T-type Ca2+ current.These changes were blocked by the tumor necrosis factor-α inhibitor XPro1595,indicating that both types of Ca2+currents may be mediated by soluble tumor necrosis factor-α.The intracellular mitogen-activated protein kinase/extracellular signal-regulated kinase pathway and nuclear factor kappa-B signaling pathway mediate the effects of tumor necrosis factor-α.TUNEL assays revealed that mibefradil,a T-type calcium channel blocker,reduced the number of apoptotic retinal ganglion cells in the rat model of chronic ocular hypertension.These results suggest that T-type Ca2+ channels are involved in disrupted Ca2+ homeostasis and apoptosis of retinal ganglion cells in glaucoma,and application of T-type Ca2+ channel blockers,especially a specific CaV3.3 blocker,may be a potential strategy for the treatment of glaucoma.
Key Words:apoptosis;CaV1.2;CaV3.3;chronic ocular hypertension;extracellular signal-regulated kinase;mitogen-activated protein kinase;nuclear factor-kappa B;patch-clamp;retina;tumor necrosis factor-α
Introduction
Glaucoma is the leading cause of irreversible blindness in the world and results from progressive retinal ganglion cell (RGC) apoptosis,optic nerve head lesion,and visual field deficit.Similar to other neurodegenerative diseases,the mechanism underlying RGC degeneration in glaucoma has not been completely clarified (Tochel et al.,2005;Pang and Clark,2007;Almasieh et al.,2012;Mead and Tomarev,2022).The disorder of intracellular Ca2+homeostasis in RGCs plays a pivotal role in the pathogenesis of glaucoma.Disruption of Ca2+homeostasis results in various intracellular events that promote RGC apoptotic death (Tochel et al.,2005;Pang and Clark,2007;Almasieh et al.,2012;Guo and Chen,2022).Excessive intracellular Ca2+levels trigger cytoskeletal degradation through enhanced acti vities of specific enzymes,such as phospholipases,proteases,and protein kinases.Multiple factors are involved in intracellular Ca2+overload,such as voltage-gated Ca2+channels (VGCCs),ionotropic glutamate receptors,metabotropic glutamate receptors,intracellular calcium stores,and non-selective ion channels(Namekata et al.,2013;Ward et al.,2014;Weitlauf et al.,2014;Cueva Vargas et al.,2015;Dong et al.,2015;Zhao et al.,2018).VGCCs play important roles in maintaining the physiological function of RGCs.The involvement of VGCCs in the degeneration of RGCs was demonstrated through evidence showing that VGCC blockers (such as iganidipine,nilvadipine,flunarizine,nimodipine,and lomerizine) attenuated RGC damage in retinal ischemia/hypoxia models (Crosson et al.,1990;Takahashi et al.,1992;Toriu et al.,2000;Osborne et al.,2002;Yamada et al.,2006;Uemura and Mizota,2008).In the glaucomatous retina,VGCC blockers exhibit neuroprotective roles (Araie and Mayama,2011;Mayama,2014).However,clinical studies showed that administration of VGCC blockers (such as nimodipine and nifedipine) improved visual functions only in a subset of glaucoma patients with blood hypertension,suggesting that the effects of VGCC blockers might be related to the reduction of blood pressure,but they may not work directly on RGCs (Harris et al.,1997;Rainer et al.,2001;Luksch et al.,2005;Müskens et al.,2007;Mayama,2014).Therefore,identifying how VGCCs are involved in RGC injury in glaucoma is critical.Previous studies showed that RGCs express a variety of Ca2+channel subtypes,including L-,T-,N-,and P/Q-types (Xu et al.,2002;Sargoy et al.,2014).N-and P/Q-type Ca2+channels are mainly localized to the synapse-enriched inner plexiform layer (IPL);these Ca2+channel subtypes may be involved in the regulation of neurotransmitter release (Xu et al.,2002).Our recent study showed that high-and low-voltage-activated Ca2+currents are primarily mediated by L-and T-type Ca2+channels,respectively,in rat RGCs (Qian et al.,2017).In this study,we explored the changes in these two types of Ca2+currents and investigated possible mechanisms in an experimental glaucoma model.We also studied how these changes may contribute to RGC damage in glaucoma.Our findings may identi fy potenti al targets for the prevention and treatment of glaucoma.
Methods
Animals and chronic ocular hypertension model
Male Sprague-Dawley rats (specific-pathogen-free level;70–100 g,4 weeks old) were provided by the SLAC Laboratory Animal Co.,Ltd.(Shanghai,China,license No.SCXK (Hu) 2017-0005).All animals were reared under a standard 12-hour light/dark cycle,22–25°C,and 40–70% humidity in an IVC system(GUX-56-25,Guxiu Experimental Equipment,Suzhou,China) with a maximum of six rats in each cage.Animal care and experiments conformed to the Guide for the Care and Use of Laboratory Animals (8thed;National Institutes of Health,2011).This study was approved by the Bioethical Committee at Fudan University on February 27,2018 (approval No.IOBS 2018 JS-008).
The COH rat model was established as previously described (Chen et al.,2011;Xu et al.,2020).Briefly,micro-magnetic beads (10 µL) (Bangs Laboratories,Inc.,Indianapolis,IN,USA) were injected into the anterior chamber of rats after anesthesia via intraperitoneal injection of pentobarbital sodium (50 mg/kg,Cat# P3761,Sigma-Aldrich,St.Louis,MO,USA).Sham-operated control rats were injected with the same volume of saline.Intraocular pressure (IOP) was measured before the injections as a baseline (control),immediately after the injection (G0d),and on the third day (G3d),1 week (G1w),2 weeks (G2w),3 weeks (G3w),and 4 weeks (G4w) after the injections using a Tonolab digital tonometer (TioLat,Helsinki,Finland).
A total of 520 rats were used in this study.We performed eight experiments with electrophysiological recordings;one to three RGCs were recorded in each rat.(1) To investigate the changes of total,L-types,and T-types of Ca2+currents,210 rats were divided into six groups (n=30–36/group): sham(control) and COH at G3d,G1w,G2w,G3w,and G4w groups.(2) To investigate the dose-response relationship of tumor necrosis factor-α (TNF-α),23 rats were divided into four groups (n=5–6/group): 0.125,1.25,12.5,and 25 ng/mL groups.(3) To investigate the effects of TNF-α on L-and T-type Ca2+currents in isolated RGCs,46 rats were divided into four groups (n=10–12/group): control,TNF-α,XPro1595,and XPro1595+TNF-α groups.(4) To investigate the effects of intravitreal injection of TNF-α on L-and T-type Ca2+currents,46 rats were divided into four groups (n=10–12/group): control(injection of saline),TNF-α,XPro1595,and XPro1595+TNF-α groups.(5)To investigate the effects of intravitreal injection of TNF-α on L-and T-type Ca2+currents in COH rats,56 rats were divided into five groups (n=10–12/group): control (injection of saline),G3d,G3d+XPro1595,G1w,and G1w +XPro1595 groups.(6) To investigate the role of signaling pathways of TNF-α on L-and T-type Ca2+currents,12 rats were divided into two groups (n=6/group): U0126 and pyrrolidinedithiocarbamate ammonium (PDTC) groups.(7) To investigate the effects of nimodipine and mibefradil on Ca2+currents,one rat was treated with each drug.(8) To investigate the effects of XPro1595 on T-type Ca2+currents in RGCs of COH rats at G3w,12 rats were divided into three groups (n=4/group): control,G3w,and G3w+XPro1595 groups.
We performed three experiments with western blot analysis.(1) To investigate the changes in expression of Ca2+channel subunits (CaV1.2,CaV3.1,CaV3.2,and CaV3.3) and Brn3a,36 rats were divided into six groups (n=6/group):control,G3d,G1w,G2w,G3w,and G4 groups.(2) To investigate TNF-α-induced changes of Ca2+channel subunits (CaV1.2 and CaV3.3),39 rats were divided into three groups (n=6–7/group): control,TNF-α,and TNF-α+XPro1595 groups.(3) To investigate the linearity of protein intensity levels with different protein loading amounts,three rats were used.
For apoptosis detection,23 rats were divided into three groups: control (n=7),G2w (n=8),and G2w+mibefradil (n=8) groups.For immunohistochemistry experiments,12 rats were divided into two groups (n=6/group): control and COH groups.
Retrograde labeling of retinal ganglion cells
RGCs were retrogradely labeled as previously described (Zhao et al.,2010;Ji et al.,2011;Dong et al.,2015;Li et al.,2016).Briefly,5–7 days before the animals were sacrificed,4% rhodamine-B-isothiocyanate (2 µL;List Biological Laboratories,Campbell,CA,USA) was injected in anestheti zed rats into the superior colliculus bilaterally (6.0 mm posterior,2.0 mm lateral to the bregma,and 4–5 mm deep from the cortical surface) to label RGCs for electrophysiological recordings (Zhao et al.,2010).
Preparation of isolated retinal ganglion cells
RGCs were harvested from sham (control) and COH rats at specific time points(G3d,G1w,G2w,G3w,and G4w) as previously described (Zhang et al.,2013;Li et al.,2016;Qian et al.,2017).Briefly,the retinas were removed quickly from anestheti zed rats and incubated in oxygenated Hank’s solution (in mM: NaCl 137,NaHCO30.5,NaH2PO41,KCl 3,CaCl22,MgSO41,2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid 20,glucose 16,sodium pyruvate 1,pH 7.4).Samples were digested in papain (1.6 U/mL) (Cat# 107144,Calbiochem,San Diego,CA,USA) and L-cysteine (0.2 mg/mL) (Cat# C7352,Sigma-Aldrich).Retinal neurons were dissociated mechanically using pipettes.Rhodamine-Bisothiocyanate labeled RGCs were used for patch-clamp recordings.
Electrophysiological recordings
Whole-cell membrane currents in rhodamine-B-isothiocyanate-labeled RGCs were monitored using a patch amplifier (Axopatch700B;Molecular Devices,Foster City,CA,USA) with a Digidata 1440A data acquisition board and pClamp 10.2 soft ware (Molecular Devices) (Dong et al.,2015;Wang et al.,2016;Qian et al.,2017;Zhao et al.,2018).Patch pipettes were prepared by pulling borosilicate glass capillaries (BF150-86-10,Sutter Instrument Co.,Novato,CA,USA) on a P-97 Flaming/Brown micropipette puller (Sutter Instrument Co.).After fire polishing (Model MF-830;Narishige,Tokyo,Japan)and filling with internal solutions,the resistance of pipettes was typically 3–6 MΩ.Fast capacitance of pipettes was fully canceled and cell membrane capacitance was parti ally canceled as much as possible.The series resistance of the recording pipettes was compensated.Analog signals were sampled at 10 kHz,filtered at 1 kHz,and stored for further analysis.All experiments were performed at room temperature (20–25°C).
Total Ca2+currents of RGCs were evoked by voltage pulses (–80 mV to+40 mV with increments of 10 mV).Both peak and steady-state current amplitudes were measured (Qian et al.,2017).Nimodipine (1 mM,Tocris Bioscience,Ellisville,MO,USA) was added to the bath solution to block possible remaining components of L-type Ca2+currents when T-type Ca2+currents were recorded (Henderson and Miller,2007;Qian et al.,2017).The external solution consisted of 115 mM NaCl,2.5 mM KCl,10 mM BaCl2,5 mM CsCl,15 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid,15 mM tetraethylammonium-Cl,10 mM glucose,and 0.5 mM tetrodotoxin,pH 7.4.The internal solution consisted of 128 mM CsCl,1 mM CaCl2,2 mM MgCl2,10 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid,10 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid,2 mM ATP-Mg,0.4 mM GTP-Na,and 10 mM phosphocreatine,pH 7.2.
Intravitreal injections
The procedure for intravitreal injections was described in our previous studies(Ji et al.,2012;Dong et al.,2015).After the pupil of the anestheti zed eye was dilated with tropicamide drops (Santen Pharmaceutical,Shanghai,China),a single dose of TNF-α (5 ng/mL,R&D Systems,Minneapolis,MN,USA) or XPro1595 (50 µg/mL;Xencor,Monrovia,CA,USA)+TNF-α (5 ng/mL) in 0.9%saline (2 µL) was intravitreally injected 3 days before the RGCs were isolated for Ca2+current recordings and western blotting using a micro-injector with a 30-gauge needle (Hamilton,Reno,NV,USA) under a stereoscopic microscope(Carl Zeiss,Oberkochen,Germany).For Ca2+current recordings in RGCs isolated from COH retinas,a single dose of XPro1595 (50 µg/mL) dispersed in 0.9% saline (2 µL) was intravitreally injected 1 day before the COH operation.For terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining,a single dose of mibefradil (3 µM;Tocris Bioscience) in 0.9% saline (2 µL) was intravitreally injected 1 day before the COH operation.In vehicle (sham) controls,eyes were injected with saline in the same manner.
Western blot analysis
Western blot analysis was performed as previously described (Chen et al.,2011;Ji et al.,2012;Miao et al.,2012;Dong et al.,2015).Briefly,retinas were homogenized and protein concentrations were determined using a standard bicinchoninic acid assay kit (Pierce Biotechnology,Rockford,IL,USA).Proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis.The primary antibodies used in this study were mouse anti-CaV1.2 monoclonal antibody (1:50,Abcam,Cambridge,MA,USA,Cat# ab84814,RRID: AB_1860052),rabbit anti-CaV3.1 polyclonal antibody(1:100,Alomone,Jerusalem,Israel,Cat# ACC-021,RRID: AB_2039779),rabbit anti-CaV3.2 polyclonal antibody (1:100,Alomone,Cat# ACC-025,RRID: AB_2039781),rabbit anti -CaV3.3 polyclonal anti body (1:100,Alomone,Cat# ACC-009,RRID: AB_2039783),goat anti-Brn-3a polyclonal antibody(1:300,Santa Cruz Biotechnology,Santa Cruz,TX,USA,Cat# sc-31984,RRID: AB_2167511),and mouse anti -β-actin (1:10,000,Sigma-Aldrich,Cat#A5316,RRID: AB_476743).The secondary anti bodies used in this study were horseradish-peroxidase-conjugated donkey anti -mouse,-rabbit and -goat IgG(1:10,000,Jackson ImmunoResearch Labs,West Grove,PA,USA,Cat# 715-035-150,711-035-152,705-035-147;RRID: AB_2340770,AB_10015282,AB_2313587).The incubation time for the primary anti bodies was overnight at 4°C,and the incubation time for the secondary antibodies was 2 hours at room temperature.Images were obtained from automatic optimized exposure programs (FluorChem E System,ProteinSimple,San Jose,CA,USA),and protein levels were quantified by Alphaview Soft ware (version 3.3.0,Cell Biosciences,Santa Clara,CA,USA).The linearity of protein intensity levels was examined by loading different amounts of protein (from 2 to 16 µg) with linear regression analysis (Pillai-Kastoori et al.,2020;Additional Figure 1).The protein expression levels were normalized by β-actin.
Immunofluorescence
Immunofluorescent staining of the retinal section was performed as previously described (Xu et al.,2020;Cheng et al.,2021).Briefly,eyecups were fixed with 4% paraformaldehyde for 2–4 hours and dehydrated with graded sucrose solutions at 4°C.The eyecups were embedded in OCT compound (Tissue Tek,Torrance,CA,USA),and retinal vertical sections were cut at a thickness of 14 µm on a Leica CM1950 freezing microtome(Leica Biosystems,Nussloch,Germany).The sections were incubated with primary antibodies: goat-anti-Brn-3a monoclonal antibody (1:400,Santa Cruz Biotechnology,Cat# sc-31984,RRID: AB_2167511),mouse-anti -CaV1.2 monoclonal antibody (1:100,Abcam,Cat# ab84814,RRID: AB_1860052),rabbit-anti -CaV3.1 polyclonal anti body (1:100,Alomone,Cat# ACC-021,RRID:AB_2039779),rabbit-anti -CaV3.2 polyclonal anti body (1:100,Alomone,Cat#ACC-025,RRID: AB_2039781),and rabbit-anti-CaV3.3 polyclonal antibody(1:100,Alomone,Cat# ACC-009,RRID: AB_2039783) at 4°C for 48 hours.The sections were then incubated with Cy3-conjugated donkey anti -goat IgG(1:400,Jackson ImmunoResearch Labs,Cat#705-165-003,RRID: AB_2340411)or Alexa Fluor 488-conjugated donkey anti -mouse/rabbit IgG (1: 400,Jackson ImmunoResearch Labs,Cat#715-545-150 or 711-547-003;RRID: AB_2340846,AB_2340620) for 2 hours at room temperature.After washing,the sections were mounted with anti-fade mounting medium (Sigma-Aldrich,Cat#SLBW7464) and stained with 4′,6-diamidino-2-phenylindole (Roche,Basel,Switzerland,Cat# 10236276001).The immunofluorescence signals in the sections were visualized with a FluoView 1000 confocal microscope (Olympus,Tokyo,Japan).
Detection of retinal ganglion cell apoptosis
TUNEL assays were used to detect RGC apoptosis in whole flat-mounted retinas (Dong et al.,2015;Xu et al.,2020) using the DeadEnd Fluorometric TUNEL System G3250 kit (Promega,Madison,WI,USA).All TUNEL-positive RGCs that were merged well with 4′,6-diamidino-2-phenylindole staining were counted in each whole flat-mounted retina using a FluoView 1000 confocal laser scanning microscope (Olympus).
Reagents and drug application
Nimodipine,mibefradil,PDTC (Tocris Bioscience),and U0126 (Tocris Bioscience) were dissolved in dimethyl sulfoxide and added to the bath solution immediately before electrophysiological recordings.The final concentration of dimethyl sulfoxide in the solution was equal to or less than 0.1%;this concentration has been demonstrated to have no significant effects on Ca2+currents in RGCs (Qian et al.,2017).The other chemicals were freshly dissolved in the bath solution.The drugs were delivered through a stepper motor-based rapid solution changer (RSC-160,Bio-Logic,Claix,France) (Zhao et al.,2010;Li et al.,2016;Qian et al.,2017).
Stati stical analysis
In this study,we did not pre-calculate sample sizes forin vivoexperiments;the sample sizes were consistent with our previous reports (Xu et al.,2020;Cheng et al.,2021).The “n” values indicate cell number in electrophysiological recordings or animal number in western blotting,immunohistochemistry,and TUNEL experiments.For the counting of TUNEL-positive signals,the raters were blinded to the groupings.The Clampfit 10.2 (Molecular Devices,Foster City,CA,USA),SigmaPlot 10.0 (Systat Soft ware,San Jose,CA,USA),and Igor 4.0 (WaveMetrics,Lake Oswego,OR,USA) soft ware were used to analyze the electrophysiological data.Data are expressed as the mean ± standard error of mean (SEM).Before performing the significance tests,the normality or the homogeneity of variance was evaluated by the Kolmogorov-Smirnov’s test or Brown-Forsythe’s test,respectively.Stati stical tests were carried out through a one-way analysis of variance,combined with Bonferroni’spost hoctest and non-parametric Kruskal-Wallis test,and with two-tailed pairedt-test (paired data).P<0.05 indicated stati stical significance.
Results
Dynamic changes of L-and T-type Ca2+ channels in retinal ganglion cells of chronic ocular hypertension retinas
Changes of IOP in the operated eyes in the COH rat model after the operation were similar to our previous reports (Chen et al.,2011;Xu et al.,2020;Figure 1).We first examined the changes of Ca2+currents in RGCs isolated from retinas from COH rats.As shown inFigure 2A–C,both the peak and steadystate Ca2+current densities recorded in RGCs of COH retinas at G3d were significantly reduced (36.0 ± 3.4 pA/pF (n=27,P=0.005) and 31.8 ± 3.2 pA/pF(n=27,P=0.005),respectively) compared with levels in the controls (65.6± 7.0 pA/pF,n=26,and 57.7 ± 6.1 pA/pF,n=26).The current densities in RGCs of COH retinas remained at reduced levels through G4w.The peak Ca2+currents were mainly composed of L-and T-type components,and the steadystate ones were mainly composed of L-type channels (Qian et al.,2017).Therefore,we explored which component(s) mediate the downregulation of Ca2+currents in RGCs of COH retinas.We confirmed that L-and T-type Ca2+channels contributed to the high-and low-voltage-activated Ca2+currents,respectively (Additional Figure 2).We first examined changes in L-type Ca2+currents.Representative recordings in RGCs isolated from retinas of control and COH rats are shown inFigure 2D.The current densities in RGCs obtained from COH retinas at G3d were reduced (19.7 ± 1.9 pA/pFn=37,P<0.001)compared with the control (32.8 ± 2.7 pA/pF,n=23) at 0 mV;this reduction was maintained at subsequent time points (G1w: 17.4 ± 1.5pA/pF,n=32,P<0.001;G2w: 18.9 ± 2.0 pA/pF,n=21,P=0.002;G3w: 17.7 ± 1.3 pA/pF,n=15,P=0.004;G4w: 21.1 ± 2.2 pA/pF,n=17,P=0.041;Figure 2E).Changes in T-type Ca2+currents,which were induced by depolarizing voltage pulses from–80 to–20 mV for 150 ms duration,showed a different trend.At early stages of IOP elevation (from G3d to G2w),the T-type Ca2+current density was unchanged (G3d: 36.3 ± 4.6 pA/pF,n=18,P>0.999;G1w: 35.9 ± 5.7 pA/pF,n=20,P>0.999;G2w: 36.5 ± 5.3 pA/pF,n=20,P>0.999) compared with the control (35.1 ± 3.4 pA/pF,n=30);it then increased to 51.4 ± 2.9 pA/pF(n=18,P=0.013) and 50.92 ± 2.3 pA/pF (n=17,P=0.011) at G3w and G4w,respectively (Figures 2FandG).These results suggest that IOP elevation in retinas of COH rats induced downregulation of L-type Ca2+currents and upregulation of T-type currents in RGCs.Thus,the decrease in total Ca2+current density may be from the L-type Ca2+current suppression.We further examined the expression of L-and T-type Ca2+channels in retinas of COH rats.Previous studies reported that rat RGCs mainly express the CaV1.2 subunit of L-type Ca2+channels and the CaV3.1,CaV3.2,and CaV3.3 subunits of T-type Ca2+channels (Yang et al.,2016;Qian et al.,2017).Western blot results showed that changes in the expression levels of the CaV1.2 subunit in COH retinas were consistent with those of L-type Ca2+current density (Figure 3A).CaV1.2 protein levels tended to reduce at G3d (79.8 ± 3.9% of control,n=6,P=0.060) and then significantly reduced to 74.2 ± 4.1 % (n=6,P=0.009),53.3 ± 7.6 % (n=6,P<0.001),55.8 ± 5.3% (n=6,P<0.001),and 65.7 ± 6.9%of controls (n=6,P<0.001) at G1w,G2w,G3w,and G4w,respectively (Figure 3B).CaV3.1 and CaV3.2 protein levels did not change during the whole period of IOP elevation (Figure 3A,C,andD).In contrast,CaV3.3 protein expression significantly increased from G2w (185.9 ± 14.7% of controls,n=6,P<0.001)and maintained higher levels thereafter (169.0 ± 15.0% of controls at G3w,n=6,P=0.015;175.7 ± 17.0% of controls at G4w,n=6,P=0.009;Figure 3AandE).Consistent with these results,immunohistochemistry showed that the expression of the CaV1.2 subunit of L-type Ca2+channels in Brn-3a-positive RGCs and the IPL were reduced in retinas of COH rats (Additional Figure 3A)compared with the controls (Additional Figure 3A).While the expressions of CaV3.1 and CaV3.2 of T-type Ca2+channels were not significantly changed(Additional Figure 3BandC),CaV3.3 expression was significantly increased in Brn-3a-positive RGCs and the IPL of COH retinas (Additional Figure 3D)compared with the controls (Additional Figure 3D).
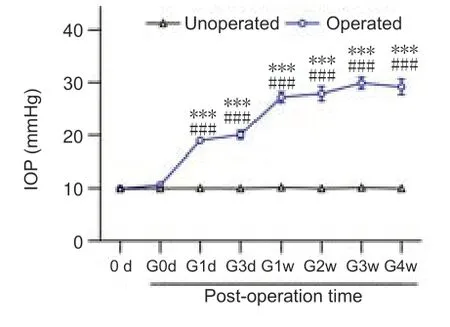
Figure 1|Changes in IOP in eyes of COH rats.
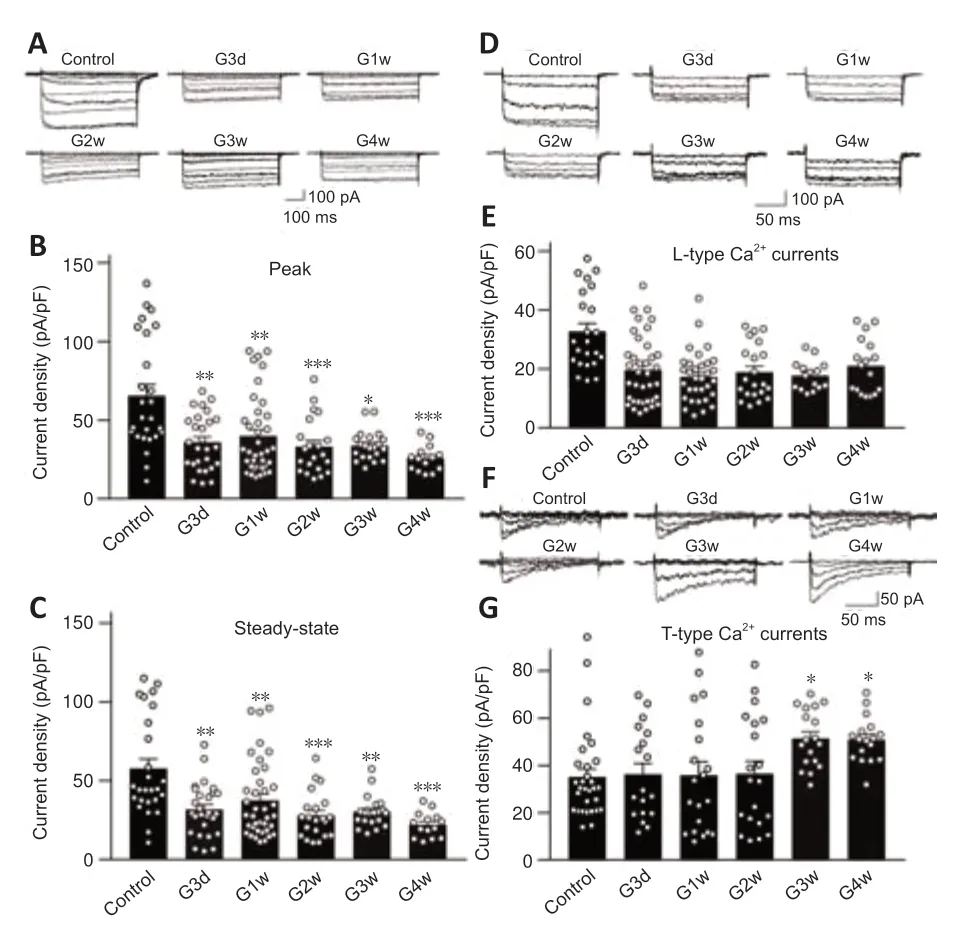
Figure 2|Dynamic changes of Ca2+ currents in RGCs of COH rats.
The decreased expression of the CaV1.2 subunit of Ca2+channels in retinas of COH rats may be because of decreased RGC density.We examined the expression of Brn3a,an RGC marker (Nadal-Nicolás et al.,2009),in COH retinas and calculated the ratios of CaV1.2/Brn3a.Brn3a expression started to decrease at G1w in COH retinas (82.7 ± 11.8% of controls,n=6,P=0.280),and a progressive and significant decline of Brn3a expression was observed from G2w to G4w (G2w: 69.2 ± 7.6% of controls,n=6,P=0.031;G3w: 48.1 ±4.6% of controls,n=6,P<0.001;G4w: 34.7 ± 5.2% of controls,n=6,P<0.001)(Figure 3FandG).However,the CaV1.2/Brn3a ratio only showed a decreased tendency from G3d to G2w (G3d: 77.8 ± 6.1% of controls,n=6,P=0.343;G1w: 96.3 ± 10.0% of controls,n=6,P>0.999;G2w: 77.9 ± 7.0% of control,n=6,P=0.310;G3w: 116.6 ± 3.5% of controls,n=6,P=0.682),and it was significantly increased at G4w (197.3 ± 12.2% of controls,n=6,P<0.001;Figure 3H).The CaV1.2 subunit of L-type Ca2+channel is widely expressed in retinal cells of vertebrates,including RGCs,bipolar cells,amacrine cells,and glial cells (Van Hook et al.,2019).Therefore,these results suggest that the CaV1.2/Brn3a ratio in whole retinal extracts cannot accurately reflect the changes of CaV1.2 proteins in RGCs of COH retinas.
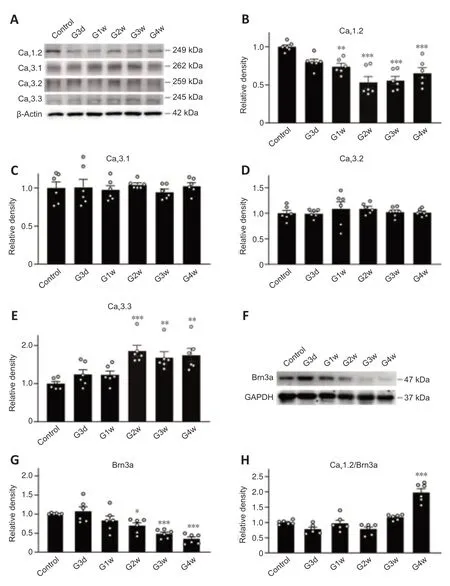
Figure 3|Changes in protein levels of L-and T-type Ca2+ channel subunits in COH retinas.
Inflammatory factor tumor necrosis factor-α mediates the dichotomous modulation of L-and T-type Ca2+ currents
We then investigated the mechanisms underlying the modulation of L-and T-type Ca2+currents in retinas of COH rats.Previous studies showed that TNF-α regulates various VGCCs in neurons of the central nervous system(Motagally et al.,2009;Hagenacker et al.,2010).TNF-α is one of the main inflammatory factors released by glial cells of the glaucomatous retina(Pasparakis et al.,1996;Arnett et al.,2001;Canault et al.,2004;Olleros et al.,2005;Alexopoulou et al.,2006;Ierna et al.,2009).Thus,the increased levels of TNF-α in glaucomatous retinas may be involved in modulating Ca2+currents in RGCs.To test this possibility,we first examined the effects of TNF-α on L-type Ca2+currents in RGCs isolated from untreated rats.As shown inFigure 4AandB,soluble TNF-α dose-dependently suppressed the current density of L-type Ca2+channels.The effects of TNF-α were reversed by co-treatment of XPro1595 (50 µg/mL),a TNF-α inhibitor (Figure 4CandD).We then tested whether TNF-α modulates L-type Ca2+currentsin vivo.Saline (control),TNF-α(5 ng/mL),XPro1595 (50 µg/mL),or TNF-α+XPro1595 was intravitreally injected into rats.Three days later,RGCs were isolated for analyses.The L-type Ca2+current density was significantly smaller in the TNF-α-injected group (19.0 ± 1.9 pA/pF,n=23,P=0.009) compared with the control group(28.8 ± 2.5 pA/pF,n=11).Co-injection of XPro1595 reversed the changes in current density induced by TNF-α (Figure 5A).No significant changes were observed in the XPro1595-injected group.Furthermore,in retinas from COH rats,intravitreal pre-injection of XPro1595 reversed the IOP elevation-induced reduction in current density of L-type Ca2+channels in RGCs at G3d (XPro1595+TNF-α: 35.9 ± 1.9 pA/pF,n=25,TNF-α: 21.9 ± 1.7 pA/pF,n=16,P<0.001)(Figure 5B).However,pre-injection of XPro1595 did not rescue the reduction of L-type Ca2+currents in RGCs at G1w (XPro1595+TNF-α: 22.0 ± 2.5 pA/pF,n=14;TNF-α: 21.4 ± 2.6 pA/pF,n=13,P>0.999),indicating that the effects of XPro1595 in retinas lasted less than 1 week,which is consistent with a previous study (Cueva Vargas et al.,2015).
The effects of TNF-α on T-type Ca2+currents were also explored.TNF-α (12.5 ng/mL) increased the current amplitudes of T-type Ca2+channels in RGCs from untreated rats to 132.4 ± 20.1% of controls (n=11,P<0.001;Figure 6AandB).Upon co-treatment of XPro1595,the current amplitudes were not changed by TNF-α (97.7 ± 18.3% of control,n=8,P>0.999vs.control;Figure 6AandB).Additionally,intravitreal injection of XPro1595 did not alter the current densities of T-type Ca2+channels in RGCs isolated at 3 days after the injections in untreated rats (P>0.05vs.control;XPro1595:n=10,control:n=10),while injections of TNF-α (5 ng/mL) significantly enhanced T-type Ca2+current densities (TNF-α: 47.7± 3.0 pA/pF,n=12;control: 34.5 ± 2.5 pA/pF,P=0.038),which was inhibited by co-injecting XPro1595 (31.4 ± 4.9 pA/pF,P=0.009,vs.TNF-α alone) (Figure 6C).T-type Ca2+currents in RGCs were upregulated from G3w in COH retinas (Figure 2).Thus,we also tested the effects of pre-injection of XPro1595 on this type of current in RGCs of COH retinas.However,pre-injection of XPro1595 did not block the increase of these currents in RGCs at 3 weeks,further demonstrating that the effects of XPro1595 in retinas were not long lasting (Additional Figure 4;Cueva Vargas et al.,2015).Together,these results suggest that TNF-α is involved in the downregulation of L-type Ca2+currents and upregulation of T-type currents in RGCs of COH rats.
We next explored the possible mechanisms of the effects of TNF-α on Ca2+currents.TNF-ɑ receptor 1 (TNFR1) regulates cell functions through intracellular mitogen-activated protein kinase (MAPK) and nuclear factor kappa-B (NF-κB) signaling pathways (Zhao et al.,2001;Chen and Goeddel,2002;Motagally et al.,2009;Zhang et al.,2014;Cheng et al.,2021).RGCs isolated from untreated control rats were pre-treated with U0126 (10 µM),a MAPK/extracellular signal-regulated kinase (ERK) signaling pathway blocker,for 30 minutes,and L-type Ca2+currents were recorded.In U0126 pre-treated cells,TNF-α (12.5 ng/mL) did not change L-type Ca2+currents (97.8 ± 3.8%,n=11,P=0.789) (Figure 7AandB).In RGCs pre-treated with PDTC (50 µM),an NF-κB blocker,for 30 minutes,TNF-α slightly reduced the amplitudes of L-type Ca2+currents (93.2 ± 2.4%,n=12,P=0.032) (Figure 7CandD),but the extent of reduction was significantly attenuated compared with that without PDTC pre-treatment (Figure 4).Additionally,after pre-incubation with U0126 (10 µM) or PDTC (50 µM) for 30 minutes,TNF-α (12.5 ng/mL) did not significantly change T-type Ca2+currents (U0126: 101.2 ± 2.4% of controls,n=10,P=0.9127;PDTC: 102.2 ± 2.2% of controls,n=11,P=0.515) (Figure 7E–H).These results indicate that MAPK/ERK and NF-κB pathways mediate the TNFα-induced downregulation of L-type Ca2+currents and upregulation of T-type currents in RGCs.
The expressions of the L-type Ca2+channel CaV1.2 subunit were downregulated and the CaV3.3 subunit expressions of the T-type Ca2+channel were upregulated in retinas of COH rats (Figure 3).We further examined whether TNF-α mediates these changes.In retinas injected with TNF-α (5 ng/mL),CaV1.2 protein levels were reduced to 56.2 ± 3.3% of controls (n=6,P=0.034) (Figure 8AandB),whereas CaV3.3 levels increased to 153.5 ± 15.8%of controls (n=7,P=0.011) (Figure 8CandD).Intravitreal co-injection of XPro1595 (50 µg/mL) reversed these changes (Figure 8A–D).
T-type Ca2+ channels contribute to retinal ganglion cell apoptosis in chronic ocular hypertension retinas
T-type VGCCs are low-voltage activated channels.Depolarization of cell membrane potential first activates these Ca2+channels,resulting in Ca2+influx (Qian et al.,2017).We previously showed that IOP elevation induced a depolarization of membrane potential in RGCs (Zhao et al.,2018),which activates T-type VGCCs,thus contributing to Ca2+overload and RGC damage.We next examined the role of T-type VGCCs in apoptosis of RGCs in COH retinas.The T-type Ca2+channel blocker mibefradil (2 µL,3 µM) was intravitreally pre-injected in COH rats and the effect on RGC apoptosis was examined by TUNEL assays.As shown inFigure 9AandB,the number of TUNEL-positive RGCs was significantly increased at G2w in COH retinas (1359± 139,n=8,P<0.001) compared with controls (67 ± 11,n=7),similar to our previous reports (Chen et al.,2011;Xu et al.,2020).Pre-injection of mibefradil significantly reduced the number of TUNEL-positive RGCs to 584 ± 42 (n=8,P<0.001vs.G2w andP=0.002vs.controls) (Figure 9AandB).
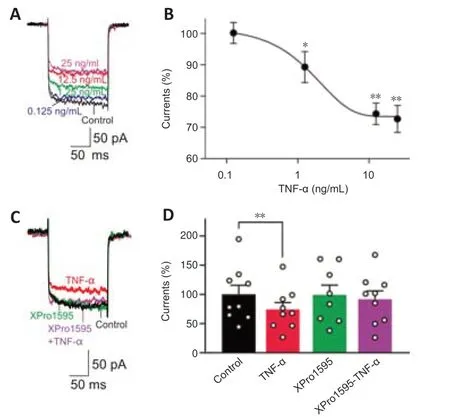
Figure 4|TNF-α induces suppression of L-type Ca2+ currents in RGCs.
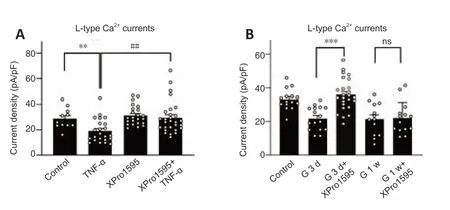
Figure 5|TNF-α mediates reduction of L-type Ca2+ current amplitudes in RGCs of untreated control and COH rats.
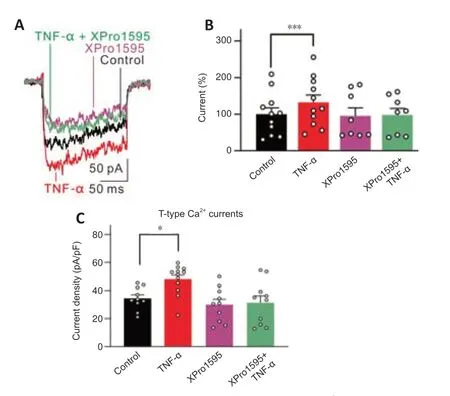
Figure 6|TNF-α induces enhancement of T-type Ca2+ currents in RGCs.
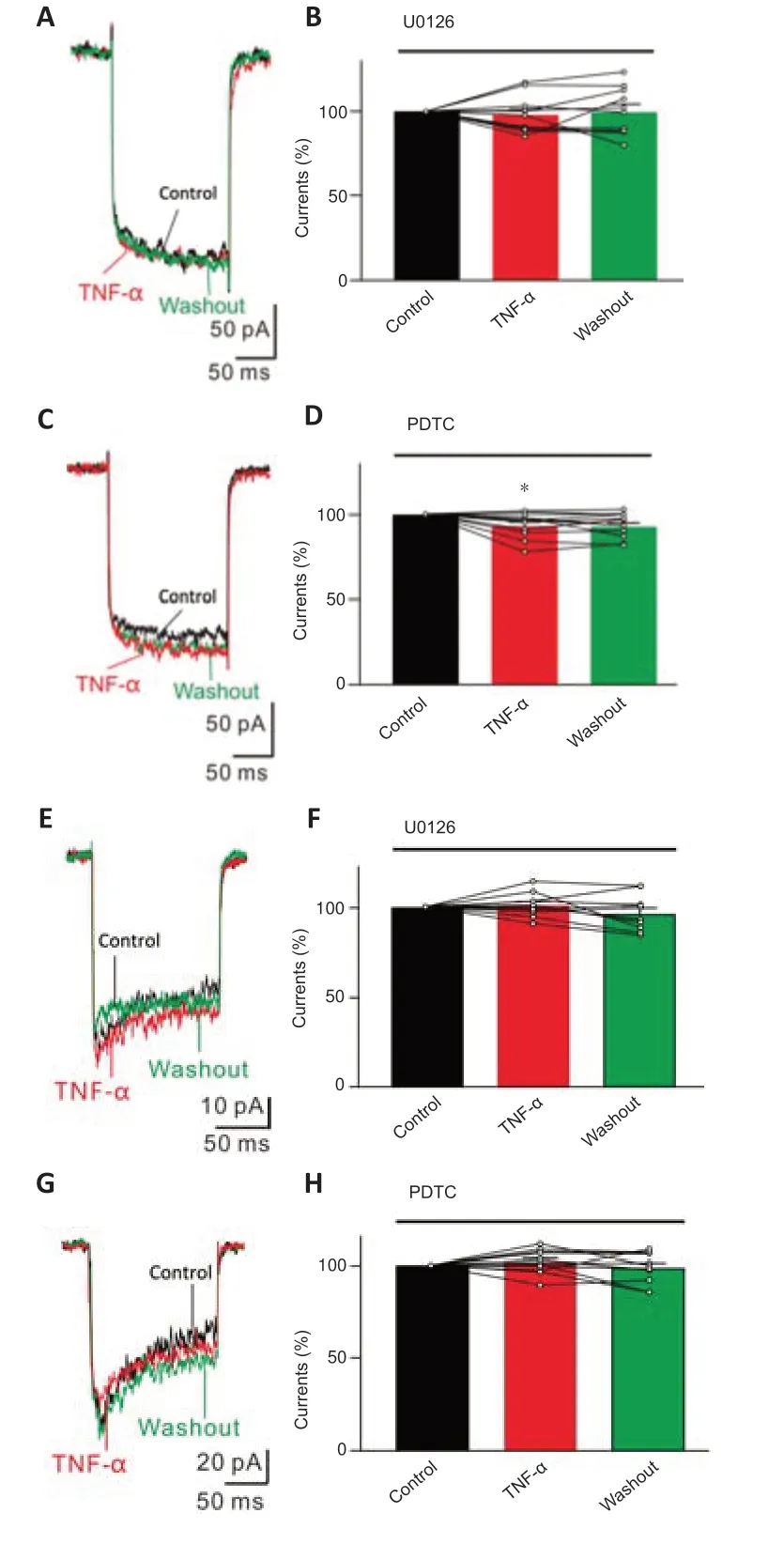
Figure 7|MAPK/ERK and NF-κB signaling pathways mediate TNF-α-induced effects on Ca2+ currents in RGCs.
Discussion
Disorder of intracellular Ca2+homeostasis is closely related to neuronal damage in neurodegenerative diseases (Alexopoulou et al.,2006;Chinopoulos and Adam-Vizi,2006;Lin and Beal,2006;Renkema et al.,2008;Chan et al.,2009;Gleichmann and Mattson,2011;Zündorf and Reiser,2011;Ryskamp et al.,2016).Here,we showed that T-type,but not L-type,VGCCs contribute to RGC apoptosis in glaucoma.First,we found that both currents and CaV1.2 subunit expression of L-type Ca2+channels were downregulated in RGCs of COH rats.RGCs of rats mainly express the CaV1.2 subunit and do not express CaV1.3 (Yang et al.,2016),and thus downregulation of CaV1.2 may contribute to the IOP elevation-induced reduction of L-type Ca2+currents.These results provide a reasonable explanation for the clinical ineffectiveness of L-type Ca2+channel blockers in glaucoma patients without blood hypertension(Araie and Mayama,2011;Mayama,2014).While VGCC blockers showed neuroprotective effects on RGCs in retinal ischemia/hypoxia models(Crosson et al.,1990;Takahashi et al.,1992;Toriu et al.,2000;Osborne et al.,2002;Yamada et al.,2006;Uemura and Mizota,2008),the L-type Ca2+channel blockers may have no direct neuroprotection on RGCs,at least in IOP elevation-induced glaucoma.The downregulated L-type Ca2+channels might be an intrinsic self-protective mechanism of RGCs in glaucoma that antagonizes the excessive Ca2+influx through ionotropic glutamate receptors,non-selective ion channels,and other types of VGCCs (Hardingham et al.,1999;Hou et al.,2002;Zhang et al.,2008;Namekata et al.,2013;Ward et al.,2014;Weitlauf et al.,2014;Cueva Vargas et al.,2015;Dong et al.,2015;Zhao et al.,2018).Second,our results showed that T-type Ca2+currents of RGCs were significantly enhanced from G3w,although the currents remained unchanged at the early stage of IOP elevation.Upregulation of T-type Ca2+currents may result from the increased expression of the CaV3.3 subunit.Notably,upregulated expression of CaV3.3 was observed from G2w,while upregulation of T-type Ca2+currents was seen from G3w.This difference in time course may be because the currents consist of all components of T-type Ca2+channels.We previously showed that IOP elevation resulted in a depolarized membrane potential in RGCs (Zhao et al.,2018).T-type VGCCs are low-voltage activated channels.These Ca2+channels are easier to be activated,which may contribute to Ca2+overload in RGCs of COH retinas.As shown inFigure 2,the total Ca2+currents,in both peak and steady state,were reduced in RGCs when IOP was elevated even though the T-type Ca2+currents were upregulated from G3w.The reason may be that the amplitude of the reduction of L-type Ca2+currents is greater than the amplitude of the increase of T-type currents.
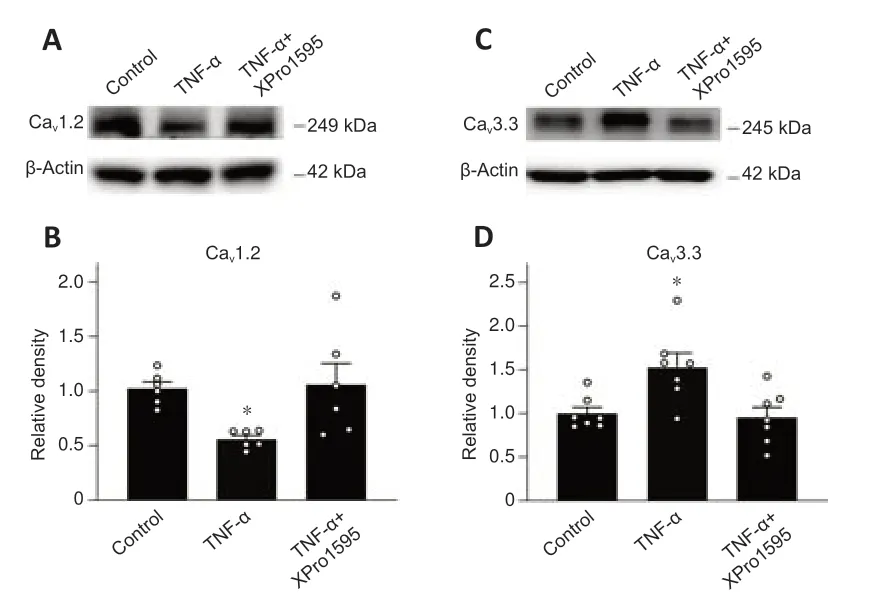
Figure 8|TNF-α induces changes in protein levels of CaV1.2 and CaV3.3 in retinas of control rats.
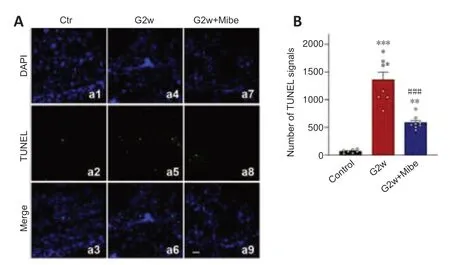
Figure 9|T-type Ca2+ channel blocker mibefradil reduces RGC apoptosis in COH retinas.
RGCs also express other Ca2+channel subtypes,such as N-and P/Q-types (Xu et al.,2002;Sargoy et al.,2014).These Ca2+channels may be mainly involved in the regulation of neurotransmitter release because of their restrictive location in the IPL and axons (Xu et al.,2002;Sargoy et al.,2014).Some evidence showed that the degeneration of RGC axons and dendrites may be a fundamental pathogenesis mechanism of glaucoma,in addition to progressive RGC apoptosis (Johnson et al.,2000;Whitmore et al.,2005;Almasieh et al.,2012;Calkins,2012;Risner et al.,2018).However,in dissociated RGCs used for recordings in this study,most of the processes were abolished during the isolation procedures.Whether and how these Ca2+channels are expressed in the processes of RGCs remain to be explored.
One of the important findings of this study is that inflammatory factor TNF-α plays a key role in the changes of L-and T-type Ca2+currents in RGCs of COH retinas.TNF-α is a major inflammatory factor in glaucomatous retinas and is released from activated retinal glial cells (Pasparakis et al.,1996;Arnett et al.,2001;Canault et al.,2004;Olleros et al.,2005;Alexopoulou et al.,2006;Ierna et al.,2009).Previous studies in the central nervous system have shown that TNF-α may regulate various VGCCs (Motagally et al.,2009;Hagenacker et al.,2010);however,ourin vivoandin vitroexperiments demonstrated that dichotomous changes of L-and T-type Ca2+currents in RGCs of COH retinas were mediated by TNF-α.TNF-α-mediated modulation of the two types of Ca2+currents may be through two pathways: change of Ca2+channel expression and direct regulation of Ca2+currents.We showed that intravitreal injection of TNF-α downregulated the expression of CaV1.2 and upregulated the expression of CaV3.3 in retinas from untreated control rats.In RGCs isolated from control rats,TNF-α downregulated L-type Ca2+currents and upregulated T-type currents,which was blocked by the TNF-α inhibitor XPro1595.In RGCs isolated from TNF-ɑ intravitreally injected rats,downregulation of L-type Ca2+currents and upregulation of T-type Ca2+currents were observed.TNF-ɑ effects on Ca2+currents were reversed by coinjection of XPro1595.Additionally,pre-intravitreal injection of XPro1595 blocked IOP elevation-induced downregulation of L-type Ca2+currents in RGCs.TNF-α concentrations in the aqueous humor,retina,and optic nerve head of glaucoma patients and animal models were significantly increased(Yan et al.,2000;Yuan and Neufeld,2001;Sawada et al.,2010;Balaiya et al.,2011;Chidlow et al.,2012;Gramlich et al.,2013;Levkovitch-Verbin et al.,2013;Cueva Vargas et al.,2015;Wilson et al.,2015).Notably,pre-injections of XPro1595 did not affect the IOP elevation-induced changes in L-type Ca2+currents at G1w and T-type Ca2+currents at G3w,which was consistent with a previous study showing that the effects of XPro1595 in retinas are not longlasting (Cueva Vargas et al.,2015).We did not repeat intravitreal injections because the manipulation would destroy the elevated IOP.
Our results showed that IOP elevation-induced changes of L-and T-type Ca2+currents in COH retinas were mediated through MAPK/ERK and NFκB pathways from activation of TNF-ɑ/TNFR1.MAPK and NF-κB pathways mediate the effects of TNF-α/TNFR1 on regulating cell functions (Chen and Goeddel,2002).In dissociated neurons from mouse superior mesenteric ganglia,TNF-α significantly decreased the omega-conotoxin GVIA-sensitive N-type Ca2+current amplitude via the NF-κB pathway (Motagally et al.,2009).In dorsal root ganglion neurons of rats,the MAPK/JNK pathway mediated TNF-α-induced mechanical allodynia following bortezomib treatment (Zhang et al.,2014).In this study,TNF-α-mediated modulation of L-and T-type Ca2+channels in RGCs of COH retina may be through two pathways.First,IOP elevation increased TNF-α release and in turn directly downregulated L-type Ca2+currents and upregulated T-type Ca2+currents through MAPK/ERK and NF-κB signaling pathways.Second,IOP elevation in COH retinas induced changes in CaV1.2 and CaV3.3 protein expression.The MAPK/ERK signaling pathway and NF-κB transcriptional factor play key roles in transcription regulation,and their activation may modulate Ca2+channel protein expression.Indeed,TNF-α-induced inhibition of N-type VGCCs in neurons of mouse superior mesenteric ganglia was related to decrease CaV2.2 mRNA and protein levels (Motagally et al.,2009).However,our results showed that preincubation of RGCs with the MAPK/ERK inhibitor U0126 or NF-κB inhibitor PDTC almost completely blocked TNF-α-induced modulation on L-and T-type Ca2+currents,suggesting that MAPK/ERK and NF-κB signaling molecules may directly modulate Ca2+channels in a transcription-independent manner.We previously showed that TNF-α directly modulates Na+currents in RGCs in this manner (Cheng et al.,2021).Notably,NF-κB blockade did not fully inhibit TNF-α-induced downregulation of L-type Ca2+currents,suggesting that other signaling molecules may also be involved.
We showed that TNF-ɑ plays a vital role in disorder of Ca2+homeostasis in RGCs of COH retinas.A previous study reported that TNF-ɑ may increase intracellular Ca2+by regulating the GluA2 subunit of AMPA receptors in COH retinas (Cueva Vargas et al.,2015).With the increased hyperexcitability by enhancing NaV1.6-mediated currents (Cheng et al.,2021),TNF-ɑ promoted retinal neuronal apoptosis (Tezel and Wax,2000,2004;Tezel,2008).However,TNF-α significantly inhibited NMDA or AMPA receptor activation-induced increase of intracellular Ca2+in hippocampal neurons (Cheng et al.,1994).We speculate that this effect may be mediated by TNFR2 activation.One study has shown that knockdown of TNFR1 inhibited RGC apoptosis,whereas knockdown of TNFR2 significantly promoted RGC apoptosis (Fontaine et al.,2002).Another possible explanation is that TNFR1 and TNFR2 may have diverse expression patterns in different neurons.
In summary,our results demonstrate that IOP elevation induces dichotomous modulation of L-and T-type Ca2+currents in RGCs,which is mediated by increased TNF-ɑ in COH retinas.We provide robust evidence showing that L-type Ca2+channel blockers may have no direct neuroprotective effects on RGCs in glaucoma because L-type Ca2+currents have been downregulated.In addition,T-type Ca2+channels contribute to RGC apoptosis in glaucoma.These findings suggest that selective application of T-type Ca2+channel blockers,especially specific CaV3.3 blockers,may be a prospective strategy for glaucoma treatment.
Acknowledgments:We would like to thank Dr.Xiong-Li Yang (Institutes of Brain Science,Fudan University) for helpful discussion and critical comments on the manuscript.
Author contributions:ZFW and XHS conceived and designed the experiments;HNW,WJQ,GLZ,FL,and YYM performed the experiments;HNW,WJQ,BL,XHS,and ZFW analyzed the data.HNW,XHS,and ZFW wrote the manuscript.All authors read and approved the final manuscript.
Conflicts of interest:The authors declare that they have no competinginterests.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Guadalupe Álvarez-Hernán,Lund University,Sweden.
Additional files:
Additional Figure 1:Identification of the linearity of protein intensity levels.
Additional Figure 2:High-and low-voltage activated Ca2+ currents are respectively mediated by L-and T-type Ca2+ channels in rat RGCs.
Additional Figure 3:Changes in expression of L-and T-type Ca2+ channel subunits in RGCs of COH retinas.
Additional Figure 4:XPro1595 cannot reduce T-type Ca2+ current densities in retinas at 3 weeks after the COH.
Additional file 1:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

