Bystanders or not? Microglia and lymphocytes in aging and stroke
Justin N.Nguyen,Anjali Chauhan
Abstract As the average age of the world population increases,more people will face debilitating agingassociated conditions,including dementia and stroke.Not only does the incidence of these conditions increase with age,but the recovery afterward is often worse in older patients.Researchers and health professionals must unveil and understand the factors behind age-associated diseases to develop a therapy for older patients.Aging causes profound changes in the immune system including the activation of microglia in the brain.Activated microglia promote T lymphocyte transmigration leading to an increase in neuroinflammation,white matter damage,and cognitive impairment in both older humans and rodents.The presence of T and B lymphocytes is observed in the aged brain and correlates with worse stroke outcomes.Preclinical strategies in stroke target either microglia or the lymphocytes or the communications between them to promote functional recovery in aged subjects.In this review,we examine the role of the microglia and T and B lymphocytes in aging and how they contribute to cognitive impairment.Additionally,we provide an important update on the contribution of these cells and their interactions in preclinical aged stroke.
Key Words:age;B lymphocytes;brain;central nervous system;cognition;inflammation;microglia;middle cerebral artery occlusion;neuroinflammation;stroke;T lymphocytes;white matter injury
Introduction
The advancement of technology and medicine increased the individual’s average age.With a decreasing fertility and mortality rate,a significant proportion of the population is becoming older (Kowal et al.,2016).Older individuals are more suscepti ble to age-associated diseases including stroke,dementia,diabetes,cardiovascular disease,and cancer (Jaul and Barron,2017).The anatomy and homeostasis of the brain change with aging (Juraska and Lowry,2012) and the aged brain respond differently to injury and stress than the brain of a young adult.Interestingly,the neuronal density remains stable throughout the lifespan however,30% of individuals suffer from severe cognitive impairment without meeting the criteria of Alzheimer’s disease (AD)or dementi a (Harada et al.,2013).Results from MRI and histological analysis studies demonstrate a decline in white matter volume which corroborated with cognitive impairment in healthy aged individuals (Fjell and Walhovd,2010;Coelho et al.,2021).The myelin sheath damage leads to white matter vulnerability resulting in axonal damage producing disconnect that could attribute to cognitive impairment (Kohama et al.,2012;Peters and Kemper,2012;Faizy et al.,2020).A complex interplay between myelin plasticity,oligodendrocyte maturation,removal and clearance of myelin debris,and remyelination supports myelin maintenance and homeostasis and these processes decline with age (Soreq et al.,2017;Spitzer et al.,2019;Sams,2021).Experimental studies have shown that oligodendrocyte precursor cells fail to mature into myelinating oligodendrocytes and aged animals demonstrate limited ability to replenish oligodendrocyte precursor cells pool thus is parti ally responsible for age-related reduced remyelination and myelin repair (Shields et al.,2000;Neumann et al.,2019;Ito et al.,2021).Microglia plays an important role in oligodendrocyte precursor cell differentiation into mature oligodendrocytes,removes myelin debris,and promotes remyelination,however,this function declines with age (Kalafatakis and Karagogeos,2021;Luan et al.,2021),thus prompting cognitive dysfunction.Several genes are expressed by microglia that promote lymphocyte infiltration in the aged brain exacerbating the white matter injury and contributing to cognitive impairments in aged individuals.Thus an interplay between microglia and lymphocytes not only contributes to age-associated brain injury but their precise role in stroke pathophysiology in older subjects has not been fully explored.The objective of this review is to summarize the evidence that both microglia and lymphocytes are involved in the process of aging.The interplay of microglia and lymphocytes negatively modulates cognition in older subjects after stroke.Finally,we present the studies targeting therapeutic strategies toward microglia or lymphocytes in older animals after stroke.The review will aid in better understanding the contribution of microglia and lymphocytes in post-stroke functional outcomes that happen to be a major concern in older individuals.
Retrieval Strategy
The studies cited in this review were retrieved from Google scholar and PubMed databases.The relevant literature published from January 1994 to September 2022 was screened.A combination of the following words(MeSH terms) was used to maximize search specificity and sensitivity: age,cognition,microglia,T lymphocytes,B lymphocytes,stroke,white matter,neuroinflammation,and brain.The results were further screened by title and abstract and studies exploring the relationship between microglia,and lymphocytes in aging were included.Furthermore,the role of microglia and lymphocytes and their contribution to stroke neuroinflammation was included.No language or study type restrictions were applied.Articles involving studies investigating the contribution of young microglia and lymphocytes in stroke were excluded.No limit was used on the year of publication or authorship.
Aged Microglia Contributes to Cognitive Impairment
One of the cell types that is profoundly affected by age is brain resident macrophages-microglia.Microglia are primary immune cells that constitute 10% of all cells in the adult brain (Pessac et al.,2001).Microglia acquire a dystrophic and dysfunctional phenotype with age (Safaiyan et al.,2016;Cantuti-Castelvetri et al.,2018;Deczkowska et al.,2018).A multitude of factors including cellular aging,transcriptomic changes,senescenceassociated secretory phenotype,dysregulated microglia metabolism,and the milieu that these cells reside could influence the microglia behavior(Minhas et al.,2021).Activated microglia secrete multiple pro-inflammatory cytokines including interleukin (IL)-1β (Figure 1).Cathepsin B is associated with the production and secretion of IL-1β and leakage of cathepsin B,a lysosomal enzyme from the endosomal/lysosomal system is observed with aging thus,cathepsin B plays a major role in brain aging (Nakanishi,2020).Genetic depletion of cathepsin B in middle-aged mice resulted in decreased microglia-dependent reactive oxygen species (ROS) generation,inflammation,and improvement in age-dependent cognitive impairments (Ni et al.,2019).Overexpression of cathepsin B in hippocampus microglia of middle-aged mice leads to cognitive impairments validating the contribution of microglia lysosomal function in cognitive impairment in old animals (Ni et al.,2019).With age,microglia and macrophages have increased lipid messenger prostaglandin E2(PGE2) signaling (Breyer et al.,2001;Minhas et al.,2021).PGE2promotes the conversion of glucose to glycogen resulting in reduced glucose influx and mitochondrial respiration thus enhancing inflammatory responses (Casolini et al.,2002;Wu and Meydani,2004).Enhanced PGE2 signaling has been shown to promote cognitive impairment in rodents (Xiao et al.,2018;Zhao et al.,2019;Minhas et al.,2021).Increased macrophages and microglia PGE2 signaling have been shown to induce age-associated cognitive impairment (Minhas et al.,2021;Figure 1).Inhibition of peripheral PGE2 signaling in aged mice restored hippocampal CA1 long-term potenti ation and improved mitochondrial coupling of electron transport and ATP synthesis(Minhas et al.,2021) thus confirming the detrimental role of PGE2 signaling in age-associated cognitive functions.
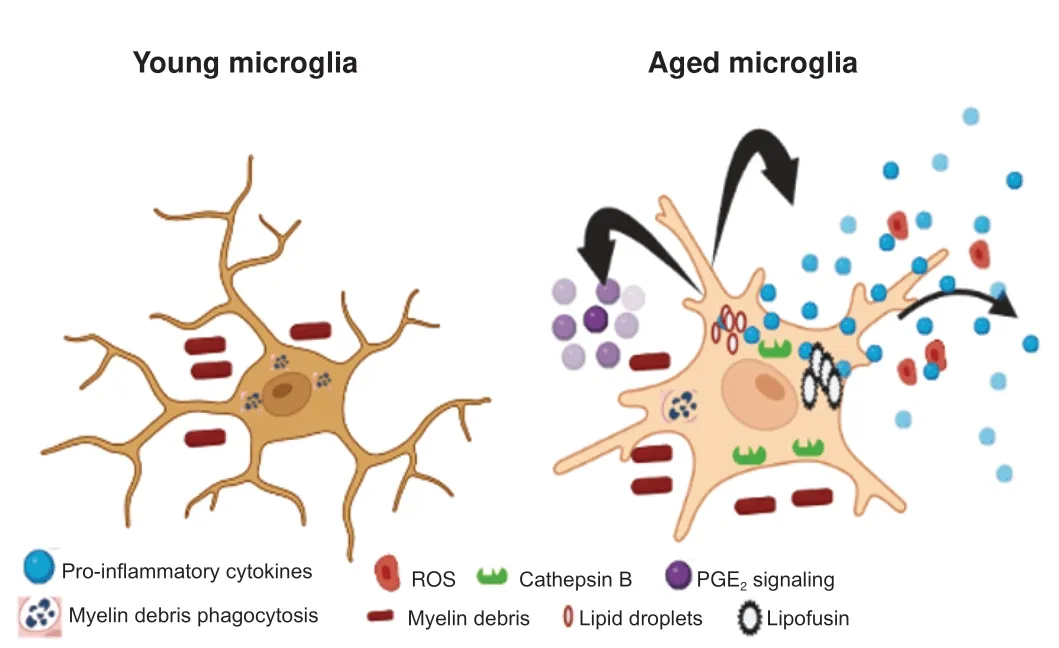
Figure 1|Microglia become dysregulated with aging.
A decline in microglia phagocytic function has been shown to increase in aged microglia (Pluvinage et al.,2019;Yanguas-Casas et al.,2020).CD36,a protein involved in phagocytosis is decreased in senescent microglia (Rawji et al.,2020).Upregulation of CD36 in cultured microglia improved myelin phagocytic activity and treatment with niacin in 9–12-month-old (middleaged) demyelinated mice promoted myelin debris clearance,increased oligodendrocyte precursor cells,and improved remyelination (Rawji et al.,2020).Additionally,CD22,a sialic acid binding immunoglobin-like lectin expressed on B cells was found to be upregulated in aged microglia (Pluvinage et al.,2019).CD22 negatively regulates BCR signaling and has been shown to modulate leucocyte phagocytosis in fish (Nitschke et al.,1997;Li et al.,2019).A recent study by Pluvinage et al.(2019) showed inhibition of CD22 promoted myelin debris removalin vitroand central nervous system (CNS) delivery of antibodies against CD22 reprogrammed microglia towards homeostatic transcriptional state and improved cognitive functions in older mice,thus suggesting that restoration of microglia phagocytosis might improve memory in older individuals.
Macrophages depend on colony-stimulating factor-1 receptor (CSF-1R)signaling for survival,proliferation,and differentiation (Stanley and Chitu,2014;Pluvinage et al.,2019).Inhibition of CSF-1R signaling has been shown to result in a reversible decline in the microglia population (Elmore et al.,2014;Spangenberg et al.,2016,2019).Depletion of aged microglia by using CSF-1R inhibitor,PLX5622,and repopulation restored age-associated cognitive impairment and increased neurogenesis in older rodents (Elmore et al.,2018).However,a recent study showed that the depletion of microglia by PLX5622 and repopulation resulted in a partial reversal of primed microglia in aged mice (Elmore et al.,2018).As the repopulated microglia demonstrated exaggerated pro-inflammatory responses when challenged with lipopolysaccharide.Furthermore,inflammatory gene transcriptome persisted in the brain even after microglia was repopulated in aged animals thus suggesting the presence of an inflammatory environment in older brains (Elmore et al.,2018;O’Neil et al.,2018).Similar results were observed by microglia depletion and repopulation in aged BALB/c mice.Microglia repopulation reversed age-associated defects in microglia CD68+lysosome enlargement and lipofuscin accumulation (O’Neil et al.,2018).Lipofuscin accumulation has been seen in aged microglia and leads to impaired microglia functions and cell death (Streit et al.,2014;Safaiyan et al.,2016;Burns et al.,2020).The lipopolysaccharide challenge following repopulation in both young and aged animals demonstrated heightened microglia responses compared to young mice (O’Neil et al.,2018) thus reflecting that microglia renewal does not influence the microenvironment in the aged brain.Additionally,microglia repopulation failed to restore synaptic transmission and cognition in aged animals suggesting that aged microglia support synaptic and cognitive functions in older subjects (Yegla et al.,2021).
Microglia and Lymphocytes Interact with Age
The detrimental contribution of microglia towards age-associated cognitive decline is well characterized,however,little is known about how innate immune cells including T and B lymphocytes might in involved in brain aging.As microglia and lymphocytes communicate in the aged brain it becomes imperative to understand the underlying mechanisms that lead to ageassociated cognitive impairments.
Several genes associated with microglia activation interact with infiltrated T lymphocytes and could play a role in neurodegenerative diseases and aging (Schetters et al.,2017;Groh et al.,2021).With aging,microglia tend to shift towards pro-inflammatory status and secrete TNF-α,IL-6,and IL-1β(Hickman et al.,2013;Ritzel et al.,2015;Zhang et al.,2022b).The expression of adhesion molecules,chemokines,and their receptors control the immune cell migration across the blood-brain barrier (BBB) (Marchetti and Engelhardt,2020).TNF-α has been shown to upregulate the expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 (ICAM-1) on venous endothelial cells and promoted T cell transmigration (Zhang et al.,2022b).Aged microglia release CCL-3 resulting in the recruitment of CD8+memory T cells into the SV zone thus resulting in age-related neuroinflammation (Zhang et al.,2022b).A study by Groh et al.(2021)reported an increase in CD8+T cells in the WM regions in both aged mice and old humans.The microglia acting as anti gen-presenting cells contributed to CD8+T cell-mediated axonal degeneration,and cognitive and motor impairment in aged animals.Interestingly in their Sc-RNA sequencing data,the authors reported an age-related increase in CD8+T cell subgroups some of which could be protective (Urban et al.,2020).
Tumor necrosis factor-α-induced protein 3 or A20 is expressed on microglia and has been shown to maintain brain homeostasis (Urban et al.,2020).A20 is a key regulator in NF-κB signaling which has been shown to promote inflammation and aging (Tilstra et al.,2011;Liu et al.,2017).Loss of A20 in microglia resulted in a microglial pro-inflammatory phenotype (Voet et al.,2018;Mohebiany et al.,2020).A20 deficient microglia upregulated IL-6,CCL-2,and CCL-3 and promoted brain infiltration of CD8+T cells.CD8+T cells have been shown to produce IFN-γ which leads to neuroinflammation and neurological damage (Akwa et al.,1998;Campbell et al.,1999).IFN-γ has been shown to have detrimental effects in the neurogenic niche during aging(Dulken et al.,2019;Kalamakis et al.,2019).Interestingly,the loss of microglia A20 with aging has not been investigated however it is plausible that aged microglia acquire an inflammatory phenotype due to deficiency of A20,and this needs further investigation.
Role of T Lymphocytes in Aging and Cognition
Recent studies have derailed the conventional wisdom that CNS is immuneprivileged tissue and peripheral immune cells only invade the brain when there is damage to the BBB.With aging the BBB becomes leaky and T cells may be able to infiltrate the brain parenchyma (Yang et al.,2020;Figure 2) and contribute to age-associated cognitive impairment.Incidentally,the presence of T cells has been demonstrated in WM,subventricular zone (SV)zone,meninges,and choroid plexus (CP) and exerts pathological effects with aging in both humans and non-human primates and rodents (Baruch et al.,2013;Dulken et al.,2019;Moreno-Valladares et al.,2020a,b;Batterman et al.,2021;Berry et al.,2021;Brioschi et al.,2021;Schafflick et al.,2021;Korf et al.,2022;Zhou et al.,2022).Rustenhoven et al.elegantly demonstrated an increase in sinus and non-sinus T lymphocytes with age in mice (Rustenhoven et al.,2021).T lymphocytes upon brain-derived anti gen presentation could be recruited to meninges and infiltrate brain parenchyma (Ellwardt et al.,2016;Rustenhoven et al.,2021) migrating to myelin-rich regions (Groh et al.,2021) and negatively regulating cognition.Da Mesquita et al.(2021)demonstrated an increase in meningeal CD4+FOXP3+T regs in aged animals thus linking meningeal immunity to cognitive impairment.Removal of these cells by anti-CD25 antibodies resulted in a decrease in meningeal CD4+FOXP3+T regs and improvement in cognition in aged mice reflecting that modulation of meningeal immunity could improve age-associated cognitive dysfunction (Da Mesquita et al.,2021).CNS-specific effector memory CD4+T cells have been shown to increase in aged CP and are robust producers of IL-4 (Baruch et al.,2013).Increased levels of IL-4 promoted CCL-11 by CP epithelium,a chemokine associated with cognitive impairment with aging(Villeda et al.,2011).However,there is less known about how CD4+T cells infiltrate aged brain parenchyma and modulate normal aging processes.Batterman et al.(2021) found an age-associated increase in the infiltrated T cells in the cingulum bundle and WM of aged rhesus monkeys.Furthermore,the T cell density in the cingulum bundle correlated with microglia reacti vity and cognitive impairment (Batterman et al.,2021).On the contrary,Berry et al.(2021) reported no difference in the human cortex T cells with age reflecting that regional differences could influence T cell infiltration in the aged brain.Tissue-resident memory T cells are seen in human and rodent brains (Ritzel et al.,2016;Smolders et al.,2018).A recent study demonstrated oligoclonal T cells with tissue-resident memory gene signatures in the neurogenic niches in the aged brain (Dulken et al.,2019).These anti gen-specific CD8+T resident memory T cells were found in the CNS after peripheral immunizations and showed protection against brain infections (Urban et al.,2020) suggesting some T lymphocytes can have protective roles.Studies showed elevated infiltrated CD8+T cells in the SV zone with age in both mice and humans (Dulken et al.,2019;Moreno-Valladares et al.,2020a).The exact mechanism that leads to the infiltration of CD8+T cells in the aged brain is not fully delineated.However,studies report the critical role of microglia.
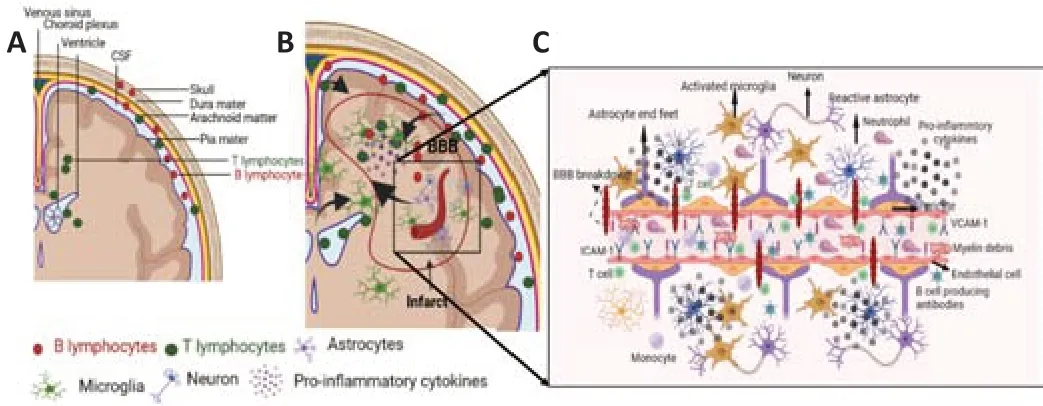
Figure 2|The presence of T and B lymphocytes in the healthy brain and after stroke.
Role of B Lymphocytes in Aging
A small number of B and plasma cells have been reported in healthy human cerebrospinal fluid (CSF) (Prasad,1983;Rojas et al.,2020) and brain parenchyma (Frischer et al.,2009;Machado-Santos et al.,2018;Jain and Yong,2022).They are usually observed in perivascular spaces including meninges and dura mater (Frischer et al.,2009;Howell et al.,2011;Machado-Santos et al.,2018).Unfortunately,there is only one study that has reported B cell density in the human-aged brain (Berry et al.,2021).In this study,the authors reported a lower B cell density (7.8 ± 1.4 cells/cm2) in youngversusaged human subjects (6.2 ± 1.0 cells/cm2) (Berry et al.,2021).In mice,B cells are rarely present in the healthy brain parenchyma but are found in small numbers in CSF,CP,and subdural meninges (Korin et al.,2017;Brioschi et al.,2021;Schafflick et al.,2021).B lymphocytes have been shown to occur in dural meninges and account for 15–30% of all CD45hileukocytes (Korin et al.,2017;Brioschi et al.,2021;Schafflick et al.,2021).Most of these cells in the dural meninges are B2 B cells and the vast majority are immature B cells generated in the skull bone marrow (Brioschi et al.,2021;Schafflick et al.,2021).B cells in the brain are tissue-resident,generated locally (Brioschi et al.,2021;Schafflick et al.,2021).Mature CNS B cells are IgM+cells with unmutated BCRs although few IgA+B cells are also observed (Brioschi et al.,2021;Schafflick et al.,2021).In the young mice,the integration of peripheral B cells into the resident CNS pool is very limited however it increases with age and tends to demonstrate T bet+B cells which is consistent with ageassociated B cells (Rubtsova et al.,2015;Korf et al.,2022).In young mice,IgA+plasma cells comprise most of the B cells in the meninges that are parti ally derived from the gut (Fitzpatrick et al.,2020).However,with an increase in age,IgG+and IgM+plasma cells become common in the meninges (Brioschi et al.,2021).Recently,we have shown the presence of CD11bhiB cells in the mouse brain that have a distinct phenotype and are robust producers of TNF-α (Korf et al.,2022).CD11b is expressed by myeloid cells as well as B cells and regulates cell motility and phagocytosis (Liu et al.,2015;Schittenhelm et al.,2017).
CD11bhiB cell counts increased in the spleen,skull bone marrow,and brain of the aged mice compared to young animals.These cells had higher expression of CD138 (expressed by plasma cells) (Sanderson et al.,1989),memory markers (CD80 and CD27) (Wu et al.,2011;Good-Jacobson et al.,2012),CD73(class switched) (Schneider et al.,2019),and CD268 shown to be expressed by autoreactive B cells (Liu et al.,2015;Qian et al.,2019).Additionally,a decrease in IgD and no change in IgM were consistent with age-associated B cells (Hao et al.,2011;Rubtsov et al.,2011;Rubtsova et al.,2015).Upon lipopolysaccharide stimulation,sorted CD45+CD19+B cells increased CD11b expression and had higher T-bet expression and increased uptake of beads in phagocytic assay suggesting enhanced phagocytic acti vity.B cells have been shown to increase the production of TNF-α on activation (Ma et al.,2019) and we observed higher expression of TNF-α on CD45+CD19+CD11bhiB cells hence reflecting a mixed,activated,and heterogeneous B cell population.
To understand the contribution of CD45+CD19+CD11bhiB cells in modulating the functions of microglia we used PepBoy mice which has a distinguishable CD45 haplotype (CD45.1) than C57BL/6 express Ptprc (CD45.2) allele.The CD11bhiand CD11blowB cells were sorted from aged spleens and retroorbitally transferred to young PepBoy mice.Twenty-four hours post-injection,the microglia expression of CD11b increased additionally an increase in bead uptake was observed consistent with phagocytic activity in mice treated with aged CD11bhiB cells than the aged CD11blowB cells.These results suggested that the presence of CD11bhiB cells could be partially responsible for microglia dysfunction (Korf et al.,2022).Microglia-mediated neuroinflammation contributes toward age-associated cognitive impairment.However,further research is warranted to delineate the functions of CD11bhiB cells in aging and age-associated cognitive impairment.
Aged Microglia in Stroke
Stroke is the second leading cause of death and disability worldwide(Collaborators,2019),and approximately two-thirds of all strokes occur in older adults (Feigin et al.,2014).Increased risk of stroke and poor strokerelated outcomes in older individuals already represent major challenges to the current healthcare system (Go et al.,2014).Findings from our laboratory and others demonstrate differential infarct and functional outcomes after stroke in young and aged animals (Sutherland et al.,1996;Kharlamov et al.,2000;Chauhan et al.,2017;Ritzel et al.,2018).Some studies demonstrate larger infarcts in older animals whereas others have shown smaller infarct sizes than those observed in young subjects (Sutherland et al.,1996;Kharlamov et al.,2000;Chauhan et al.,2017;Ritzel et al.,2018).Irrespective of the discrepancy in infarct size,aged animals have worse functional outcomes and increased mortality post-stroke in comparison to young animals (DiNapoli et al.,2008;Tan et al.,2013;Wang et al.,2013;Suenaga et al.,2015).In response to cerebral injury,microglia are some of the first responders,who quickly develop an activated phenotype (M1),generate reactive oxygen species,phagocytize,and produce pro-inflammatory cytokines and proteases (Macrez et al.,2011;Taylor and Sansing,2013).In addition,microglia also produce anti -inflammatory cytokines (M2),including IL-4 and IL-10,which play important role in the resolution and the restorative phase of stroke.Dystrophic changes and age-associated microglia activation have been reported in aged rodents,humans,and non-human primates (Perry et al.,1993;Streit and Sparks,1997;Sheffield and Berman,1998).Removal of microglia in young mice by PLX 3397 prior to stroke resulted in increased infarct size,due to dysregulated neuronal calcium responses and increased neuronal cell death (Szalay et al.,2016).Additionally,the young microglia do not have dysfunctional phenotype hence we tested our hypothesis that the removal of activated microglia in aged mice will improve acute functional recovery.The aged mice were fed with CSF-1R inhibitor (PLX 5622) 3 weeks before the stroke.Depletion of aged microglia resulted in increased infarct volume and acutely after the middle cerebral artery occlusion (MCAO)model.We further validated our findings by administering a monoclonal anti body against CSF-1R directly into the brain.An increase in infarct volume and increased infiltrated monocytes in the aged mice after stroke was seen in animals in which microglia were depleted (Marino Lee et al.,2021).The increase in post-stroke injury after microglia removal in aged could parti ally be explained by a higher number of infiltrating monocytes in the mice that were fed with PLX5622.Acute monocyte infiltration has been shown to worsen stroke outcomes in young mice (Fang et al.,2018).Additionally,the removal of microglia by PLX5622 promoted GFAP+reactive astrocytes which could be responsible for enchased brain injury after stroke (Stadler et al.,2022).
RNA sequencing studies have shown upregulation or downregulation of different sets of genes after stroke in aged microglia (Jiang et al.,2020;Shi et al.,2020).After distal MCAO,upregulation of genes involved in cell motility,cell interactions,angiogenesis,and inflammatory responses was observed in young microglia whereas the aged microglia had reduced or no changes in the genes involved in these processes (Jiang et al.,2020;Shi et al.,2020).Reduced chemotaxis and failure of increased expression of genes involved in cell-to-cell interaction,tissue remodeling,and angiogenesis were apparent in the aged microglia during the recovery phases of the stroke (Jiang et al.,2020;Shi et al.,2020) thus highlighting the difference in the post-stroke transcriptome in aged microglia.
WM damage is present after stroke in both rodents and humans (Fu et al.,2005;Li et al.,2013;Suenaga et al.,2015;Marin and Carmichael,2018;Etherton et al.,2019;Faheem et al.,2019;Hong et al.,2021).Incidentally,aged animals are more sensitive to WM damage,increased oligodendrocyte death,oxidative stress,and poor functional recovery after stroke (Rosenzweig and Carmichael,2013;Suenaga et al.,2015).Increased myelin fragmentation with age resulted in the accumulation of undegradable lysosomal aggregates in aged microglia (Safaiyan et al.,2016).Lysosomes attached to lipid droplets have been reported in aged microglia suggesting lipid cycling through lipophagy (Vaughan and Peters,1974).Lipid droplet accumulating microglia has been identified in the aged human and rodent brain and represents a dysfunctional phenotype (Foley,2010;Marschallinger et al.,2020;Arbaizar-Rovirosa et al.,2022).Studies have shown that stroke induces lipid droplet biogenesis in both young and aged microglia and plays a role in lipid metabolism and phagocytosis (Beuker et al.,2022;Arbaizar-Rovirosa et al.,2022).Accumulation of lipid droplets in chronic stroke has been shown to impair post-stroke recovery (Becktel et al.,2022).Repopulation of microglia after cessation of the PLX5622 diet in aged stroke animals increased expression of genes involved in long-chain fatty acid metabolism and cholesterol biosynthesis and metabolism (Arbaizar-Rovirosa et al.,2022).Renewal of microglia in old stroke mice resulted in reduced microglia lipid droplet content and improvement in functional outcomes (Arbaizar-Rovirosa et al.,2022) reflecting rejuvenation of certain metabolic pathways after microglia repopulation.Suenaga et al.(2015) demonstrated greater WM damage,and reduced M2 polarized microglia in the aged mice correlated with worse functional outcomes.M2 microglia promotes recovery processes including neurogenesis,angiogenesis,WM repair,and release of neurotrophic factors thus resolving inflammation (Hu et al.,2015;Suenaga et al.,2015).However,all these processes have been shown to be impaired in aged mice after stroke and could be responsible for worse functional outcomes (Walter et al.,2010;Manwani et al.,2011;Tang et al.,2016;Ritzel et al.,2018).
T and B Lymphocytes in Aged Stroke
T and B lymphocytes in clinical stroke in older individuals
A rapid decline in circulating lymphocytes is seen early after a stroke (Haeusler et al.,2008;Wang et al.,2017;Juli et al.,2021).At the stroke onset,a decrease in the frequency of circulating T cells was seen in acute ischemic stroke (AIS) patients that negatively correlated with infarct volume and NIHSS on day 1 after stroke (Wang et al.,2017).In the same study,although no difference between the frequency of B cells in AIS and controls was observed a negative correlation between B cells percentage and stroke outcomes was evident (age 63.36 ± 13.01 years) (Wang et al.,2017).A recent study showed a decrease in lymphocyte count in patients with leukocytosis was associated with poor NIHSS scores in AIS patients (age <60 years) (Juli et al.,2021).Circulating CD4+CD28–T cells increased within the first 48 hours after stroke and were associated with an increased risk of recurrent stroke and death(age 75.0 ± 13.5 years) (Nadareishvili et al.,2004).Systemic IL-17 secreting T cells were found to be increased in stroke patients 30 days after stroke and were associated with poor cognitive function (mean age 71.8 ± 14.4 years)(Swardfager et al.,2014).Peripheral Treg proportions declined in the patients within 72 hours of stroke onset (mean age stroke cohort,65.8± 15.4 years;control cohort,54.0 ± 10.8 years) (Noh et al.,2018).Additionally,the Treg proportion positively correlated with age but not infarct volume (Noh et al.,2018).Urra et al.(2009) reported a decline in circulating T lymphocytes and Tregs after stroke (mean age 78.9 ± 11.4 years)versuscontrols (age 65.9 ±18.6 years).Furthermore,a decrease in circulating B cells was observed after the stroke and was associated with poor stroke outcomes at 3 months (Urra et al.,2009).A study of Dolati et al.(2018) showed that the frequency of CD4+CD25+Tregs reduced whereas the proportion of Th-17 cells was higher in AIS patients (age 67.3 ± 8.9 years) on days 1 and 5 after stroke,suggesting an imbalance in Th17/Treg cells might contribute to stroke pathophysiology.On the contrary,circulating Tregs were shown to be higher in ischemic stroke versus controls (age >18 years) at admission (Santamaria-Cadavid et al.,2020).Patients with lower Tregs at 48 hours showed reduced circulating IL-10,higher frequency of early neurological deterioration,and risk of infections.Additionally,higher Treg levels during the acute phase of stroke were associated with better functional outcomes at 3 months (Santamaria-Cadavid et al.,2020).
In a study by Mantani et al.(2014) showed that no difference in the circulating CD19+B cells between controls and stroke cases was observed however,they identified an association between the high level of CD19+CD40+B cells and a decrease in the risk of stroke incidence.Moreover,higher CD19+CD86+B cells were associated with increased stroke risk suggesting different B cell subsets may have the opposite impact on stroke risk (Mantani et al.,2014).CD19highIgD+CD38highCD24highCD5highsubset has been identified in humans and has shown to have a suppressive role by inducing Foxp3+CD4+CD25+Tregs(Lemoine et al.,2011).Similarly,B-regulatory cells secreting IL-10 have been identified in rodent models (Yanaba et al.,2008) and have been shown to play a protective role in stroke outcomes in young animals (Offner and Hurn,2012;Seifert et al.,2018;Ortega et al.,2020).Incidentally,in most studies,an age difference in circulating B and T cells was not reported as only older individuals were included however,the peripheral immune responses to stroke have shown to differ between young and aged humans and rodents(Ritzel et al.,2018;Sykes et al.,2021;Zhang et al.,2022a).
T and B lymphocytes in preclinical stroke in older subjects
Preclinical stroke studies using older animals though limited have shown an increase in brain immune cells infiltration after stroke leading to neuroinflammation (Ritzel et al.,2016;Chauhan et al.,2017;Vogelgesang et al.,2019;Harris et al.,2020;Figure 2).Neuroinflammation contributes to impaired functional recovery in older animals after stroke (Manwani et al.,2011;Buga et al.,2014;Tang et al.,2016;Ritzel et al.,2018).Spleen harbors the largest pool of peripheral immune cells and splenectomy in aged mice reduced infarct size and improved acute functional outcomes (Chauhan et al.,2017).Although,removal of the spleen is not a therapeutic strategy,blocking the egress of lymphocytes from the spleen could reduce brain injury after stroke.Siponimod treatment,an S1PR inhibitor that selectively inhibits lymphocyte egress from the spleen (Arnon and Cyster,2014),reduced brain T lymphocyte infiltration without improving infarct volume or motor outcomes in middle-aged mice (Vogelgesang et al.,2019).Brain CD4+T and CD8+T cell counts were reduced in the middle-aged mice that received the S1PR inhibitor(Vogelgesang et al.,2019).The other source of peripheral immune cells in aged animals after stroke is the gut (Lee et al.,2020).Aged mice receiving a fecal transplant of young microbiome had reduced inflammatory brain IL-17+γδ T cells and improved functional recovery (Lee et al.,2020).In contrast to γδ T cells,T regs cells promote tissue recovery after stroke by reducing neuroinflammation (Liesz et al.,2009).A recent study by Cai et al.(2022)identified CD8+CD122+CD49dloT cells as CD8+regulatory T cells (CD8+TRLs)that promoted neuroprotection after stroke.Post-stroke administration of CD8+TRLs resulted in reduced infarction and improved long-term functional recovery in older animals (Cai et al.,2022).
Brain-infiltrated CD4+T cells secrete IFN-γ which stimulates monocytes,macrophages,neutrophils,and endothelial cells to release CXCL-10.CXCL-10 in turn stimulates T cells to secrete more IFN-γ and other cytokines (Seifert et al.,2014;Harris et al.,2020).Aged animals had higher circulating and post-stroke brain CXCL-10 levels than young mice.Removal of CD4+T cells in aged mice after stroke resulted in improved functional recovery (Harris et al.,2020).Additionally,the depletion of CD4+T cells reduced circulating levels of pro-inflammatory cytokines including IFN-γ,CXCL-10,CCL-2,and CXCL-1(Harris et al.,2020),thus reflecting a detrimental role of T lymphocytes after stroke in aged animals.A recent study demonstrated that 2-hydroxypropylβ-cyclodextrin,an Food and Drug Administration (FDA)-approved drug that solubilizes and entraps lipophilic substances not only reduced the brain lipid droplet accumulation but also reduced infarct infiltrated B220+B cells,CD3ε+T cells and IgA+anti body-producing plasma cells in the aged mice (Becktel et al.,2022).Furthermore,2-hydroxypropyl-β-cyclodextrin treatment in older mice attenuated neurodegeneration and improved functional outcomes after stroke (Becktel et al.,2022).After the stroke,BBB is breached,and myelin reactive antigens leak out and are exposed to immune cells in the periphery.The peripheral immune cells recognize them as foreign anti gens and mount an auto-aggressive immune response (Frenkel et al.,2003;Dirnagl et al.,2007).Adaptive immunity plays a detrimental role in stroke neuroinflammation and targeting brain-reactive T cells reduced infarct outcomes after stroke (Subramanian et al.,2009;Akiyoshi et al.,2011;Dziennis et al.,2011).Treatment with recombinant T cell receptor ligand 1000(RTL1000) in middle-aged mice resulted in a decrease in activated microglia/macrophages,and CD3+T cell infiltration after stroke (Zhu et al.,2015).
B cells are recruited to the brain after an ischemic stroke (Doyle et al.,2015).Loss of B cells has been shown to increase infarct size,impair functional outcomes,and mortality in mice deficient in B cells (Ren et al.,2011).Administration of IL-10-producing Bregs into B cell-deficient and wildtype B cell-sufficient young mice reduced infarct volume but also improved functional outcomes by promoting neurogenesis (Bodhankar et al.,2013,2014;Ortega et al.,2020).Depletion of B cells by humanized antibody to CD20+delayed functional recovery and reduced stroke-induced hippocampal neurogenesis (Ortega et al.,2020).Doyle et al.(2015) demonstrated B cells to be responsible for delayed cognitive impairment after stroke.In human postmortem brain tissue,the presence of B cells and auto-reactive IgG staining was observed in some patients with stroke and dementia (Doyle et al.,2015).Higher titers of MBP antibodies are associated with cognitive decline after stroke (Becker et al.,2016).Unfortunately,all these findings were observed in young mice and there is a lack of studies on age stroke models.In our recent study,we reported the presence of the CD11bhiB cells with age and stroke in aged animals (Korf et al.,2022).Furthermore,these cells regulated microglial phagocytosis.However further studies are required to clarify their role in post-stroke recovery.
Conclusion
The world population is growing old and older individuals have a higher risk for cardiovascular diseases including stroke.The aged brain responds differenti ally to stress or injury as the anatomy and homeostasis of the brain change with aging.Without any neuronal damage,a significant number of individuals suffer from severe cognitive impairment.The immune cells including microglia,T,and B lymphocytes impact the cognition functions with age and stroke.Microglia become dysfunctional with age and contributes to cognitive dysfunction in older individuals.However,depletion or renewal of microglia in older animals does not salvage the deteriorating cognitive functions completely thus supporting the notion although dysfunctional,microglia alone are not the major players.Activated microglia interact with infiltrated T lymphocytes and also negatively influence cognitive functions after stroke.The presence of T and B lymphocytes in the perivascular spaces and brain parenchyma has been documented in humans and rodents.The presence of infiltrated lymphocytes in the brain parenchyma contributes to age-associated cognitive impairment.It is imperative to unveil and understand the mechanisms that are different in aged individuals to develop therapies for age-associated disorders.
Author contributions:JNN and AC drafted the manuscript.AC critically reviewed the manuscript.Both authors have read and approved the manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis
- CMT1A current gene therapy approaches and promising biomarkers

