Transient neurogenesis in ischemic cortex from Sox2+astrocytes
Jia-Lei Yang,Hong Fan,Fan-Fan Fu,Bao-Lin GuoYing Huang,Li SunWen-Ting WangJun-Ling XingXin-Tian Hu,Yu-Qiang Ding,Kun Zhang,Ying-Zhou Hu,,Ya-Zhou Wang
Abstract The adult cortex has long been regarded as non-neurogenic.Whether injury can induce neurogenesis in the adult cortex is still controversial.Here,we report that focal ischemia stimulates a transient wave of local neurogenesis.Using 5′-bromo-2′-deoxyuridine labeling,we demonstrated a rapid generation of doublecortin-positive neuroblasts that died quickly in mouse cerebral cortex following ischemia.Nestin-CreER-based cell ablation and fate mapping showed a small contribution of neuroblasts by subventricular zone neural stem cells.Using a mini-photothrombotic ischemia mouse model and retrovirus expressing green fluorescent protein labeling,we observed maturation of locally generated new neurons.Furthermore,fate tracing analyses using PDGFRα-,GFAP-,and Sox2-CreER mice showed a transient wave of neuroblast generation in mild ischemic cortex and identified that Sox2-positive astrocytes were the major neurogenic cells in adult cortex.In addition,a similar upregulation of Sox2 and appearance of neuroblasts were observed in the focal ischemic cortex of Macaca mulatta.Our findings demonstrated a transient neurogenic response of Sox2-positive astrocytes in ischemic cortex,which suggests the possibility of inducing neuronal regeneration by amplifying this intrinsic response in the future.
Key Words:adult;astrocyte;cortex;fate-mapping;ischemia;local neurogenesis;neural stem cells;Sox2
Introduction
Once development ends,the neurogenic potenti al of the neocortex becomes latent.Because of the importance of the neocortex in brain function and its vulnerability to injuries (such as ischemia),extensive investigations have been conducted to seek and promote neurogenesis in adult cortex after injury (Kernie and Parent,2010;Ceanga et al.,2021).In the past decade,several studies have documented cortical neurogenesis under physiological and pathophysiological conditions,particularly following cerebral ischemia,whereas others have detected no neurogenesis in adult cortex (Jiang et al.,2001;Pekcec et al.,2006;Kernie and Parent,2010;Ohira et al.,2010;Vessal and Darian-Smith,2010;Ohira,2011;Huttner et al.,2014).Cortical neurogenesis,if indeed occurs,is minimal.Adult neural stem cells (aNSCs) in the subventricular zone (SVZ) respond to cerebral ischemia,so it is generally thought that the observed cortical neurogenesis is mainly derived from SVZ aNSCs (Li et al.,2010).
Some studies have indicated that there may exist potential neurogenic cells in the cortex,such as GABAnergic progenitors (Ohira et al.,2010),oligodendrocyte progenitors (Guo et al.,2010) and reactive astrocytes (Buffo et al.,2008).In particular,reactive astrocytes have been speculated to be neurogenic because (1) SVZ aNSCs and hippocampal aNSCs are astrocyte-like cells,and (2) reactive astrocytes can upregulate neural progenitor markers,form neurospheres,and are multi -potentin vitro(Robel et al.,2011;Shimada et al.,2012;Gotz et al.,2015).Recent studies have reported that in the striatum,where postischemic neurogenesis is thought to be solely derived from SVZ aNSCs,local astrocytes also give rise to neurons (Luzzati et al.,2014;Magnusson et al.,2014).These observations indicate the possibility of cortical neurogenesis from local astrocytes.However,in vivoneurogenic evidence is sti ll lacking.
Recently,Chunli Zhang’s group reported that a subpopulation of cortical astrocytes maintains a low level of Sox2 expression (Chen et al.,2019).Our previous study revealed that Sox2-positive glia in dorsal root ganglion give rise to nociceptive neurons under chronic pain (Zhang et al.,2019).In the present study,using 5′-bromo-2′-deoxyuridine (BrdU) labeling,retrovirus expressing green fluorescent protein (GFP;Retro-GFP) labeling and Cre-mediated cell fate tracing,we demonstrated transient neurogenesis in adult mouse cortex after ischemia and further explored the neurogenic potenti al of Sox2-positive astrocytes.
Methods
Study design
The experimental design and protocol is shown inFigure 1.

Figure 1|The experimental design.
Mice
Adult C57BL/6J male mice (body weight 20–23 g,8–10 weeks old) were used in our study.All mice were specific pathogen-free and housed under a 12-hour light/dark cycle and controlled temperature (22–24°C) with free access to water and standard rodent chow.Only male mice were used.The ROSA26-YFP(strain B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J,stock number 006148,RRID:IMSR_JAX:006148),ROSA26-DTA (strain B6;129-Gt(ROSA)26Sortm1(DTA)Mrc/J,stock number 010527,RRID: IMSR_JAX:010527),Sox2-CreER(strain B6;129SSox2tm1(cre/ERT2)Hoch/J,stock number 017593,RRID: IMSR_JAX:017593),PDGFRα-CreER(strain B6.129S-Pdgfratm1.1(cre/ERT2)Blh/J,stock number 032770,RRID: IMSR_JAX:032770),GFAP-CreER(strain B6.Cg-Tg(GFAP-cre/ERT2)505Fmv/J,stock number 012849,RRID: IMSR_JAX:012849),Nestin-CreER(strain C57BL/6-Tg(Nes-cre/ERT2)KEisc/J,stock number 016261,RRID:IMSR_JAX:016261) and Glast-CreERmice (strain Tg(Slc1a3-cre/ERT)1Nat/J,stock number 012586,RRID: IMSR_JAX:012586) were purchased from the Jackson Laboratory (Farmington,CA,USA).Different strains were kept in separate cages.Three to four mice were used for each experiment.All animal experiments were performed according to protocols approved by the Animal Care and Use Committees of Fourth Military Medical University (approval No.20211024,approval date: October 15,2021).All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines (Percie du Sert et al.,2020).
Routine photothrombotic ischemia (focal cerebral ischemia)
The routine photothrombotic ischemia model in mice was established as previously described (Lee et al.,2007).A total of 60 mice were anestheti zed by intraperitoneal injection of 2% pentobarbital sodium (Cat#P5178,MilliporeSigma,Burlington,MA,USA) at a dose of 50 mg/kg.Rose bengal(Cat#330000,MilliporeSigma) was injected into the tail vein at a dose of 25 mg/kg to induce photothrombotic ischemia.A cranial window was carefully made,ranging from 0.8 to 2 mm posterior to bregma and 2 mm lateral of the midline,which is the location of the somatosensory cortex according to a brain atlas (Paxinos and Franklin,2019).The cranial window was illuminated for 10–12 minutes using a FL1500 LCD cold light source (Zeiss,Oberkochen,Germany).
Mini-photothrombotic ischemia in mice
For Retro-GFP labeling and electrophysiological study,a mini-photothrombotic model was adopted to induce mild ischemic injury.Rose bengal was injected intraperitoneally at a dose of 100 mg/kg.Thirty minutes post-injection,the right cortex at 0.9–2 mm anterior to bregma and 1–1.5 mm lateral of the midline was illuminated with the skull closed for 15 minutes by a nonintrusive optical fiber connected to a laser light source for optogenetics (wavelength:473 nm,diameter of optical fiber: 200 µm) (RWD,Shenzhen,Guangdong Province,China) to induce cortical ischemia.
Mouse treatment
Daily BrdU (MilliporeSigma) intraperitoneal injection at a dose of 100 mg/kg was performed 12 hours post-ischemia for 6 consecutive days.To detect neuroblasts in the cortex,mice were sacrificed at different days post-ischemia(dpi) by intraperitoneal injection of 2% pentobarbital sodium (MilliporeSigma,Cat# P5178) at a dose of 50 mg/kg.Tamoxifen (TAM) was purchased from MilliporeSigma (Cat# T5648),dissolved in corn oil (MilliporeSigma,Cat# C8267) at a concentration of 10 mg/mL and stored at 4°C.TAM was intraperitoneally injected for genetic progenitor ablation and fate mapping at a daily dose of 20 mg/kg.The mice were given TAM treatment for 5 to 6 consecutive days (Additional Figure 1).The same volume of corn oil without TAM was injected in the control group.Retrovirus expressing GFP (Retro-GFP),lentivirus expressing DIO-EGFP,and adeno-associated virus expressing GFAP-Cre were purchased from OBiO Technology (Shanghai,China).For DIO-EGFP-based cell fate mapping,lentivirus expressing DIO-EGFP was stereotactically injected into the right somatosensory cortex (coordinates from bregma: anterior-posterior,–1.0 mm;medial-lateral,+1.0 mm;dorsal-ventral,+1.0 mm) of Sox2-CreERor Glast-CreERmice 8 days before TAM administration.TAM was injected once per day for 4 to 5 successive days.The photothrombotic ischemia model was carried out 7 days after the last TAM injection.
Monkey focal ischemia model
A healthy male rhesus macaque (Macaca mulatta) aged 19 years was maintained under a 12/12-hour light/dark cycle,controlled temperature(22–24°C),and relative humidity (30–50%).The monkey was purchased from the Kunming Primate Research Center,Chinese Academy of Sciences (Yunnan,China;license No.SYXK (Dian) K2017-0008).The monkey was anesthetized by intramuscular injection of ketamine (20–25 mg/kg,Parchem,NY,USA)for induction and 1.5% pentobarbital sodium (30 mg/kg) during surgery.To induce ischemia,endothelin (5 µg/µL;MilliporeSigma,Cat# E7764) was injected into three points (5 mm from the midline,5 µL per point) in the right motor cortex (4 mm in depth) at 5 mm apart according toMacaca mulattabrain maps (Moirano et al.,2019).Using a 3.0 Tesla magnetic resonance scanner (Shanghai United Imaging Intelligence Co.,Shanghai,China),magnetic resonance imaging (MRI) T1-and T2-weighted sequences were performed to observe the ischemic cortex as previously described (van den Brink et al.,2018).TheMacaca mulattawas perfused with 4% paraformaldehyde phosphate buffer (PFA;Cat#P0099,Beyotime,Shanghai,China) through the heart at 24 hours posti -schemia.The brain was then removed and the tissue was fixed with 4% PFA for around 2 hours.Frozen coronal sections (20 µm thick) were prepared using a cryostat (Leica,CM1950,Germany).Procedures of theMacaca mulattaexperiments were performed under the approval of the Animal Care and Use Committee of Kunming Institute of Zoology,Chinese Academy of Sciences (approval No.IACUC19009,approval date: August 8,2019).
Electrophysiological recording
To trace actively proliferating cells in mice,Retro-GFP virus was injected into the ischemic region of the somatosensory cortex at 3 dpi.Patch-clamp recording was conducted at 21–24 dpi.Biocytin labeling was used to identi fy the recorded cells when performing electrophysiological recordings.The patch-clamp electrode was filled with potassium-based intrapipette solution and the resistance was set to approximately 7–9 MΩ.Pipette offset current was turned to zero immediately before contact of the cell membrane.pCLAMP 9 soft ware and DigiData 1322 (Axon Instruments,Burlingame,CA,USA) were used for recording.For current-clamp recordings,the data were sampled at 20 kHz.For voltage-clamp recording,the data were sampled at 10 kHz.Tetrodotoxin (1 µM;MilliporeSigma,Cat# 203732) was added to block action potenti als.
Immunohistochemistry
Mice were sacrificed at each time point and perfused intracardially with 4%PFA.The brain tissue was postfixed with 4% PFA for approximately 2 hours at 4°C.Then,the tissue was cryoprotected with 25% sucrose (MilliporeSigma,Cat# S0389) and frozen in Tissue-Tek® O.C.T.Compound (Sakura,Torrance,CA,USA,Cat# 4583).Frozen serial coronal sections (12 µm thick) were prepared with a cryostat (Leica CM1950).Primary anti bodies were incubated at room temperature overnight.Detailed antibody information was shown inAdditional Table 1.For BrdU staining,HCl treatment (30–35 minutes at 37°C) was performed to expose DNA in the proliferating cell before adding BrdU primary anti bodies.Secondary anti bodies conjugated with Alexa Fluor 594 (1:800;donkey anti -rabbit,Cat# 715-585-150,RRID: AB_2 340621;anti -mouse,Cat# 705-585-003,RRID: AB_2 340854;anti-guinea pig,Cat#706-585-148,RRID: AB_2 340474;anti-goat IgG,Cat# 711-585-152,RRID:AB_2 340432;Jackson ImmunoResearch,West Grove,PA,USA),Alexa Fluor 488 (donkey anti -mouse,Cat# 711-545-152,RRID: AB_2 338845;anti -rabbit,1:800,Cat# 115-545-062,RRID: AB_2 313584;anti -goat,Cat# 705-545-003,RRID: AB_2 340428;Jackson ImmunoResearch),and Alexa Fluor 647 (donkey anti-rabbit IgG,1:800,Jackson ImmunoResearch,Cat# 711-605-152,RRID:AB_2492288) were incubated for 3 hours at room temperature.
To perform TUNEL staining,DeadENDTMTUNEL system (Cat#G3250,Promega,Madison,WI,USA) was used according to the product manual.For the combined staining of TUNEL with doublecortin (DCX),TUNEL staining was performed before DCX immunostaining.
Images were captured using a confocal microscope (FV1000,Olympus,Tokyo,Japan).Cell counting was carried out by an investigator blinded to the group assignments.The ischemic region was defined as the infarct area with marked morphological changes compared with the contralateral cortex.The number of cells in six randomly selected coronal sections from the entire ischemic region was counted in one mouse,and cells were counted for at least three mice from each group.The percentages of specific marker-positive cells were calculated as (specific marker– positive cell count/total cell count) × 100%.
Western blotting
Cortical samples including the lesion site and 1–2 mm of the surrounding tissue were dissected out.Each sample was homogenized in radioimmunoprecipitation assay (RIPA) buffer (Beyotime,Cat# P0013B) for about 20 min,allowed to stand for 40 minutes on ice,then centrifuged at 12,000 ×gat 4°C.The protein samples were then boiled and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis.Next,the proteins were electrotransferred to a polyvinylidene difluoride membrane that was incubated with anti-Nestin antibody (as described inAdditional Table 1)overnight at 4°C,then incubated with HRP-conjugated anti-goat antibody(1:5000;Bioss,Woburn,MA,USA,Cat# bs-0294R,RRID: AB_10855262) at room temperature for 50 minutes.The bands were visualized using an ECL kit (MilliporeSigma,Cat# WBULS0100).β-actin (1:5000;MilliporeSigma,Cat#A5441;RRID: AB_476744) was used as an internal control.All blots and gels were derived from the same experiment and were processed in parallel.
Stati stical analysis
GraphPad Prism (version 7.01,GraphPad,San Diego,CA,USA) was used for stati stical analysis.Data were presented as the mean ± SEM.The stati stical differences between groups were analyzed using the Student’st-test when comparing two groups,or by one-way analysis of variance (ANOVA) with Student-Newman-Keulspost hocanalysis when comparing three or more groups.P<0.05 was considered stati stically significant.
Results
Focal ischemia induces transient neuroblast generation in mouse cerebral cortex
We induced focal ischemia in the somatosensory cortex through photothrombosis.TUNEL staining confirmed the limited injury within cortex(Additional Figure 2A).In the intact cortex,there was no expression of nestin and approximately one-third of astrocytes expressed Sox2 (Figure 2A,left panel).After ischemia,the expression of both Sox2 and nestin was upregulated in the ischemic region (Figure 2A,right panel andFigure 2B).In the area surrounding the ischemic core (200 µm from the lesion site),approximately 86.7 ± 4.6% and 68.2 ± 6.3% of GFAP-positive astrocytes expressed Sox2 and nestin,respectively (Figure 2B,left panel).Approximately 80% of Sox2-positive cells expressed nestin,and almost all of the nestinpositive cells expressed Sox2 (Figure 2B,right panel).In addition,most of the Sox2-positive cells expressed Pax6 (neural progenitor marker) in this region (Additional Figure 2B).These data indicated that reactive astrocytes,particularly Sox2-positive ones may acquire some properties of neural progenitor cells in ischemic cortex.
We then examined whether focal ischemia leads to the appearance of neuroblasts in the cortex.At 7 dpi,intermediate neural progenitor marker Tbr2 was expressed in some nestin-positive cells and was also colocalized with BrdU in some cells (Figure 2C).Furthermore,neuroblast marker DCX was also expressed in the boundary between the infarct area and the surrounding normal tissue.DCX was coexpressed with neuronal markers polysialylated neuronal cell adhesion molecule (PSA-NCAM) and Tuj-1 (Figure 2C).These data indicated the induction of neuroblasts by ischemia in the cortex.
We next examined if these neuroblasts were newly born BrdU/DCX doublepositive cells and if they were detected at both 7 and 14 dpi.The BrdU/DCX cell number decreased significantly at 14 dpicompared with that at 7 dpi (P<0.01;Figure 2D).Considering that DNA repairing or DNA damaged cells may also uptake BrdU (Zheng et al.,2011),we double-stained DCX with γ-H2AX(marker of DNA damage) and APEX-1 (marker of DNA repair) in the ischemic region.Cells expressing γ-H2AX were detected in irradiated cerebellum(positive control,Additional Figure 2C),but no γ-H2AX-positive cells were observed in the ischemic cortex (Figure 2E).APEX-1 was expressed in the ischemic cortex,but no DCX/APEX-1 colabeled cells were detected (Figure 2E).These results indicated that the presence of BrdU/DCX double-positive cells was not the result of DNA damage or repair-induced BrdU incorporation.To test if the decrease of DCX-positive cells was due to cell death,we performed TUNEL staining.There was a significant increase of TUNEL/DCX colabeled cells in the ischemic cortex at 10 dpicompared with that at 7 dpi (P<0.01;Figure 2FandG),indicating this reduction was caused by secondary cell death.To explore if there were BrdU-labeled mature neurons,we examined ischemic cortex at 30 dpi.Only one BrdU/NeuN-positive cell was found in all of the cortical sections of three mice (data not shown).This is consistent with the commonly accepted view that there is no neurogenesis in ischemic cortex (Huttner et al.,2014).Nevertheless,our results indicated that a transient wave of neuroblast generation did occur in the mouse ischemic cortex.
SVZ aNSCs contribute to a small proportion of the neuroblasts in the early phase
To investigate the contribution of SVZ aNSCs to the ischemia-induced neuroblasts,we used Nestin-CreER:ROSA26-DTA mice,in which TAM treatment induced the expression of diphtheria toxin in nestin-positive cells to lead to cell death (Imayoshi et al.,2008).To test the effects of depleting SVZ aNSCs on cortical neurogenesis,TAM was injected 7 days before ischemia.Depletion of nestin was confirmed by western blots (Additional Figure 1).DCX-positive cells were almost completely eliminated in the SVZ of TAM-treated Nestin-CreER:ROSA26-DTA mice at 7 dpi.However,there were still DCX/BrdUcolabeled cells present in ischemic cortex (Figure 3AandB).These data indicated that there might be other cells that give rise to neuroblasts besides SVZ aNSCs.
Given that reactive astrocytes also express high levels of nestin,we compared the effects of depleting SVZ aNSCs (injecting TAM before injury) and the effects of depleting both SVZ aNSCs and nestin-positive reactive astrocytes(injecting TAM after injury) on cortical neuroblast generation.The number of BrdU/DCX-colabeled cells was significantly reduced in the cortex of Nestin-CreER:ROSA26-DTA mice treated with TAM after injury compared with that in mice treated with TAM before ischemia (P<0.05;Figure 3C).
To further assess the contribution of SVZ aNSCs to new cortical neuroblasts,cell fate tracing was carried out using Nestin-CreER:ROSA26-YFP mice.TAM was injected before injury to label SVZ aNSCs-derived cells,or after injury to label both SVZ aNSCs and reactive astrocyte-derived cells.Less than 20%of cortical BrdU/DCX-colabeled cells were YFP-positive in mice treated with TAM before injury.Approximately 60% of cortical BrdU/DCX-colabeled cells were YFP-positive in mice treated with TAM after injury (Figure 3D).These data indicated that,with regard to the neuroblast generation after cerebral ischemia in the early phase,SVZ aNSC-derived cells may account for a small portion of new neuroblasts in the cortex.
Mild ischemia allows maturation of local generated neuroblasts
We next explored whether newborn neuroblasts differenti ate into functional neurons.This is challenging to investigate in standard ischemic models(photothrombosis and middle cerebral artery occlusion) because (1) the number of new neuroblasts is very small in the late phase (3 weeks or later post-ischemia),and (2) the severely damaged cortex makes it technically difficult to perform successful patch-clamp recording.We adopted a modified mini-photothrombosis model,which induced consistent mild focal cortical ischemia using a nonintrusive optical fiber without opening the skull (Additional Figure 3).We then injected Retro-GFP at 3 dpi,performed double-immunostaining at 8,11 and 28 dpi and patch-clamping at 28–30 days after retrovirus injection (Figure 4A).At 8 and 11 dpi,Retro-GFP-labeled DCXpositive cells with bipolar or multi -processes could be observed (Figure 4B).At 28 dpi,Retro-GFP/MAP2 and Retro-GFP/NeuN-positive cells with typical neuronal morphology could be detected (Figure 4C).
During patch-clamp recordings,biocytin was injected to confirm the recording of GFP-positive cells (Figure 4D).Of 13 successfully patched neuron-like Retro-GFP cells,11 cells fired action potentials and seven cells exhibited spontaneous excitatory postsynaptic currents (Figure 4EandF).The average input resistance and resting membrane potenti al of these cells were (270.8± 56.3) MΩ and (−59.7 ± 4.6) mV,respectively.Taken together,these data indicated that local newborn neuroblasts differentiated into functional neurons under the mild ischemic condition.
Sox2-positive astrocytes are the major potenti al neurogenic cells
We next investigated the local cellular origin of these neuroblasts.Previous studies have reported that some oligodendrocyte progenitors express DCX under ischemic conditions (Diaz et al.,2013).We examined if DCX-positive cells expressed oligodendrocyte markers.Double-immunostaining showed that approximately 13% of DCX-positive cells expressed NG2 and 14%expressed Olig2 (Figure 5AandB).To trace if oligodendrocyte precursor cells could give birth to DCX-positive cells,we injected TAM into PDGFRα-CreER:ROSA26-YFP mice before injury to label resident oligodendrocytes progenitor cells (Figure 5C).In ischemic cortex,approximately 21% of DCXpositive cells were labeled by YFP at 7 dpi.Most of these YFP/DCX-colabeled cells expressed NG2 (Figure 5C).However,none of these YFP/DCX cells expressed Tuj-1 or PSA-NCAM (data not shown).These results demonstrated that PDGFRα-expressing oligodendrocyte precursor cells contributed to a small portion of DCX-expressing cells,but these cells retained the identi ty of oligodendrocyte lineage.
To investigate whether local reactive astrocytes contributed to neuroblast generation,we injected adeno-associated virus expressing GFAP-Cre into cortex of ROSA-YFP mice.Three weeks later,focal ischemia was established and the fate of YFP-labeled cells analyzed.At 5–7 dpi,YFP-labeled Pax6-,Tbr2-and DCX-expressing cells were observed,indicating the generation of neuroblasts (Figure 6A).To confirm this phenomenon,we injected lentivirus carrying a DIO-EGFP cassette into the cortex of GFAP-CreERmice,and administered TAM to label virus-infected cells.At 7 dpi,DIO-EGFP/DCXpositive cells were detected in ischemic cortex (Figure 6B),indicating that local reactive astrocytes generated neuroblasts after cerebral ischemia.
To further investigate the identi ty of the “neurogenic” astrocytes in cortex,we focused on Sox2-positive astrocytes because Sox2,which is expressed by a subpopulation of astrocytes,can be upregulated after ischemia and Sox2 overexpression is sufficient to reprogram astrocytes into neuroblasts(Niu et al.,2013,2015).We tested the neurogenic potenti al of Sox2-positive astrocytes by fate mapping of Sox2-CreER:ROSA26-YFP mice.The mice were first irradiated by 15 Gy60Co to eliminate SVZ Sox2-positive aNSCs,and then treated with TAM before injury to label resident Sox2-positive astrocytes(Figure 7AandB).In the contralateral cortex,over 90% of YFP-labeled cells were GFAP-positive and exhibited typical astrocytic morphology.In the ischemic region,most YFP-labeled cells showed dramatic morphological changes,becoming bipolar and extending longer processes (Figure 7C).In addition to YFP/GFAP-positive cells,YFP/NG2-and YFP/DCX-positive cells were observed in the peri-infarction area (Figure 7C),suggesting the plasticity of Sox2-positive astrocytes after ischemia.Approximately 75.7% ± 1.6% of the total population of cortical DCX-positive cells was labeled by YFP.To further analyze the neurogenic potential of local Sox2-positive cells,DIOEGFP lenti virus was injected into the cortex of Sox2-CreERmice to trace the fate of cortical Sox2-positive astrocytes (Figure 7D).At 7 dpi,Pax6/EGFP-,Tbr2/EGFP-,and DCX/EGFP-colabeled cells were detected in the ischemic cortex (Figure 7D).These data indicated that Sox2-positive astrocytes were the major contributor of neuroblasts in ischemic cortex.Finally,we tested if this ischemia-induced plasticity change could be observed in other species.We performed the focal ischemia inMacaca mulattacortex by injecting endothelin.Successful ischemia modeling was confirmed by MRI (Figure 8A).In normal cortex,approximately 40% of GFAP-positive cells expressed Sox2.This percentage increased to 60% in the ischemic region at 10 dpi (Figure 8B).Further analysis revealed sparsely distributed DCX/Tuj-1-and DCX/PSA-NCAM-colabeled cells in the ischemic cortex (Figure 8C).
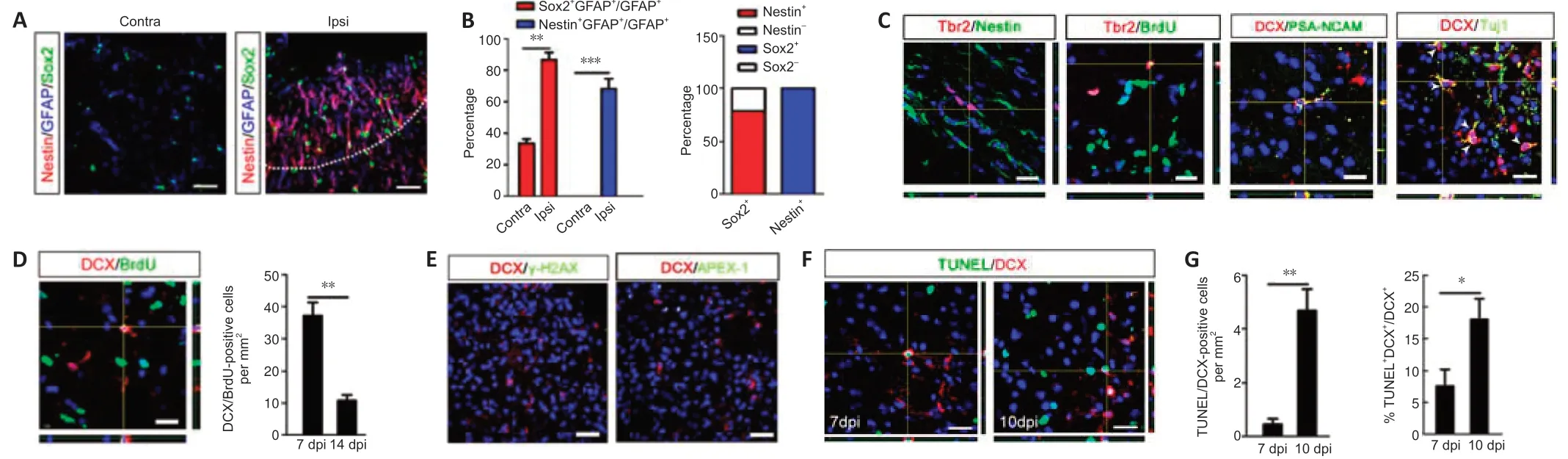
Figure 2|Transient neuroblast generation in adult cortex after focal ischemia.
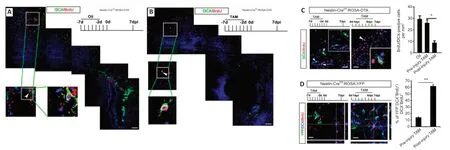
Figure 3|Contribution of SVZ aNSCs to neurogenesis in adult cortex.
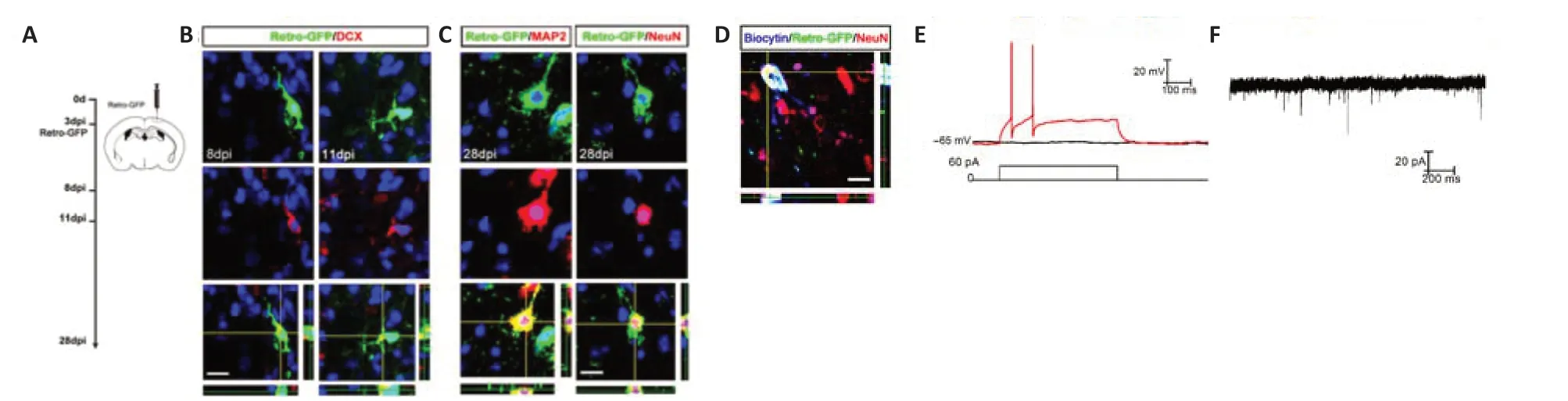
Figure 4|Neuronal marker expression and electrophysiology of Retro-GFP-labeled cells.
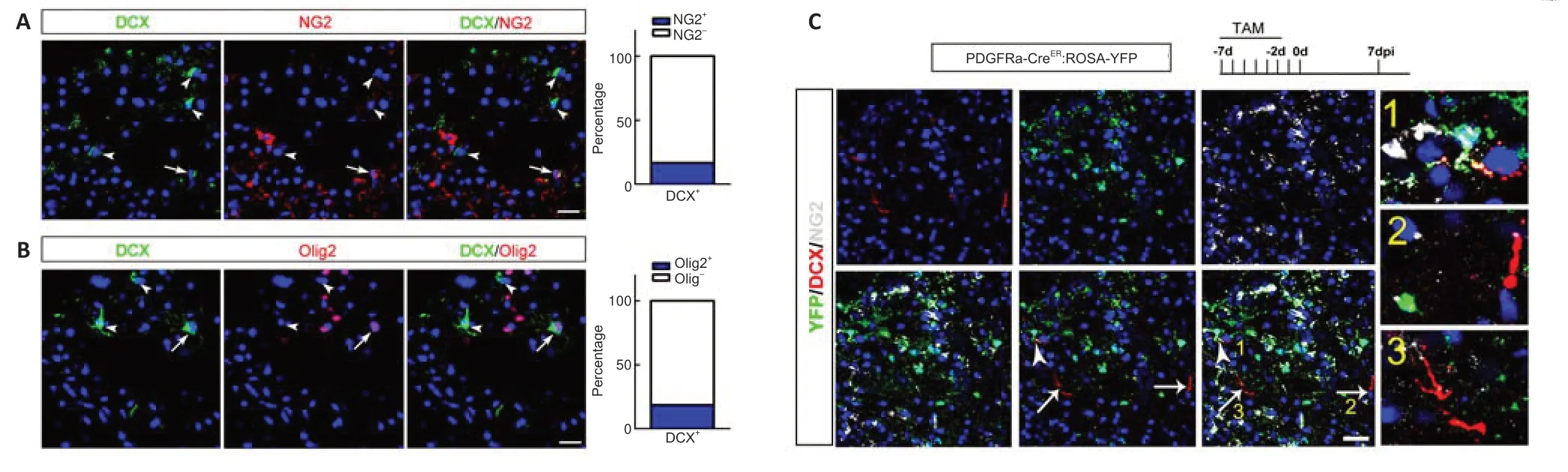
Figure 5|Contribution of oligodendrocyte precursor cells to DCX-positive cells.
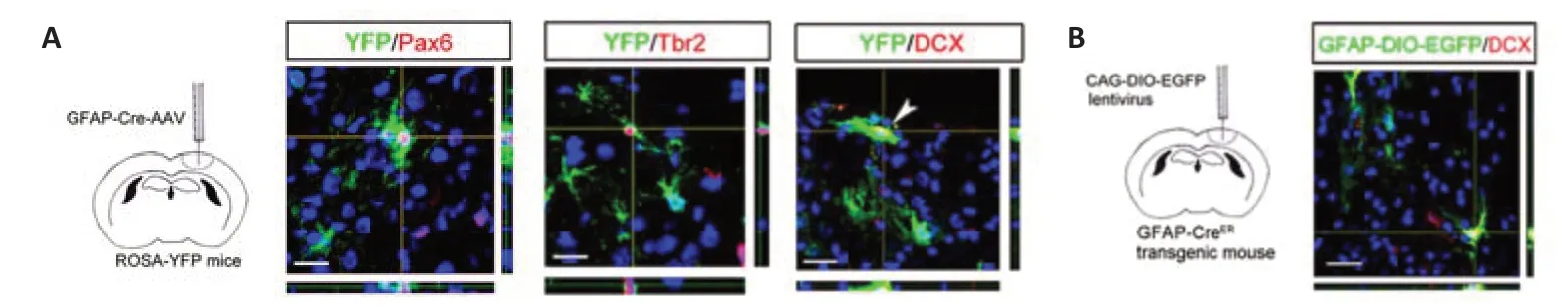
Figure 6|Fate mapping of astrocytes.
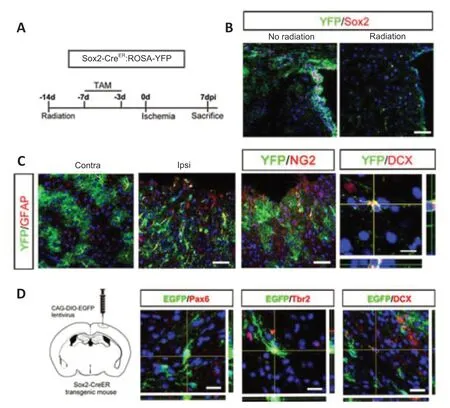
Figure 7|Neurogenic response of Sox2-positive astrocytes.
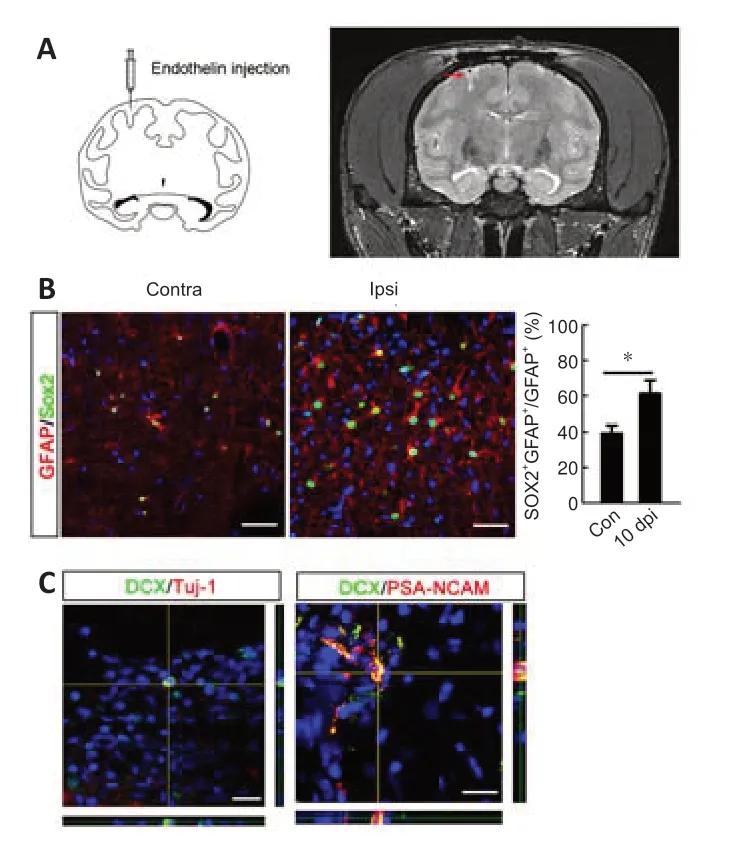
Figure 8|Response of Sox2-positive astrocytes and appearance of neuroblasts in the ischemic cortex of Macaca mulatta.
Discussion
Although the topic has been investigated for years,whetherin situneurogenesis occurs in adult cortex under certain pathological circumstances remains controversial.Here,we reported a transient wave of neuroblast generation in cerebral cortex after focal ischemia and identified Sox2-positive astrocytes as the potenti al source of these neuroblasts.
The major concerns for neurogenesis include whether newly detected DCXpositive cells post-ischemia are truly neuroblasts and whether there are new mature neurons.The expression of DCX in Olig2-positive cells has been reported in a previous study (Diaz et al.,2013),so we analyzed the doublestaining of DCX with both oligodendrocyte markers and neuroblast markers.Our results demonstrated that only a small portion of DCX-expressing cells were colabeled with oligodendrocyte markers Olig2 and NG2.In contrast,DCX coexpressed well with neuroblast markers PSA-NCAM and Tuj-1.These data were consistent with a previous report that identified two populations of DCX-expressing cells in ischemic cortex (Kunze et al.,2015),and indicated that the majority of the DCX-positive cells we observed were neuroblasts.Our finding that DCX-positive cells die quickly may reflect the harsh microenvironment in the ischemic cortex,and this may account for the very weak or absence of neurogenesis at 14 dpi or later as reported by previous studies (Jiang et al.,2001;Parent et al.,2002;Pekcec et al.,2006;Vessal and Darian-Smith,2010;Ohira,2011;Huttner et al.,2014).By developing a modified mini-photothrombosis model,which induces reliable ischemia without opening the skull (Additional Figure 3) and thereby enhances the integrity of the lesion cortex and the survival of retrovirus-labeled cells,we analyzed the electrophysiological properties of Retro-GFP-labeled cells at 1 month post-ischemia.The results that Retro-GFP-labeled cells fire action potenti als indicated that mild ischemia may allow for the survival of newborn neuroblasts.In clinical work,focal ischemia is a high risk factor of seizure development (Castro-Apolo et al.,2018).Our previous study demonstrated a possible role of reactive astrocytes in posti schemic seizures (Yang et al.,2016).It is possible that new neuroblasts that survive and differenti ate into neurons are also involved in this process.
To elucidate the origin of these newborn neuroblasts in ischemic cortex,we first analyzed the contribution of SVZ aNSCs to DCX-expressing cells.Although a previous study reported that neurosphere-forming reactive astrocytes in the infarct region are mainly derived from SVZ (Faiz et al.,2015),the neurogenic ability of local astrocytes remains untested.Our nestin-Cre-based cell depletion and fate tracing studies showed that cortical nestin-positive reactive astrocytes contributed to a larger portion of DCX-positive cells than did SVZ aNSCs in the first 7 days after ischemia.The retrovirus labeling of DCXpositive cells added evidence for the de novo generation of neuroblasts.Fate mappings using GFAP-Cre,Sox2-Cre and PDGFRα-Cre mice provided further evidence for the cellular origin of the two populations of DCX-positive cells.Most DCX-positive cells were derived from Sox2-positive astrocytes and a few were from oligodendrocyte lineage cells.Considering that quiescent aNSCs in the SVZ are GFAP+Sox2+nestin–,rarely form neurospheresin vitro,and express nestin only upon activation (Codega et al.,2014;Mich et al.,2014),it is possible that Sox2-positive astrocytes are the potenti al quiescent neurogenic cells in adult cortex,which are transiently activated by ischemia.Our recent study in dorsal root ganglion demonstrated similar neurogenic potential of Sox2-positive satellite cells under chronic pain (Zhang et al.,2019).How the neurogenic potenti al of Sox2-positive astrocytes can be used is of great interest and is worth further investigation.
Limitations
This study was limited by the lack of detailed analysis of newborn neurons (for example,determination of the type of new-born neurons),as well as the lack of a detailed time-course study of neurogenesis.In addition,the mechanism by which the observed neurogenesis occurs should be fully investigated in the future.
Contribution to the field
Adult cerebral cortex is usually regarded as non-neurogenic.Whether ischemia can induce local neurogenesis remains controversial.By adopting a modified mini-ischemic model and using retrovirus labeling and genetic cell fate tracing,we demonstrated a transient wave of neuroblast generation in mild ischemic cortex and identified Sox2-positive astrocytes as the potenti al neurogenic cells.Our data indicate the possibility of in situ neurogenesis in adult cortex and suggest that stimulating endogenous regeneration may be feasible in the future.
Acknowledgments:The authors thank Prof.Xu Lin (Kunming Institute of Zoology,Chinese Academy of Sciences) for his technological assistance in monkey study.
Author contributions:JLY,HF and FFF performed most of experiments.BLG,WTW,and JLX contributed to eletrophysiological study.YH and XTH contributed to monkey experiments.YZH and YQD contributed to fatemapping.KZ contributed to mini-ischemia and data analysis.YZW designed the experiments,overviewed the progress of experiments,analyzed data,wrote manuscript and provided grant support.All authors approved the final version of the manuscript.
Conflicts of interest:The authors claim no conflicts of interests.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative CommonsAttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:Primary anti bodies used in this study.
Additional Figure 1:Western blotting of Nestin expression.
Additional Figure 2:Establishment of photothrombosis and marker expression.
Additional Figure 3:Mini-photothrombotic ischemia.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

