Emodin attenuates inflammation and demyelination in experimental autoimmune encephalomyelitis
Yue-Ran Cui,Zhong-Qi Bu,Hai-Yang Yu,Li-Li Yan,Juan Feng
Abstract Emodin,a substance extracted from herbs such as rhubarb,has a protective effect on the central nervous system.However,the potential therapeutic effect of emodin in the context of multiple sclerosis remains unknown.In this study,a rat model of experimental autoimmune encephalomyelitis was established by immune induction to simulate multiple sclerosis,and the rats were intraperitoneally injected with emodin (20 mg/kg/d) from the day of immune induction until they were sacrificed.In this model,the nucleotide-binding domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome and the microglia exacerbated neuroinflammation,playing an important role in the development of multiple sclerosis.In addition,silent information regulator of transcription 1 (SIRT1)/peroxisome proliferator-activated receptor-alpha coactivator (PGC-1α) was found to inhibit activation of the NLRP3 inflammasome,and SIRT1 activation reduced disease severity in experimental autoimmune encephalomyelitis.Furthermore,treatment with emodin decreased body weight loss and neurobehavioral deficits,alleviated inflammatory cell infiltration and demyelination,reduced the expression of inflammatory cytokines,inhibited microglial aggregation and activation,decreased the levels of NLRP3 signaling pathway molecules,and increased the expression of SIRT1 and PGC-1α.These findings suggest that emodin improves the symptoms of experimental autoimmune encephalomyelitis,possibly through regulating the SIRT1/PGC-1α/NLRP3 signaling pathway and inhibiting microglial inflammation.These findings provide experimental evidence for treatment of multiple sclerosis with emodin,enlarging the scope of clinical application for emodin.
Key Words:demyelination;emodin;experimental autoimmune encephalomyelitis;microglia;multiple sclerosis;neuroinflammation;NLRP3 inflammasome;PGC-1α;pyroptosis;SIRT1
Introduction
Multiple sclerosis (MS) is a demyelinating autoimmune disease of the central nervous system (CNS) (Filippi et al.,2018).MS affects more than two million people worldwide (Browne et al.,2014) and is more common in young adults.The pathological features of MS include focal demyelinating plaques in the brain,spinal cord,and optic nerve,as well as diffuse changes such as axonal injury and astrogliosis (Lassmann,2018).Although the eti ology of MS has not been fully elucidated,environmental,genetic,and epigenetic factors have been implicated in its pathogenesis (Olsson et al.,2017).Existing drugs for MS,such as interferon beta,dimethyl fumarate,and fingolimod,have strong side effects and cannot reverse the chronic progressive disabilities associated with this condition (Tintore et al.,2019;Bhargava,2021;D’Amico et al.,2022).Therefore,there is an urgent need to develop effective and safe therapeutic drugs for MS.Experimental autoimmune encephalomyelitis (EAE) is currently the most widely used animal model of MS (Lassmann and Bradl,2017);it partially simulates MS pathogenesis and is a useful tool for exploring the underlying molecular mechanisms of MS.
Inflammasomes are signaling complexes that sense injury and stress signals and induce inflammatory cytokine maturation and secretion.The nucleoti debinding domain-like receptor family pyrin domain containing 3 (NLRP3)inflammasome is the most widely studied inflammasome and is involved in many autoimmune and inflammatory diseases (Duewell et al.,2010;Heneka et al.,2013).NLRP3 inflammasome-related molecules are involved in MS pathogenesis,as indicated by altered levels of NLRP3 inflammasome complex components in patients with MS and in animal knockout models(Gris et al.,2010;Walsh et al.,2014;Burm et al.,2016;McKenzie et al.,2018).Inflammasome component expression is increased in the CNS tissues of MS patients.Furthermore,inflammasome activation and pyroptosis occur in human glial cells,which may affect the occurrence and development of MS.Moreover,abnormal microglial activation leads to demyelination and neurodegenerative changes (Baecher-Allan et al.,2018).Microglia,which are the intrinsic immune cells of the CNS,can exacerbate neuroinflammation by secreting chemokines,which in turn recruit immune cells to the CNS,and can also act as anti gen-presenting cells to activate immune cells (Ferrari et al.,2004;Mallucci et al.,2015).In addition,microglia can be activated by pro-inflammatory cytokines such as interleukin (IL)-1β,thus creating a vicious cycle (Correale and Farez,2015).These studies illustrate the key roles of inflammasome and microglial activation in MS.In addition,studies have demonstrated that silent information regulator of transcription 1 (SIRT1) and the downstream molecule peroxisome proliferator-activated receptor-alpha coactivator (PGC-1α) can inhibit NLRP3 inflammasome activation (Park et al.,2020;Wang et al.,2021b;Zhang et al.,2021).Research has shown that SIRT1-mediated responses are involved in a variety of physiological processes,such as oxidative stress,inflammation,and apoptosis (Yang et al.,2022).Several studies have reported the inhibitory effect of SIRT1 on the inflammatory response (Yeung et al.,2004;Planavila et al.,2011;Bai et al.,2020).These results suggest that SIRT1 can reduce neuroinflammation by inhibiting NLRP3 inflammasome activation.Moreover,related studies have found that activation of SIRT1 can reduce the severity of EAE (Guo et al.,2021,2022;Wang et al.,2021a).Therefore,the SIRT1/PGC-1α/NLRP3 signaling pathway may be a key pathway mediating the beneficial effects of emodin treatment on EAE.
Emodin (1,3,8-trihydroxy-6-methylanthraquinone;Additional Figure 1) is a substance extracted from traditional herbs that can alleviate the symptoms of autoimmune diseases such as ulcerative colitis and rheumatoid arthritis(Luo et al.,2018;Zhu et al.,2019).In addition,a recent study reported that emodin exhibits potent neuroprotective effects in a variety of CNS disorders such as Alzheimer’s disease,Parkinson’s disease,and cerebral ischemia(Mitra et al.,2022).Emodin inhibits the acti vity of the protein kinase casein kinase 2 (CK2).Systemic pharmacological inhibition of CK2 or knockdown of CK2b in CD4+T cells exerts neuroprotective effects in EAE (Gibson and Benveniste,2018).Based on these studies,it seems likely that emodin would have neuroprotective effects in the context of CNS autoimmune conditions.However,there are currently no reports on the protective effects of emodin in these diseases.While a recentin vivostudy using an EAE model reported the protective effects of emodin,the exact mechanism remains unclear (Zheng et al.,2022a).A previous study found that emodin enhances SIRT1 signaling,which in turn inhibits silica-mediated lung fibrosis (Yang et al.,2016).Another study showed that emodin inhibits NF-κB signaling and attenuates sepsisinduced lung injury through SIRT1 signaling (Liu et al.,2022a).In addition,anin vitrostudy reported that emodin decreases the level of microglia activation(Park et al.,2016).Therefore,we hypothesized that emodin may ameliorate EAE disease severity through regulation of the SIRT1/PGC-1α/NLRP3 signaling pathway and microglia.The aim of this study was to determine the role of emodin in alleviating inflammation and demyelination in an EAE rat model and its association with microglial activation and the SIRT1/PGC-1α/NLRP3 signaling pathway.
Methods
Animals
Multiple sclerosis is an autoimmune,inflammatory demyelinating disease of the CNS that is more common in women than in men (Krysko et al.,2020).Thus,basic research related to multiple sclerosis is typically carried out in female rodents (Dayani et al.,2022;Guo et al.,2022;Packialakshmi et al.,2022).We therefore used female rats to establish an EAE model.Thirty-two 6-to 8-week-old female Sprague-Dawley rats (used to establish the EAE rat model) weighing 180–200 g were purchased from the HFK Company,Beijing,China (license No.SCXK (Jing) 2019-0008).In addition,ten guinea pigs (300–350 g,used for obtaining spinal cord tissue to establish the EAE rat model) were purchased from Changsheng Company,Benxi,Liaoning,China (license No.SCXK(Liao) 2020-0001).Animals were housed in a specific pathogen-free animal laboratory and had ad libitum access to water and food.Rats and guinea pigs were maintained under a 12/12-hour light/dark cycle.The experiments were performed in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals (8thed) (National Institutes of Health,2011).This study was approved by the Medical Ethics Committee of the Shengjing Hospital of China Medical University on December 1,2021 (approval No.2021PS843K).Rats were randomly assigned to the following four groups(n=8 per group): (1) control group (not immunized),(2) EAE group,(3) EAE +emodin group,and (4) emodin group (not immunized).
EAE model establishment
The EAE rat model was established as reported previously (Li et al.,2019).Briefly,after the guinea pigs were anestheti zed with 2% sodium pentobarbital(0.35 mL/100 g,intraperitoneal injection,Shangyao,Shanghai,China),the spinal cords were harvested and mixed with 0.9% normal saline to make a homogenate.Incomplete Freund’s adjuvant (equal volume to the homogenate,Sigma,St.Louis,MO,USA) containing Mycobacterium tuberculosis H37Ra (10 mg/mL,Difco;BD Biosciences,Franklin Lakes,NJ,USA) was added to prepare an emulsion mixture.Then,0.4 mL of the emulsion mixture was injected rat subcutaneous into both hind footpads of the rats.The day of induction was defined as Day 0 (D0).The emulsion mixture was subcutaneously injected on D0 and D6,and pertussis toxin (Sigma)was subcutaneously injected on D0 and D2.According to our preliminary experiments and relevant studies (Pilipović et al.,2020;Dąbrowska-Bouta et al.,2021),EAE progression in this rat model is spontaneously reversible,so we sacrificed the rats at the peak of disease (on D14) before disease regression occurred.The experimental design is illustrated inAdditional Figure 2.
Drug treatment
Emodin was purchased from Meilun,Dalian,China.The doses of emodin used were based on previous studies (Ye et al.,2019;Mei et al.,2020).The animals in the control and EAE groups received intraperitoneal injections of an equal amount (8 mL/kg) of vehicle (0.9% normal saline).The EAE+emodin and emodin groups received intraperitoneal injections of emodin at a dose of 20 mg/kg at the same time each day,starting on the day after induction of EAE (D1) and conti nuing unti l the day before sacrifice (D13).All rats were sacrificed at the peak of EAE disease,and blood samples and spinal cords were collected for subsequent procedures.
Body weight and neurobehavioral analysis
Two observers blinded to the group assignments assessed the body weights and neurological deficits of animals every morning.Neurobehavioral scores were assessed (n=8 per group) based on a five-point scoring system (Urban et al.,1988): 0=no signs;1=disappearance of tail tension,reeling gait;2=loss of muscle tone in both hind limbs;3=paralysis of hind limbs;4=paraplegia;and 5=pre-death stage;± 0.5,clinical symptoms were between the two criteria.High neurobehavioral scores indicated more severe neurological impairment and motor dysfunction.
Tissue preparation
On D14,the rats were anestheti zed with 2% sodium pentobarbital (0.3 mL/100 g,intraperitoneal injection) for sample collection.The animals (n=4 per group) used for histopathological assessment and immunohistochemistry were sacrificed,and their blood was drawn from the left cardiac ventricle via heart puncture.Next,the animals were subjected to cardiac perfusion with 0.9%normal saline and then 4% paraformaldehyde,and the lumbar enlargements of the spinal cords were harvested.The spinal cord sections were then embedded in paraffin and sliced into 5-µm thick sections.For the animals (n=4 per group) used for polymerase chain reaction (PCR) and western blotting,blood was drawn from the left cardiac ventricle via heart puncture.Then,they were sacrificed and subjected to cardiac perfusion with 0.9% normal saline,and the lumbar enlargements of the spinal cords were harvested.The blood samples obtained were left at room temperature for 30 minutes and then centrifuged at 1200 ×gfor 15 minutes to obtain serum samples.All samples were stored at–80°C and used for subsequent experiments.
Histopathological assessment
To assess inflammatory cell infiltration and demyelination,spinal cord lumbar enlargement sections were subjected to hematoxylin-eosin (HE) and Luxol Fast Blue (LFB) staining.Briefly,HE staining was carried out in accordance with the manufacturer’s protocol (Beyotime,Shanghai,China).For LFB staining,sections were first stained with 0.1% LFB solution (Solvent Blue 38,Sigma) at 60°C for 4 hours,then at 37°C for 4 hours,followed by differenti ation.Finally,the sections were observed and images were acquired using a Nikon light microscope (Minato-ku,Tokyo,Japan).The sections were scored according to previously published criteria (Li et al.,2019): 0=no infiltration;1=scattered infiltration;2=inflammatory cell infiltration around blood vessels;and 3=extensive infiltration.Demyelination was scored as follows: 0=none;1=focal demyelination;2=a few areas of demyelination;and 3=large areas of demyelination.
Immunohistochemistry
Immunohistochemistry was performed to assess the degree of microglial aggregation and activation,using ionized calcium-binding adaptor molecule 1 (Iba-1) and CD68,respectively,as markers.After spinal cord sections were deparaffinized,hydrated,and subjected to antigen retrieval,blocking was performed using endogenous peroxidase and serum.Subsequently,the sections were incubated with anti-Iba-1 (rabbit polyclonal antibody against rat,1:200,ProteinTech Group,Chicago,IL,USA,Cat# 10904-1-AP,RRID:AB_2224377) and anti-CD68 (mouse monoclonal antibody against rat,1:100,Abcam,Cambridge,UK,Cat# ab31630,RRID: AB_1141557) primary antibodies overnight at 4°C (14–16 hours).Sections were incubated with bioti n-labeled anti -mouse/rabbit secondary anti body (Maixin,Fuzhou,Fujian,China,Cat# KIT-9710) at 37°C for 30 minutes the following day,followed by 3,3′-diaminobenzidine staining (Cat# DAB-0031,Maixin).Finally,the sections were observed and images of the immunocomplexes were captured using a Nikon light microscope (Minato-ku,Tokyo,Japan).
Real-time PCR
On D14,all rats were anesthetized,and the lumbar enlargements of the spinal cord were harvested.RNAiso Plus (Takara,Shiga,Japan) was used to extract total RNA from the lumbar enlargements.Reverse transcription and real-time PCR were performed in accordance with the manufacturer’s protocols (Vazyme,Nanjing,China).The PCR conditions were as follows: stage 1,repeats: 1,95°C,30 seconds;stage 2,repeats: 40,95°C,10 seconds,60°C,30 seconds;stage 3,repeats: 1,95°C,15 seconds,60°C,60 seconds,95°C,15 seconds.The 2–∆∆Ctmethod (Livak and Schmittgen,2001),normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH),was used to analyze the results.The primer sequences are listed inTable 1.
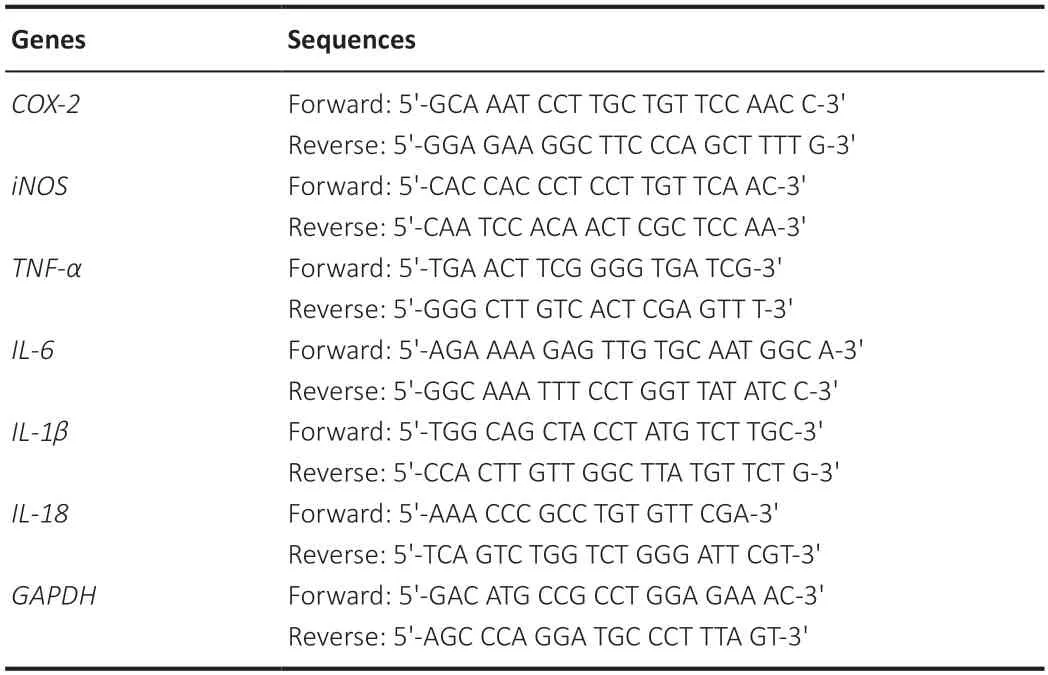
Table 1|Sequences of primers for real-time polymerase chain reaction
Western blot analysis
Total protein was extracted from the lumbar enlargements using radioimmunoprecipitation assay lysis buffer (Beyotime) and phenylmethanesulfonyl fluoride (Beyotime).Protein concentrations were measured using a bicinchoninic acid protein assay kit (Beyotime).Protein samples (40 µg each) were separated by electrophoresis on a 12%sodium dodecyl sulfate-polyacrylamide gel (Beyotime) and transferred to polyvinylidene fluoride membranes (Millipore,Billerica,MA,USA).Then,5%non-fat milk was used to block the membranes at 37°C for 30 minutes.To detect the expression levels of SIRT1,PGC-1α,and NLRP3 signaling pathway components,the membranes were incubated with following primary anti bodies overnight at 4°C: anti -NLRP3 (rabbit polyclonal anti body against rat,1:1000,Abclonal,Woburn,MA,USA,Cat# A5652,RRID: AB_2766412),anti -caspase-1 (rabbit polyclonal anti body against rat,1:1000,Abclonal,Cat# A0964,RRID: AB_2757485),anti-apoptosis associated speck-like protein containing CARD (ASC;rabbit polyclonal anti body against rat,1:1000,Zenbio,Chengdu,China,Cat# 340097,RRID: AB_2921364),anti-SIRT1 (rabbit monoclonal anti body against rat,1:1000,Zenbio,Cat# R25721,RRID: AB_2921365),anti -PGC-1α (rabbit polyclonal anti body against rat,1:1000,Zenbio,Cat# 381615,RRID: AB_2921366),anti -gasdermin D N-terminal fragment (pyroptosis marker,rabbit monoclonal anti body against rat,1:1000,CST,Danvers,MA,USA,Cat#46451S,RRID: AB_2921367);and anti-β-actin (mouse monoclonal antibody against rat,1:10,000,ProteinTech Group,Chicago,IL,USA,Cat# 66009-1-Ig,RRID: AB_2687938).Next,the membranes were incubated with goat antirabbit secondary antibodies (1:10,000,ProteinTech Group,Cat# SA00001-2,RRID: AB_2722564) and goat anti -mouse secondary anti bodies (1:10,000,ProteinTech Group,Cat# SA00001-1,RRID: AB_2722565) at 37°C for 30 minutes.Enhanced chemiluminescence reagents (Tanon,Shanghai,China)were used to visualize protein expression.The results were semi-quantified using ImageJ soft ware 1.46r (National Institutes of Health,Bethesda,MD,USA)(Schneider et al.,2012) and normalized to β-actin.
Cell culture and treatment
BV2 microglial cells (Cat# CL-0493,RRID: CVCL-0182) were purchased from Procell,Wuhan,Hubei,China,and their identity was confirmed by short tandem repeat analysis.Cells were cultured in Dulbecco’s modified Eagle medium/high glucose (KeyGENE,Nanjing,Jiangsu,China) supplemented with 10% fetal bovine serum (Lonsera,Canelones,Uruguay) and 1% penicillin/streptomycin solution (Procell) at 37°C and 5% CO2(humidified atmosphere).Lipopolysaccharide (LPS;1 µg/mL,Sigma) and adenosine triphosphate (ATP;5 mM,MCE,Shanghai,China) were used to activate BV2 cells as previously described (Liu et al.,2021b).The cells were divided into the following three groups for thein vitroexperiments: (1) control group (no treatment);(2)model group (treated with LPS for 3 hours followed by ATP for 30 minutes);and (3) treatment group (emodin pretreatment for 24 hours followed by LPS+ATP treatment).The experimental design is illustrated inAdditional Figure 3.
Cell viability detection
The effect of emodin on BV2 cell viability was assessed to determine the appropriate concentration of emodin for use in subsequent experiments.We used a cell counting kit-8 assay (APExBIO,Houston,TX,USA) to detect cell viability.Briefly,cells were seeded into 96-well plates at a density of 7 × 103per well and incubated for 24 hours.The cells were then treated with different concentrations of emodin (0,5,10,25,50,100 µM) for 24 hours,after which 10 µL of cell counting kit-8 solution was added to each well,and the plates were incubated for 2 hours at 37°C in the dark.Absorbance was then measured at 450 nm using a microplate reader (BioTek,Winooski,VT,USA).
Lactate dehydrogenase analysis
To assess cell pyroptosis (Jia et al.,2019;Liu et al.,2019;Wang et al.,2022a),lactate dehydrogenase (LDH) acti vity in serum and cell culture supernatants was detected using an LDH testing kit (Jiancheng,Nanjing,China) according to the manufacturer’s protocol.Cell culture supernatants were collected and centrifuged at 900 ×gfor 20 minutes,and the centrifuged supernatants were stored at–80°C and used for subsequent experiments.
Enzyme-linked immunoassay
Serum samples and cell culture supernatants were obtained to evaluate the levels of inflammation-related cytokines.Enzyme-linked immunoassay(ELISA) kits (Meilian,Shanghai,China) were used in accordance with the manufacturer’s protocol.The results were obtained by measuring the absorbance at 450 nm using a microplate reader.The concentrations were quantified by comparison to a standard curve.The anti bodies and the linear dynamic range of the standard curve used for ELISA quantifications are shown inAdditional Table 1.
Nitrite oxide detection
Nitric oxide (NO) reacts with O2and H2O to generate nitrite.The concentration of NO in serum and cell culture supernatants was indirectly measured by detecting the nitrite content using colorimetric NO testing kits(serum: Jiancheng;cell culture supernatants: Beyotime) according to the manufacturers’ instructions.
Stati stical analysis
We used the resource equation approach described previously (Arifin and Zahiruddin,2017) to calculate the optimal sample size,which was 4–6 rats per group.Considering that each rat can only be used for histochemistry or western blotting,and based on a previous animal study (Liu et al.,2019),we chose to include 8 rats in each group.No animals or data points were excluded from the analysis.The evaluators were blinded to the assignments.Data are presented as mean ± standard deviation (SD).GraphPad Prism 7.00 (GraphPad Software,San Diego,CA,USA,www.graphpad.com) was used for statistical analysis.All data were evaluated using one-way analysis of variance followed by Tukey’s multiple comparison test.Stati stical significance was set atP<0.05.
Results
Effects of emodin on EAE rat body weight and neurobehavior
Decreased body weight and increased neurobehavioral scores indicate an increase in EAE severity.We recorded body weights and neurobehavioral scores from D0 to D14 (Figure 1).The body weights of all groups decreased from D0 to D3 and then began to increase (Figure 1A).From D10,the body weights of the control and emodin groups conti nued to increase,whereas the body weights of the EAE and EAE+emodin groups began to decrease.The body weights of the EAE+emodin group were between those of the control and EAE groups.There was a significant difference in body weights between the EAE+emodin and EAE groups from D4 onwards,suggesting that emodin can alleviate body weight loss in EAE rats.Both the EAE+emodin and EAE groups exhibited onset of disease symptoms on D9 and reached the peak of disease at D14,indicating that emodin did not delay the onset of EAE (Figure 1B).From D10,the neurobehavioral scores of the EAE+emodin group were decreased compared with those of the EAE group,implying that emodin alleviated neurobehavioral deficits in EAE rats.
Effects of emodin on histopathological changes in EAE rats
EAE rats were sacrificed on D14 (the peak of the disease) for HE and LFB staining to assess histopathological changes in the spinal cord (Figure 1CandD).HE staining of the lumbar enlargement of the spinal cord revealed clear inflammatory cell infiltration in EAE rats,while the degree of infiltration in the EAE+emodin group was markedly reduced (Figure 1CandE).LFB staining of the lumbar enlargement of the spinal cord showed that the degree of demyelination in the EAE+emodin group was markedly reduced compared with that in the EAE group (Figure 1DandF).Collectively,these results demonstrate that emodin alleviated inflammatory cell infiltration and demyelination in an EAE rat model.
Effects of emodin on inflammatory cytokine expression in the lumbar enlargements of EAE rats
To evaluate the effects of emodin on the degree of inflammation in EAE rats,we performed real-time PCR,ELISA,and NO detection;the results from these analyses are shown inFigure 2,respectively.We found a marked increase in the level of inflammation in EAE rats,while emodin markedly reduced the degree of inflammation.
Effects of emodin on microglial aggregation and activation in the lumbar enlargements of EAE rats
Iba-1 and CD68 levels in the lumbar enlargement of the spinal cord were assessed by immunohistochemistry.Iba-1 is mainly expressed in macrophages and reflects the degree of microglial aggregation.CD68 plays a role in the phagocytic activity of tissue macrophages and indicates the microglial M1 phenotype and degree of microglial activation (Brown and Neher,2010).We observed a clear increase in Iba-1 and CD68 levels in the EAE group compared with those in the control group,whereas treatment with emodin inhibited this increase (Figure 3AandB).
We next performed a series ofin vitroexperiments to further investigate the effect of emodin on microglial activation.The results of the cell counting kit-8 assay showed a concentration-dependent effect of emodin on BV2 cell viability: cell viability increased with treatment with up to 50 µM emodin and decreased at concentrations greater than 50 µM (Figure 3C).LDH analysis demonstrated that emodin (25 µM) markedly attenuated LDH acti vity in cell culture supernatants from BV2 cells induced with LPS+ATP (Figure 3D).In subsequent experiments,25 µM emodin was used as the standard treatment concentration.Follow-up ELISA and NO assays showed a marked decrease in the activation of pro-inflammatory factors (tumor necrosis factor (TNF)-α,and NO) in BV2 cells pretreated with emodin (Figure 3EandF) and a clear increase in the expression of anti -inflammatory cytokines (interleukin (IL)-10,and arginase-1) (Figure 3GandH).These findings suggest that emodin can inhibit microglial activation.
Effects of emodin on the NLRP3 signaling pathway in the lumbar enlargements of EAE rats
Our results suggested that emodin alleviates disease severity in EAE rats,potenti ally through the NLRP3 signaling pathway.Thus,western blotting was carried out to assess the level of NLRP3-related molecules in the lumbar enlargement of the spinal cord (Figure 4A).We observed a markedly higher level of related molecules in the EAE group than in control group,and treatment with emodin markedly reduced the expression of these molecules(Figure 4B–D).Next,we assessed the expression levels of IL -1β and IL-18,two key inflammatory cytokines that function as downstream effectors of the NLRP3 signaling pathway.Real-time PCR analysis showed that the mRNA levels of IL-1β and IL-18 in the lumbar enlargement were clearly higher in EAE rats than in the control group (Figure 4E).ELISA analysis yielded similar results at the protein level (Figure 4F).However,treatment with emodin markedly attenuated IL-1β and IL-18 expression levels (Figure 4EandF).As a control,we treated healthy rats with emodin and found that emodin did not markedly affect clinical signs,histopathology,the expression of inflammatory cytokines,microglial aggregation and activation,or the NLRP3 signaling pathway in healthy rats.
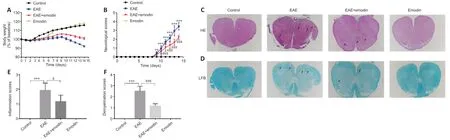
Figure 1|Emodin alleviates the clinical signs and degree of inflammation and demyelination of experimental autoimmune encephalomyelitis rats.
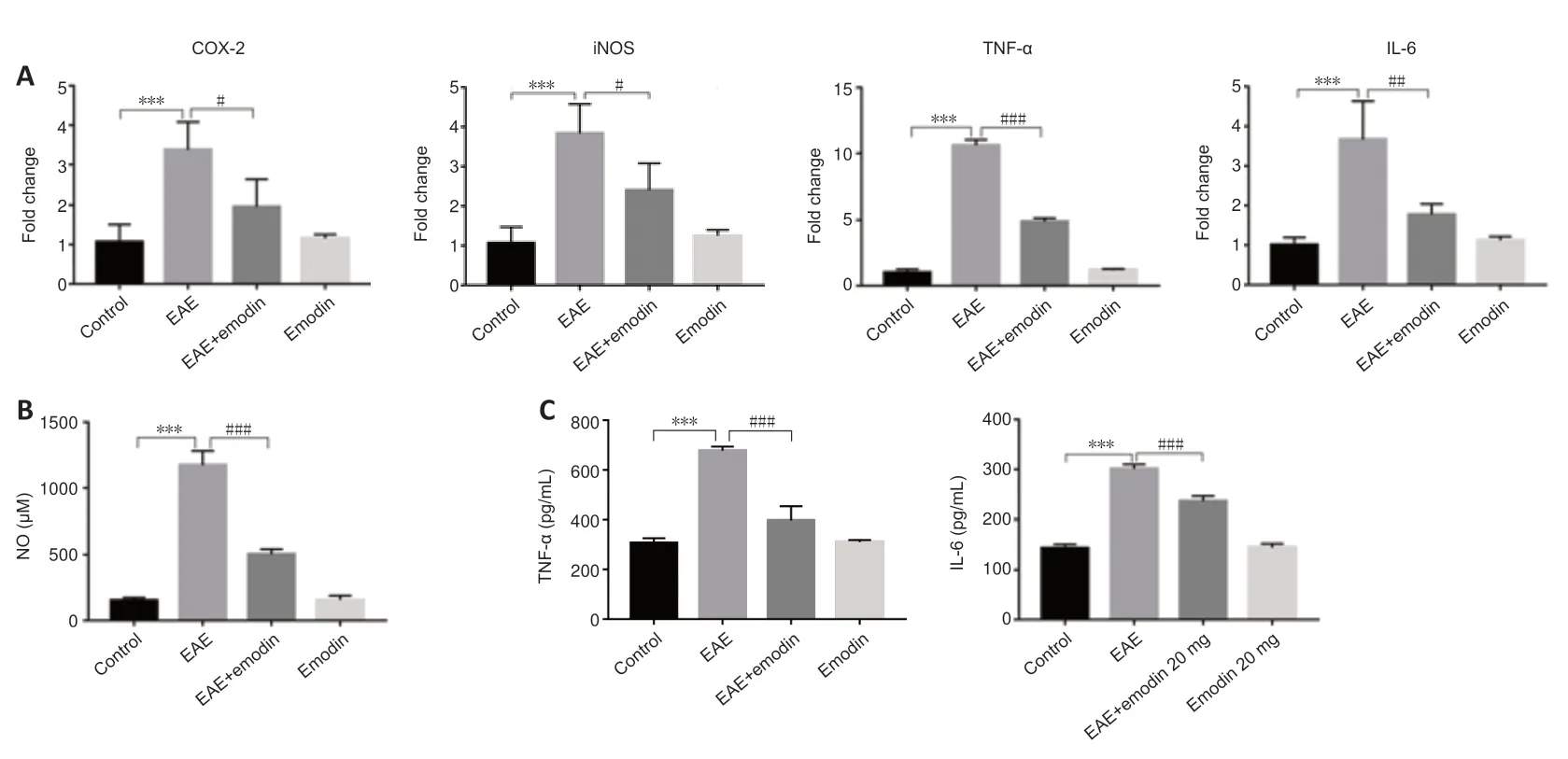
Figure 2|Emodin decreases inflammatory cytokine levels in the lumbar enlargements of experimental autoimmune encephalomyelitis rats.
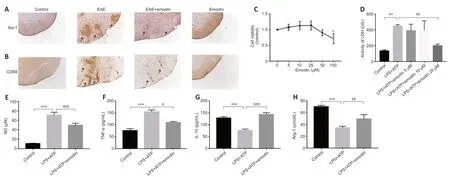
Figure 3|Emodin inhibits microglial aggregation and activation in the lumbar enlargements of experimental autoimmune encephalomyelitis rats and attenuates inflammation in BV2 cells.
Effects of emodin on pyroptosis in the lumbar enlargements of EAE rats
Pyroptosis is involved in EAE pathogenesis (Govindarajan et al.,2020).To assess the effects of emodin on pyroptosis in EAE rats,we performed LDH and western blotting assays.LDH acti vity was markedly higher in serum from EAE rats than that from controls,but was markedly lower in the EAE+emodin group than in the EAE group (Figure 5A),which suggests that emodin can alleviate the degree of cell damage.Western blotting detection of the level of the gasdermin D N-terminal fragment in the lumbar enlargement of the spinal cord yielded similar results (Figure 5B).Collectively,these results indicated that emodin reduced the degree of pyroptosis in EAE rats.Moreover,emodin did not markedly affect pyroptosis in healthy rats.
Effects of emodin on SIRT1/PGC-1α expression in the lumbar enlargements of EAE rats
SIRT1 and PGC-1α are associated with the NLRP3 inflammasome and participate in a variety of inflammatory diseases (Tang et al.,2017;Xia et al.,2021).We therefore detected SIRT1 and PGC-1α expression levels in the lumbar enlargement (Figure 5C).SIRT1 and PGC-1α levels were clearly lower in EAE rats than in controls,and markedly higher in the EAE+emodin group compared with those in the EAE group.However,SIRT1 expression in the lumbar enlargement of healthy rats treated with emodin was 1.3 times higher than in the control group.
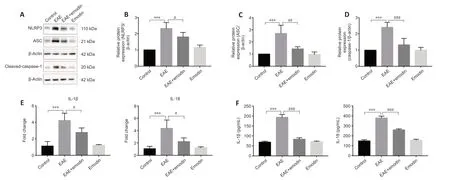
Figure 4|Emodin inhibits the expression of NLRP3 signaling pathway components in the lumbar enlargements of experimental autoimmune encephalomyelitis rats.
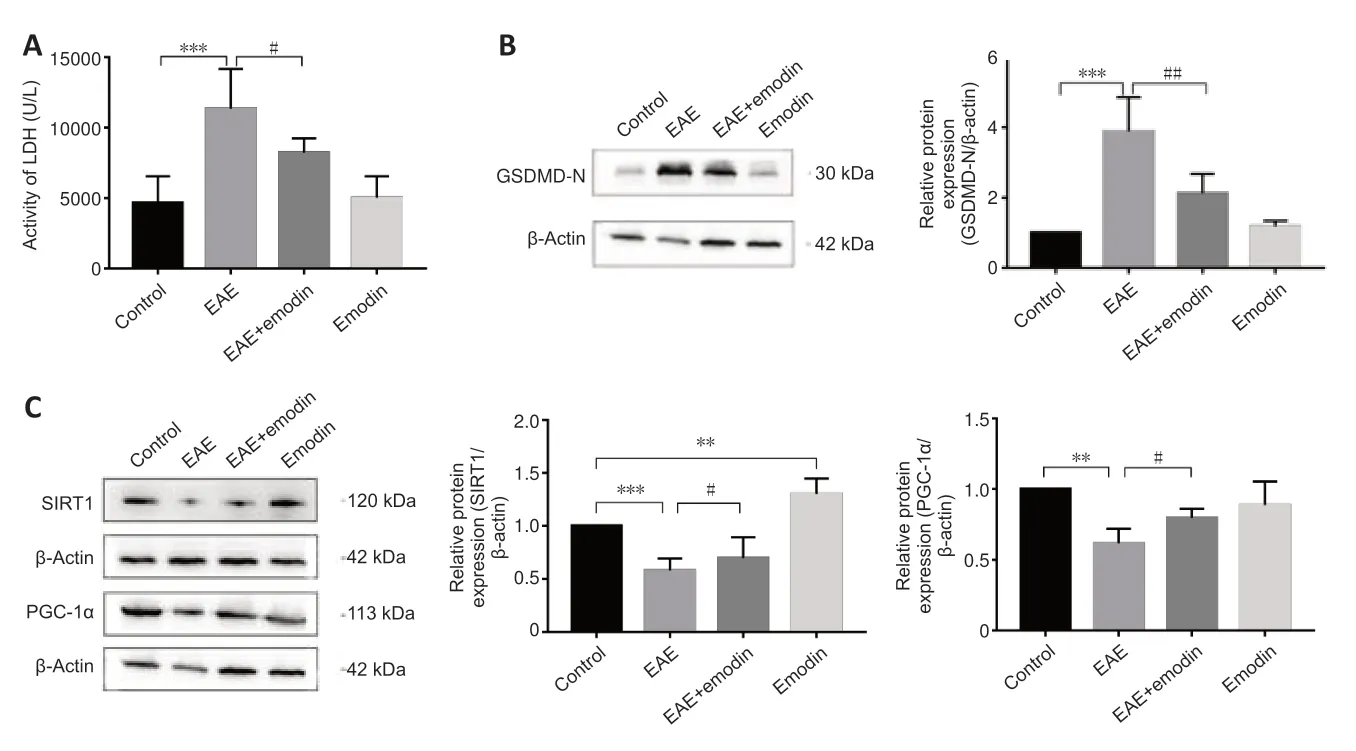
Figure 5|Emodin alleviates pyroptosis and SIRT1/PGC-1α expression in the lumbar enlargements of experimental autoimmune encephalomyelitis rats.
Discussion
In this study,we established an EAE rat model and investigated the effects of emodin on inflammation and demyelination,as well as the underlying molecular mechanism.The results demonstrated that emodin can alleviate the clinical signs and the degree of inflammation and demyelination in EAE rats.In addition,we found that the therapeutic effects of emodin in EAE rats may be related to the inhibition of NLRP3 inflammasome activation.Furthermore,SIRT1 and PGC-1α expression level were higher in the EAE+emodin group than in the EAE group,which suggests the possible involvement of SIRT1 and PGC-1α in the inhibition of NLRP3 inflammasome activation.Moreover,our findings revealed that emodin can inhibit microglial aggregation and activation in EAE rats and attenuate inflammation in BV2 cells,thus exerting neuroprotective effects.Taken together,our results demonstrate the therapeutic effects of emodin in EAE rats and suggest that emodin may be a promising drug for the clinical treatment of MS in the future.
MS is an autoimmune disease of the CNS that mainly affects young adults(Kobelt et al.,2017;Filippi et al.,2018).Emerging evidence has implicated several pathophysiological processes in MS,such as apoptosis (Mohamed et al.,2022),autophagy (Berglund et al.,2020),and pyroptosis (Govindarajan et al.,2020).Recent studies have described the role of NLRP3 inflammasome in the pathogenesis of MS and shown that overactivation of the NLRP3 inflammasome can aggravate disease severity (Walsh et al.,2014;Burm et al.,2016).Another study found that manoalide suppresses NLRP3 inflammasome activation and alleviates EAE symptoms (Li et al.,2022).Consistent with these studies,we found that the expression levels of NLRP3 signaling pathway components were higher in the EAE group than in the control group.These findings suggest that targeting the NLRP3 signaling pathway may be a potent therapeutic approach to treating MS patients.
Emodin is derived from a variety of natural plants such as rhubarb(Huang et al.,2020).Studies have demonstrated that emodin has various pharmacological effects,such as antioxidant,immunomodulatory,and anticancer effects (Zheng et al.,2021),involving autophagy,apoptosis,and pyroptosis (Gao et al.,2022;Liu et al.,2022b).Moreover,emodin has been shown to have neuroprotective effects in the CNS.For example,emodin was reported to alleviate hyperhomocysteinemia-induced dementi a (Zeng et al.,2019).In addition,emodin exerts neuroprotective effects in SH-SY5Y cells by alleviating synaptic damage and oxidative stress (Lai et al.,2020).To date,no studies have reported the clinical application of emodin in MS.A recent study found that emodin inhibited inflammation in a mouse model of EAE,thereby reducing symptoms in mice (Zheng et al.,2022a).Nevertheless,the underlying molecular mechanism is not clear,and the potential therapeutic effects of emodin in EAE rats have not been explored.Our study aimed to investigate the potenti al therapeutic benefit of emodin in EAE rats and the underlying molecular mechanism.The results demonstrated that EAE severity was alleviated after treated with emodin;furthermore,the expression levels of NLRP3 signaling pathway components were lower in the EAE+emodin group than in the EAE group.These inhibitory effects of emodin on NLRP3 signaling pathway are consistent with those seen in previous studies (Liu et al.,2022b;Shen et al.,2022).In addition,the expression levels of pro-inflammatory cytokines such as cyclooxygenase (COX)-2,TNF-α,and IL-6 were lower in the EAE+emodin group than in the EAE group.COX-2 is released by immune cells,its increased expression can,in part,indicate activation of immune cells and elevated levels of inflammation,and several studies have shown that it plays an important role in the inflammatory response (Barnett et al.,1994;Kim et al.,2005).It has been shown that activation of inflammasomes can lead to increased expression of COX-2 (Zhang et al.,2022).TNF-α and IL-6 are also released by immune cells,and an increase in their expression occurs as a consequence of immune cell activation and serves as an indication of elevated levels of inflammation.Inflammasome activation has been found to increase the expression levels of TNF-α and IL-6 (Latz et al.,2013);thus,these molecules are used as markers of altered inflammation levels following inflammasome activation.Similarly,the emodin-induced reduction in the levels of pro-inflammatory cytokines has been reported in other studies (Gao et al.,2021a).Moreover,NO can regulate many physiological processes,such as synaptic transmission and immune responses.However,many studies have found that high NO levels induced by inducible nitric oxide synthase (iNOS)are related to the pathogenesis of MS.NO and iNOS expression levels are elevated in EAE,and,given that NO is associated with blood-brain barrier disruption and oligodendrocyte damage,these pathological alterations all exacerbate EAE severity (Smith and Lassmann,2002).Several studies have reported the relationship between inflammasomes and iNOS and NO and suggested that inhibition of inflammasome activation can attenuate iNOS and NO levels (Liu et al.,2019;Hou et al.,2022).In our study,treatment with emodin restored iNOS and NO production,which is consistent with the effects of emodin treatment seen in other animal models of disease,supporting the therapeutic effects of emodin in EAE rats and the potential involvement of the NLRP3 signaling pathway (Song et al.,2019;Hu et al.,2021).Moreover,released inflammatory cytokines can promote the aggregation and activation of resting microglia,which in turn release inflammatory cytokines,creating a vicious cycle (Wu et al.,2021).Microglia are involved in the progression of MS.Studies have demonstrated that microglia can exert neuroprotective effects under normal circumstances (Yamasaki et al.,2014),but if activated,they can play a role in the pathogenesis of EAE through mechanisms such as inflammatory cytokine release,antigen presentation,and phagocytosis(Dendrou et al.,2015).In our study,we discovered that emodin treatment reduced microglial aggregation and activation in EAE rats and attenuated inflammation levels in BV2 cells,thus resulting in neuroprotective benefits,similar to previous studies (Kim et al.,2009;Park et al.,2016).These findings provide further insight into the pharmacological mechanism of emodin in the treatment of EAE.
SIRT1 is a nuclear and cytoplasmic sirtuin that deacetylates downstream PGC-1α and is involved in many cellular processes (Tang et al.,2017),such as oxidative stress,inflammation,and mitochondrial function (Wang et al.,2022b).Growing evidence has highlighted the protective effects of SIRT1 in several diseases,including those affecting the CNS.For instance,SIRT1 activation alleviates neurodegeneration in Alzheimer’s disease (Zhao et al.,2013),and SIRT1 overexpression is protective against EAE (Nimmagadda et al.,2013).Research has found that SIRT1 exerts its neuroprotective effects by reducing the expression of NLRP3 inflammasome components (Liu et al.,2021a;Xia et al.,2021).The classical activation pathway for NLRP3 is Tolllike receptor 4/NF-κB;NF-κB plays an important role in inflammation as a transcription factor that regulates the expression of NLRP3 inflammasome–related components.NF-κB can be regulated by different factors.Studies have found that SIRT1 can act as an upstream regulator of NF-κB acti vity (Gao et al.,2021b;Zheng et al.,2022b).We observed lower levels of SIRT1 and PGC-1α expression and higher levels of NLRP3 inflammasome component expression in EAE rats compared with those in rats in the EAE+emodin group.However,emodin treatment resulted in increased SIRT1 and PGC-1α levels and alleviation of symptoms in EAE rats.In addition,other studies have reported similar effects of emodin on SIRT1 and PGC-1α (Yang et al.,2016;Gao et al.,2020).These results suggest that emodin may exert neuroprotective effects in EAE by increasing SIRT1 and PGC-1α levels and inhibiting NLRP3 inflammasome activation.
This study had several limitations.Although the NLRP3 signaling pathway is crucial in MS pathogenesis,other signaling pathways are also involved;therefore,further in-depth research is necessary to clarify the role of other signaling pathways in MS.In addition,all rats were sacrificed at the peak of disease (D14),so we did not observe the long-term effects of emodin;this should be investigated in future experiments.
In this study,we established an EAE rat model by immune induction,resulting in inflammatory cell infiltration and demyelination in EAE rats.Our findings suggest that emodin alleviates inflammation and demyelination in an EAE rat model,possibly through the SIRT1/PGC-1α/NLRP3 signaling pathway and the microglia.We also demonstrated emodin-mediated inhibition of microglial activation in BV2 cells.In conclusion,our results show that emodin alleviates signs of disease in EAE rats,providing a sound experimental basis for the therapeutic use of emodin in the clinical treatment of MS patients.
Acknowledgments:We gratefully thank members of Feng’s lab for their intellectual input.
Author contributions:Study design: JF,YRC;experiments performance: YRC,ZQB;manuscript preparation: YRC;data collection and stati stical analysis:ZQB,LLY;literature search and collection: HYY;manuscript revision: JF.All authors have read and approved the final manuscript.
Conflicts of interest:The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potenti al conflict of interest.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:The two-dimensional structure of emodin.
Additional Figure 2:Schematic diagram of the in vivo experimental design.
Additional Figure 3:Schematic diagram of the in vitro experimental design.
Additional Table 1:The linear dynamic range of the standard curve for enzyme-linked immunoassay quantifications.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

