Epigenetic modifications and metabolic memory in diabetic retinopathy: beyond the surface
Dan-Dan Liu ,Chao-Yang Zhang ,Jing-Ting Zhang ,Li-Min Gu ,Guo-Tong Xu,,Jing-Fa Zhang,
Abstract Epigenetics focuses on DNA methylation,histone modification,chromatin remodeling,noncoding RNAs,and other gene regulation mechanisms beyond the DNA sequence.In the past decade,epigenetic modifications have drawn more attention as they participate in the development and progression of diabetic retinopathy despite tight control of glucose levels.The underlying mechanisms of epigenetic modifications in diabetic retinopathy still urgently need to be elucidated.The diabetic condition facilitates epigenetic changes and influences target gene expression.In this review,we summarize the involvement of epigenetic modifications and metabolic memory in the development and progression of diabetic retinopathy and propose novel insights into the treatment of diabetic retinopathy.
Key Words:diabetic retinopathy;DNA methylation;epigenetics;histone modification;metabolic memory;M6A modification;non-coding RNAs;review
Introduction
Diabetic retinopathy
Diabetic retinopathy (DR) is a slowly progressive ocular complication of diabetes mellitus,a metabolic disorder characterized by elevated blood glucose levels.DR is a leading cause of preventable blindness in working-aged people and is recognized as a disease of progressive neuro-vascular unit damage,with neuronal dysfunction proceeding to microvascular damage (Hammes,2018;Sinclair and Schwartz,2019;Oshitari,2022).It can affect almost all types of retinal cells,including neurons (e.g.,ganglion cells,amacrine cells,bipolar cells,and photoreceptor cells),vascular cells (pericytes and endothelial cells),glial cells (Müller cells,astrocytes,and microglia),and the retinal pigment epithelium (RPE) (Sti tt et al.,2016).DR can be divided into two broad categories based on clinical findings: the early stage non-proliferative DR and advanced stage proliferative DR (PDR).An additional important categorization of DR is diabetic macular edema,which occurs across all severity levels and remains the most common cause of vision loss in patients with DR (Wong et al.,2016;Duh et al.,2017).Although some new therapies have been applied in the treatment of diabetic macular edema (e.g.,intraocular steroid and anti -vascular endothelial growth factor [VEGF] injections),up to 50% of patients respond poorly.In addition,for people with PDR,laser photocoagulation remains a mainstay of treatment,despite its destructive nature (Cheung et al.,2010).The prospects for future treatment modalities,such as inhibiting other angiogenic factors,targeting reversible epigenetics,regenerative therapy,and topical therapy,are promising (Duh et al.,2017).
Metabolic memory
The persistent adverse effects of hyperglycemia on the progression of diabetic complications even after termination of the glycemic episode are defined as“metabolic memory,” which could be due to epigenetic changes in target cells under the diabetic condition and inherited through multiple cell divisions.Thus,good glycemic control has long-term beneficial effects,while a prior history of hyperglycemia induces the continuous progression of DR,even after good glucose control has been achieved,which indicates the important contribution of epigenetic modifications in the development and progression of metabolic memory (Natarajan,2021).For example,the landmark Diabetes Control and Complications Trial (1983–1993) showed that intensive glycemic control therapy effectively delayed the onset and slowed the progression of diabetic microvascular complications (such as retinopathy,neuropathy,and nephropathy) compared with conventional therapy in patients with insulindependent diabetes mellitus (Nathan et al.,1993).In addition,a study of experimental models showed that vascular smooth muscle cells derived from type 2 diabetic db/db mice exhibited a preactivated phenotype and metabolic memory of a previous diabetic environment when compared with vascular smooth muscle cells derived from nondiabetic mice,even after several generations ofin vitroculture under similar conditions (Villeneuve et al.,2008).Similarly,the genes involved in this inflammatory effect also had epigenetic alterations,suggesting a close correlation between metabolic memory and epigenetic mechanisms (Villeneuve et al.,2008).
Epigenetic modifications
“Genetic information” traditionally refers to the genetic information stored in DNA sequences,while epigenetic information refers to the instructions on when,where,and how to carry out the DNA genetic information (Harvey et al.,2018).Epigenetic modifications alter gene expression without affecting the DNA sequence and occur dynamically in response to environmental,developmental,and nutritional cues (Chen et al.,2017).They regulate gene expression,phenotype,and metabolic abnormalities,and some are heritable.In diabetes,epigenetic modifications may persist even after tight glycemic control,resulting in metabolic memory (Handy et al.,2011).Epigenetic modifications mainly include DNA and histone methylation,histone acetylation,histone lactylation,and N6-methyladenosine (m6A) modification.Moreover,chromatin changes are not only regulated by these epigenetic marks,but also modified by the activity of noncoding RNAs (ncRNAs),and all are involved in the development of diabetic complications,especially DR (Zhong and Kowluru,2013a;Reddy et al.,2015;Kowluru et al.,2016).Currently,there is a lack of biomarkers for early detection of DR,as well as effective treatments to control disease progression at an early stage.However,patients with type 1 diabetes and PDR have exhibited altered epigenetic factor patterns in the blood and vitreous humor,suggesting that some epigenetic changes may predict the progression of PDR and might be used as prospective markers for DR screening (Agardh et al.,2015).The reversible nature of epigenetics also provides key opportunities for therapeutic intervention.At present,numerous inhibitors targeting epigenetic modifiers are being tested in cancer,some of which have been tested in experimental models of diabetes complications,such as inhibitors of histone deacetylation enzymes (HDACs) and histone methyltransferases (HMTs),but with mixed results (Kato and Natarajan,2019).
There is a complex interrelationship between epigenetics and metabolism in DR.On one hand,some intermediate metabolites in metabolic pathways often act as modification enzyme cofactors,modification donors,and excitatory or antagonistic molecules to affect the activity of epigenetic modification enzymes,such as the ratio of S-adenosylmethionine to S-adenosylhomocysteine (SAM/SAH),which affects methyltransferase activity and regulates DNA and histone methylation (Mentch et al.,2015).Furthermore,the levels of acetyl CoA and NAD+affect histone acetylation(Li and Kazgan,2011).On the other hand,epigenetic modifications can directly change the expression of metabolic enzymes,transporters,and signal transduction and transcription factors to regulate cell metabolism.The complex interplay between metabolism and regulation of epigenetics is widely studied in the field of cancer diseases.
In this article,we discuss the mechanisms of different epigenetic processes affecting and regulating the metabolic memory of DR,including DNA methylation,histone modification,mRNA modification,and ncRNA activity,and propose their relationship with metabolic memory.We also summarize the potenti al therapies that could target epigenetic modifications to treat or prevent the progression of DR and provide novel therapeutic targets in DR management.
Database Search Strategy
The inclusion criteria for this review were the following: studies that discussed epigenetic modifications and metabolic memory in DR,English language,and full-text articles published between August 2001 and August 2022.We searched the PubMed database to identify relevant publications.The literature search strategy was conducted as follows: each of two phrases,i.e.,(1) diabetic retinopathy and (2) epigenetic modifications,were combined with each of: (a) metabolic memory and (b) therapy,e.g.,“diabetic retinopathy”and “metabolic memory,” viz.,(1)+(a);“diabetic retinopathy” and “therapy,”viz.,(1)+(b);“epigenetic modifications” and “metabolic memory,” viz.,(2)+(a);and “epigenetic modifications” and “therapy,” viz.,(2)+(b).Thus,four queries were obtained.We screened the reference list of included studies to identi fy other potenti ally useful studies.First,we screened the ti tles and abstracts,then the full texts for keywords,such as “diabetic retinopathy” and“epigenetic modifications,” to find those that were potenti ally suitable.
DNA Methylation in Diabetic Retinopathy
DNA methylation,catalyzed by the DNA methyltransferase (DNMT) enzyme family,usually represses gene expression by interfering with transcription factor binding (Kohli and Zhang,2013;Wu and Zhang,2014) or recruiting transcriptional corepressors (Jones,2012).DNA methylation on cytosinephosphate-guanine (CpG) islands often changes DNA-protein interactions,resulting in alterations in chromatin structure and interference with transcriptional machinery binding,leading to gene silencing (Jones and Takai,2001).This methylation state can be transferred from DNMT to the newly synthesized single strand of DNA during DNA replication,thus completing the inheritance between generations (Yeivin and Razin,1993).A clinical study demonstrated a significant association between global DNA methylation modifications and the progression of DR (Maghbooli et al.,2015).The retinal DNA methylation-hydroxymethylation machinery remained active even after the glycemic insult had ceased,indicating that DNA methylation is probably involved in the metabolic memory of DR (Mishra and Kowluru,2016;Maugeri et al.,2018).DNA methylation leads to differential gene expression in patients with DR.A study conducted genome-wide DNA methylation analysis of 485,577 sites in blood from patients with PDR and controls,showing DNA methylation differences at 349 CpG sites of 233 unique genes,where the majority (79%) of these sites showed decreased DNA methylation and abnormal gene upregulation,e.g.,matrix metalloproteinase-9 (MMP-9),nucleoti de-binding oligomerization domain-like receptor family pyrin domaincontaining-3,transforming growth factor-β1,monocyte chemoattractant protein-1,and tumor necrosis factor superfamily 2 (Chen et al.,2020).Differenti al DNA methylation contributes to the pathogenesis of DR,possibly serving as a novel predictive biomarker for DR (Agardh et al.,2015).
DNA methylation is a dynamic biological process resulting from differences in the enzyme activity of methyltransferases and demethylases.Diabetes activates both DNMT and demethylases,and the activity of DNMT is regulated by the SAM/SAH ratio (Zhang et al.,2014;Maugeri et al.,2018).SAM is a common methyl donor for DNMT,which passes its methyl group to synthesize SAH and a methylated substrate,thus inhibiting DNMT acti vity(Finkelstein,1990;Mentch et al.,2015).A population-based study illustrated that the plasma SAM/SAH ratio in patients with type 2 diabetes was inversely associated with retinopathy and vascular abnormalities (Van Hecke et al.,2008),reflecting the reduction in genome-wide DNA transmethylation reactions.This is similar to the phenomenon of methylation in tumor tissues(Ikegami et al.,2009).
DNMT1 is the only enzyme in the DNMT family that is activated and overexpressed in DR (Kowluru et al.,2016).Previous studies showed that DNMT1 hypermethylated the promotors of mitofusin 2 (Duraisamy et al.,2019) and MutL homolog 1 (Mohammad et al.,2019),which decreased the binding of transcription factor specificity protein 1 in human retinal endothelial cells (HRECs).Downregulation of mitofusin 2 resulted in increased mitochondrial fragmentation,membrane hyperpermeability,mitochondrial DNA damage,and cell apoptosis;similarly,downregulation of MutL homolog 1 led to mitochondrial dysfunction.DNMT1 inhibition protected mitochondria from the above changes (Kowluru and Mohammad,2020).Moreover,high glucose (HG)-induced DNMT1 enhanced its methylation in peroxisome proliferator-activated receptor alpha and inhibited its expression in human retinal capillary pericytes.Downregulation of peroxisome proliferatoractivated receptor alpha increased cell apoptosis and reactive oxygen species(ROS) levels,while inhibition of peroxisome proliferator-activated receptoralpha methylation could relieve retinal tissue damage in a mouse model of DR(Zhu et al.,2021).Thus,therapies targeted at repressing DNA methylation by inhibition of DNMT1 have the potenti al to prevent the development of DR.
The demethylation of 5-methyl cytosine can be catalyzed by the family of teneleven translocation proteins and thymine DNA glycosidase (Kohli and Zhang,2013).Diabetes induced the activation of ten-eleven translocation family protein 2 in endothelial cells and increased its binding to the promotors of Ras-related C3 botulinum toxin substrate 1 (Rac1) (Duraisamy et al.,2018) and MMP-9 (Kowluru et al.,2016),which facilitated the binding of transcription factor nuclear facto kappa B (NF-κB) and activation of Rac1 or MMP-9,ultimately increasing ROS production,mitochondrial damage,and cell apoptosis.HG could induce the expression of methyl-CpG binding domain protein 2 and demethylate its binding to miR-345-5p promoter in primary retinal ganglion cells.The elevated miR-345-5p expression further inhibited its target gene,activating transcription factor 1 expression and promoting cell apoptosis and neurodegeneration in DR (Ge et al.,2021).
The above studies showed the vital role of DNA methylation in terms of oxidative stress,mitochondrial dysfunction,cell apoptosis,and neurodegeneration in the development and progression of DR;therefore,regulation of DNA methylation could provide clues for DR management.
Histone Modification in Diabetic Retinopathy
Histones play a vital role in the dynamic and long-term regulation of genes(Stoll et al.,2018) and can be modified by reactions such as methylation,acetylation,acylation,and ubiquitination (Kouzarides,2007).In general,histone methylation is correlated with gene activation or repression.The result of histone methylation depends on the amount of methyl groups added,the specific histone residue modified,and its position in the N-terminal region of H3 or H4 (Kaelin and McKnight,2013).For instance,four methylation sites on histones are implicated in transcription activation: H3K4me1/2/3,H3K36me2/3,H3K48me3,and H3K79me3.In contrast,H3K9me3,H3K27me3,and H4K20me3 are connected to transcriptional repression.For arginine residues,H3R17me2 induces activation,while H3R2me2 induces transcriptional repression (Etchegaray and Mostoslavsky,2016).Histone acetylation (e.g.,H3K9ac,H3K14ac,and H4K5ac) at gene promoters has been implicated in transcriptional activation,and its removal is connected to gene repression (Kouzarides,2007).In recent years,histone lactylation has emerged as a new research field for studying metabolic dysregulation in various diseases,including DR.The varying degrees of histone modification greatly increase the complexity of gene expression regulation.Histone modification directly or indirectly affects cellular metabolism by regulating the expression of related genes and playing a regulatory role (Sabari et al.,2017).
Histone methylation in diabetic retinopathy
A meta-analysis concluded that an HMTs SUV39H2,which catalyzed the methylation of H3K9,was protective for diabetic microvascular complications in 2991 patients with type 1 diabetes and DR (Syreeni et al.,2011).The SUV39H2-mediated epigenetic modifications were closely related to gene activation events that promoted enhanced expression of pivotal proinflammatory molecules,resulting in vascular injury in DR (Syreeni et al.,2011).SUV39H1 is a close homolog of SUV39H2 that has been identified as being involved in the hyperglycemia-induced inflammatory response.DNA methylation is also regulated by histone modifications.A study demonstrated that HG increased H3K9me3 and SUV39H1 binding at Rac1 promoters in HRECs,activating Rac1 transcription.SUV39H1-siRNA attenuated upregulation of 5-hydroxymethylcytosine (5hmC) and Rac1 mRNA in diabetic mice retinas and maintained retinal mitochondria (Kowluru et al.,2021).Diabetes also hypomethylated histone H3K9me2 at the MMP-9 promoter by elevating the enzyme activity and transcripts of lysine-specific demethylase 1 and increased acetylH3K9 in the retina,increasing the accessibility to recruit NFκB.This facilitated MMP-9 activation,mitochondrial damage,and capillary cell apoptosis.As such,the molecular or pharmacological regulation of lysinespecific demethylase 1 may have been beneficial to DR (Zhong and Kowluru,2013a).
In the retinal capillary endothelial cells of diabetes,mitochondrial manganese superoxide dismutase (MnSOD),an enzyme responsible for scavenging superoxide radicals in the mitochondria,was downregulated,leading to elevated superoxide levels (Jang et al.,2000).The mitochondrial MnSODencoding gene Sod2 was epigenetically modified,with increased H4K20me3,acetyl H3K9,and p65 subunit of NF-κB at its promoter and enhancer,which contributes to its inactivation.This suggests the possibility of targeting epigenetic changes to prevent mitochondrial MnSOD inhibition and potenti ally mitochondrial damage (Zhong and Kowluru,2011).Additionally,other researchers pointed out that HG reduced the gene transcripts of Sod2,which was accompanied by decreases in H3K4me1/2 at the promoter and the enhancer of Sod2 in cultured RECs and retina from human donors (Zhong and Kowluru,2013b).Thus,targeting histone methylation serves as a potential therapy to halt the development of DR.
Histone acetylation in diabetic retinopathy
Histone acetylation mainly occurs at lysine residues in the amino-terminal tails of core histone proteins,which changes the charge quality and microenvironment of local chromatin and leads to less compact chromatin(a state of euchromatin).This process allows entry of transcription factors and other transcription coactivators and is almost invariably associated with activation of transcription by opening the chromatin structure (Verdin and Ott,2015;Bansal and Simmons,2018).Histone acetylation is regulated by the action of histone acetyltransferase (HAT) and HDACs,which maintains a dynamic balance between acetylation and deacetylation (Shafabakhsh et al.,2019).The acti vity of HAT is sensitive to the concentration of the intracellular metabolite acetyl-coenzyme A (acetyl-CoA) and glucose availability (Kaelin and McKnight,2013).Acetyl-CoA not only maintains mitochondrial oxidative metabolism but also transfers an acetyl group to the histone N-terminal tails to yield acetylation (Wong et al.,2017).HDACs are divided into two families(zinc and NAD+dependent) according to the catalysis mechanism (Segré and Chiocca,2011).Specifically,decreased free and cytosolic mitochondrial NAD+/NADH ratios and increased acetyl-CoA were found in diabetic rat retinas caused by retinal hypoxia,which led to a decrease in the enzymatic acti vity of sirtuins and acetylation of target genes (Segré and Chiocca,2011).
Histone acetylation acts as a post-translational modification,the abnormal functions of which are closely related to the occurrence of disease (Uzdensky and Demyanenko,2021).Studies have uncovered that diabetes increases histone acetylation in most tissues,which corresponds to its vigorous transcription activity (Kadiyala et al.,2012).Natarajan demonstrated that diabetic conditions increased acetylation of lysines on histones H3 and H4 in monocytes and led to inflammatory gene (tumor necrosis factor alpha [TNFα]and cyclooxygenase-2) transcription (Miao et al.,2004).Histone acetylation in retinal tissuesin vivoand HRECs/Müller cellsin vitrowas also significantly increased under diabetic conditions,with elevated histone acetyltranferase and decreased histone deacetylase levels,which played important regulating roles in the inflammatory response (Perrone et al.,2009;Wang et al.,2012).Inhibitors of histone acetyltransferase or activators of histone deacetylase could prevent the acetylation and induction of inflammatory proteins in the diabetic retina (Kadiyala et al.,2012).
Sirtuins1 (Sirt1),an NAD+dependent HDAC,transfers acetyl groups from proteins to NAD+and is expressed in various ocular tissues,including the cornea,lens,and retina.It is considered to play a crucial role in ageing,stress resistance,inflammation,and metabolic regulation (Winnik et al.,2015).Downregulation of Sirt1 was observed in many chronic ocular diseases,such as cataract,retinal degeneration,and optic neuritis (Jaliffa et al.,2009;Mimura et al.,2013).In DR,another chronic ocular disease,the suppression of Sirt1 expression and acti vity contributes to mitochondrial dysfunction in the retina and capillary cells.For example,diabetes downregulated Sirt1 in mice retinas and retinal microvasculature from human donors with DR (Kowluru et al.,2014),which was reported by methylating its promoter in retinal microvessels from diabetic mice.Downregulation of Sirt1 promoted DNMT1 activation by activating H3K9 acetylation at the DNMT1 promoter (Mishra et al.,2018).In addition,Sirt1 downregulation could increase H3K9 acetylation at the p66Shc(a 66 kDa adaptor protein,sensor of oxidative stress-induced apoptosis)promoter (Mishra et al.,2019) and increase binding of p65 at the MMP-9 promoter (Kowluru et al.,2014) due to p65 acetylation,which promoted p66Shc expression and MMP-9 activation in diabetic RECs.The accumulation of MMP-9 and p66Shc in the cytoplasm activated Rac1 and induced NADPH oxidase 2-mediated ROS production and mitochondrial DNA and membrane damages,ultimately activating the apoptotic machinery and promoting the progression of retinopathy (Mishra et al.,2019).Sirt1 downregulation also regulated the transcription activity of NF-κB and mitochondria homeostasis under diabetic conditions (Mishra and Kowluru,2017).Sirt6 deficiency in DR caused retinal transmission defects,increased the levels of H3K56 acetylation and the expression of glycolytic genes in Müller cells,and elevated neurodegenerative events (Zorrilla-Zubilete et al.,2018).Increased glycolytic proteins (i.e.,6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase,isoform 3,pyruvate kinase M2 [PKM2]) and metabolites (lactate,Ac-CoA,proangiogenic factors) in oxygen-induced retinopathy mice induced the histone acetylation and activation of macrophages/microglia,which facilitated retinal neovascularization (Liu et al.,2020c).However,in the retinas of type 1 diabetic mice,HIST1H1C,an important variant of the linker histone H1,was increased and maintained the deacetylation status of H4K16 by upregulating Sirt1 and HDAC1,then promoted autophagy and lesions in the early stage of DR.Silencing Hist1H1C significantly attenuated the diabetes-induced retinal autophagy,glial activation,inflammation,and neuron loss (Wang et al.,2017).
Lysine acetyltransferase 1 (KAT1) remained downregulated in diabetic mice retinas,which promoted retinal inflammation,neovascularization,and vascular leakage in the progression of DR through the KAT1/YTHDF2-ITGB1/FAK/PI3K/AKT signaling pathway (Qi et al.,2021).
In summary,modulating histone acetylation by controlling key enzyme acti vity remains a novel therapeutic approach for treating or preventing DR.
Histone lactylation in diabetic retinopathy
Lactate is an abundant metabolite produced during glycolysis,and it is found to exert a novel function in histone lysine lactylation to control target gene expression in macrophages (Izzo and Wellen,2019).Lactate is produced by lactate dehydrogenase in cells with increased glycolysis.Lactate dehydrogenase also regenerates NAD+from the decreased form of nicotinamide adenine dinucleoti de (NAD),maintaining the flow of glycolysis(Bonuccelli et al.,2010).
Barba et al.(2010) verified that lactate levels were higher in the vitreous fluid of patients with PDR than in nondiabetic individuals,reflecting increased anaerobic glycolysis in the retina.In addition,lactate dehydrogenase was increased in the serum of patients with diabetes (Huang et al.,2006).Therefore,retinal hypoxia in PDR changes the microcirculation and stimulates lactate secretion by glycolysis to compensate for the decreased production of oxidative adenosine 5’ triphosphate (ATP) (Barba et al.,2010).However,lactate modification in DR has been rarely reported.In Alzheimer’s disease(AD),another neurodegenerative disease,elevated histone lactylation,especially H4K12la,was first found in brain microglia from individuals with AD and experimental animals,which formed a positive feedback loop(glycolysis/H4K12la/PKM2),leading to amyloid-β plaque deposition and cognitive deficits.Blocking this loop could be a potenti al therapeutic method for AD (Pan et al.,2022).In retinal neovascular diseases (i.e.,retinopathy of prematurity),transcription factor Yin Yang-1 (YY1) was lactylated at lysine 183(K183) in microglia,which contributed to angiogenesis by upregulating the expression of fibroblast growth factor 2,leading to retinal neovascularization(Wang et al.,2021).Moreover,lactate acted as a significant signaling and immunomodulatory molecule,suppressing antitumor responses and promoting inflammatory responses (Certo et al.,2020).The above findings expand the field of protein lactylation in neurodegenerative and retinal neovascular diseases,providing new insights into potential targets for the treatment of DR.
m6A Modification of mRNA in Diabetic Retinopathy
Like DNA and histone modifications,various RNA modifications and their functions have attracted more attention in recent years.Among all types of RNA modifications,m6A,a representative type of mRNA modification,is reversible and controls gene expression in diverse physiological processes with the highest abundance (Zaccara et al.,2019).For precise regulation of m6A modifications,various enzymes or binding proteins catalyze,eliminate,or recognize these modifications after translation;these proteins are known as “writers,erasers,and readers” (Strahl and Allis,2000;Jenuwein and Allis,2001).
Dynamic m6A mRNA deposition is catalyzed by the m6A methyltransferase complex “writer,” composed of methyltransferase-like 3 (METTL3),methyltransferase-like 14 (METTL14),and Wilms’ tumour 1-associated proteins 6–10 and removed by the m6A demethylase “eraser”,including fat mass and obesity-associated protein and alkB homolog 5 (ALKBH5).Then,the m6A-modified RNA is recognized by m6A reading proteins,the “readers,”including YT521-B homology domain family (YTHDF) proteins 1–3 and YT521-B homology domain containing proteins 1/2,after transportation into the cytoplasm to promote or inhibit target mRNA translation (Zhao et al.,2017).These enzymes greatly influence post-transcriptional protein regulation and RNA decay and splicing,further accelerating mRNA metabolism and translation (Wang et al.,2016;Zhao et al.,2017).Dysfunction of m6A modification has been shown to be involved in many human diseases,such as cancers,neurological diseases,diabetic complications,and cardiovascular diseases (Klungland and Dahl,2014).However,the role of m6A modification in pathological DR is an emerging concept and remains unclear.
A recent study has uncovered the vital role of m6A RNA methylation on pericyte dysfunction in diabetes-induced retinal vascular complications.They revealed that the upregulation of m6A RNA methylation in pericytes and mouse retinas after diabetic stress was mediated by METTL3 methyltransferases.Specific depletion of METTL3 in pericytes reversed hyperglycemia-induced pericyte dysfunction and vascular complications in mice retina (Suo et al.,2022).Another study suggested that diabetic conditions induced higher m6A modification levels and faster degradation rates of tumor necrosis factor-α induced protein 3 in retinal microglia,which was mediated by the decrement of m6A demethylase ALKBH5.Downregulation of tumor necrosis factor-α induced protein 3 contributed to the enhancement of M1 polarized retinal microglia and promoted the inflammatory response in DR (Chen et al.,2022).Also,hypoxic stress could increase the level of m6A modifications in lipoprotein receptor-related protein 6 and dishevelled-1 mRNA in endothelial cells and mice retinas,mediated by METTL3 upregulation.Then,the YTHDF1 selectively recognized the m6A site and promoted their translation.Overexpression of lipoprotein receptorrelated protein 6 and dishevelled-1 subsequently activated the Wnt/β-catenin signaling pathway and upregulated downstream VEGF and erythropoietin expression,which ultimately promoted retinal neovascularization.METTL3 deletion could reduce avascular areas and pathological neovascular tuft s in oxygen-induced retinopathy mice (Yao et al.,2020).This research suggested that targeting of m6A epigenetic alterations remains an innovative strategy for the treatment of retinal angiogenic diseases,such as PDR and retinopathy of prematurity (Yao et al.,2020).
At present,there are few and not entirely consistent reports on m6A modification in retinal diseases.For example,clinical studies unveiled that METTL3 mRNA was low-expressed in the peripheral venous blood samples of patients with type 2 diabetes mellitus compared to nondiabetic individuals(Zha et al.,2020).Overexpression of METTL3 attenuated HG-induced RPE pyroptosis (Zha et al.,2020).Also,downregulation of METTL3 and m6A abundance was involved in the course of epithelial-mesenchymal transition in RPE during proliferative vitreoretinopathy (PVR),and METTL3 overexpression suppressed the ARPE-19 epithelial-mesenchymal transitionin vitroand prevented the PVR processin vivothrough inhibition of the Wnt/β-catenin pathway (Ma et al.,2021).
m6A modification and its “reader” proteins YTHDF2 also precisely control retinal ganglion cell dendrite maintenance and growth,which is valuable in developing new therapy strategies for glaucomatous eyes and other retinal ganglion cell degeneration diseases (Niu et al.,2022).m6A modification has also been reported to affect various ncRNAs,including microRNAs,long ncRNAs (lncRNAs),and circular RNAs (circRNAs),which were responsible for DR onset and progression.Many lncRNAs have m6A modifications that affect the interaction of specific DNA sites of lncRNA and RNA-binding proteins.For example,lncRNA metastasis-associated lung adenocarcinoma transcript 1 has a series of m6A modification sites that can alter its localization as well as affinity to some proteins,which then affects the regulation of aerobic glycolysis (Zhou et al.,2016).
The m6A level change is an important indicator of the physiological state of cells,and the maladjusted m6A signal will affect the physiological metabolic process of cells,leading to a disorder of normal physiological processes.
ncRNAs and Diabetic Retinopathy
ncRNAs are RNAs that do not encode protein sequences and cannot be translated into proteins.They are divided into housekeeping ncRNAs(ribosomal RNAs and transfer RNAs) and regulatory ncRNAs,which play a pivotal role in epigenetic modification.The regulatory ncRNAs are divided into two categories based on size: short chain ncRNAs (including microRNAs[miRNAs],endogenous small-interfering RNAs [siRNAs],circular RNAs[circRNAs],and Piwi-interacting RNAs [piRNAs]) and lncRNAs.The regulatory ncRNAs are found to exert transcriptional regulation through involvement in epigenetic modifications in DR,although ncRNAs are not considered epigenetic components (Zaratiegui et al.,2007;Ponting et al.,2009).The newly discovered noncoding RNAs appear to be ideal candidates for novel molecular markers and therapeutic strategies for DR (Wawrzyniak et al.,2018).
miRNAs and diabetic retinopathy
miRNAs are a class of small single-stranded non-coding RNAs encoded by endogenous genes,with a length of about 22 bp,which can target the mRNA of protein-coding genes and perform base pairing,leading to either degradation or post-transcriptional repression (Bartel,2009).miRNAs have multiple targets implicated in different pathogenic pathways in DR.Moreover,a specific gene might be modulated by multiple miRNAs.Dysregulated levels of different miRNAs underlie the various pathogenic mechanisms resulting in DR by affecting angiogenesis,microvascular complications,apoptosis of pericytes and RPE,inflammation,and oxidative stress (Mastropasqua et al.,2014).Therefore,the role of important miRNAs can be used as biomarkers for the diagnosis and prognosis of DR.Also,miRNAs have been reported to hold great potenti al as therapeutic modalities for DR in the future (Kaur et al.,2022).
Kovacs et al.(2011) verified a series of differentially expressed miRNAs in the pathogenesis of DR,including the upregulated NF-κB-responsive miRNAs(miR-21,miR-132,miR-146,miR-155),VEGF-responsive miRNAs (miR-17-5p,miR-18a,miR-20a,miR-21,miR-31,miR-155,miR-191),p53-responsive miR-34 family,other upregulated miRNAs (miR-15b,miR-19b,miR-142-3p,miR-339-5p,miR-342-3p,miR-450a,miR-184,miR-199a,miR-200a,miR-200b,miR-205,miR-223,miR-335-3p,miR-378,miR-488,miR-574-3p),and the downregulated miRNAs (miR-20b,miR-499,miR-690,miR-29c,miR-181c,miR-136,miR-376c).The results suggest a vital role for miRNAs in the pathogenesis of DR by modulating multiple pathogenetic pathways,which remain as potenti al therapeutic targets for DR.
In terms of retinal neovascularization,downregulation of miR-126,miR-200,and miR-15b,VEGF-responsive miRNAs,and NF-κB-responsive miRNAs all contribute to the increase of VEGF,hypoxia-inducible factor-1,insulinlike growth factor,or NF-κB in the progression of DR (Lu et al.,2020;Yang et al.,2020),which are vital factors in promoting retinal angiogenesis.In terms of neuronal and glial dysfunction,upregulation of miR-200b,miR-29b,and p53-responsive miR-34 family is reportedly involved in this process.In terms of the blood-retinal barrier (BRB),downregulation of miR-146a,miR-200bm,and miR-590-3p and upregulation of miR-195,accompanied by VEGFresponsive miRNAs and NF-κB-responsive miRNAs,caused BRB breakdown in DR (Mastropasqua et al.,2014;Gu et al.,2019).
miRNAs now represent a new and powerful class of gene expression modulators in the pathogenesis of DR,and miRNA mimics or antagonists are helpful in effectively modulating the progression of DR.
lncRNAs and diabetic retinopathy
lncRNAs are a class of non-translatable transcripts with a length of more than 200 nucleoti des generated by RNA polymerase transcription.lncRNAs can be divided into five types according to the source of expression,i.e.,intergenic lncRNA (located between two encodable transcripts);bidirectional lncRNA(reverse transcription of the promoter of protein-coding genes);sense/anti sense lncRNA (segments homologous to the protein-coding gene exon or its antisense chain);intronic lncRNA (homologous to the intron of proteincoding genes);and enhancer lncRNA (transcripts of enhancers) (Nojima and Proudfoot,2022).lncRNAs can participate in gene expression regulation through a variety of possible mechanisms,including scaffold function(acts as a scaffold during the formation of ribonucleoprotein complexes);guide function (binds to transcriptional regulators to guide them to target sequences);decoy function (binds to transcriptional regulators to prevent their interaction with target genes);miRNA sponge;and enhancer lncRNA acting as trans-activating RNA (Ulitsky and Bartel,2013).
DR is regulated by many lncRNAs (Figure 1).For example,recent findings showed that diabetes induced an increase in lncRNA myocardial infarctionassociated transcript,which interfered with miR-150-5p and promoted VEGF translation,leading to vascular inflammation and leakage and endothelial cell death (Jaé and Dimmeler,2015;Yan et al.,2015).Additionally,the myocardial infarction-associated transcript promoted human retinal pericyte pyroptosis by inhibiting miR-342-3p and antagonizing the depressive effect of miR-342-3p on its target,caspase 1,in DR (Yu et al.,2021).The upregulation of lncRNA-OGRU mediated inflammation and oxidative stress in the diabetic retina by competing for miR-320 to upregulate ubiquiti n-specific pepti dase 14 expression (Fu et al.,2021).The expression of lncRNA HOTAIR was significantly enhanced in hyperglycemia-treated HRECs,retinas from diabetic animals,and vitreous humor/serum from patients with PDR,which subsequently promoted retinal angiogenesis,mitochondrial aberrations,and oxidative damage (Zhao et al.,2020;Biswas et al.,2021).Diabetes induced downregulation of H19 in mice retina and vitreous humor from individuals with PDR,which caused endothelial-mesenchymal transition through transforming growth factor-β and the mitogen-activated protein kinase-extracellular signal-regulated kinase (ERK)1/2 signaling pathway in DR (Thomas et al.,2019).The lncRNA maternally expressed gene 3 promoter was methylated by DNMT1 in DR rats and cell models,and maternally expressed gene 3 downregulation accelerated the endothelial-mesenchymal transition through activation of the PI3K/Akt/mTOR signaling pathway in ECs (He et al.,2021).The vascular endothelial-associated lncRNA 2 (VEAL2) maintained endothelial permeability by modulating junctional dynamics in human umbilical vein endothelial cells by controlling protein kinase C beta acti vity (Sehgal et al.,2021).Inhibition of lncRNA metastasis-associated lung adenocarcinoma transcript 1,via Keap1-Nrf2,protected the retina from oxidative damage in DR (Radhakrishnan and Kowluru,2021).As the lncRNA field is still in its infancy,its role in the pathogenesis of DR needs to be further explored.
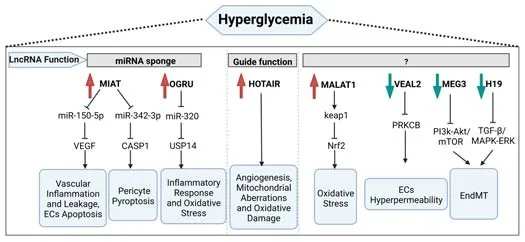
Figure 1|Schematic of hyperglycemia-induced regulation of several lncRNAs on retinal function in DR.
circRNAs and diabetic retinopathy
circRNAs are covalently closed single-stranded RNAs formed by cyclization of mRNA introns or exons,which exist widely in eukaryotic cells (Jeck and Sharpless,2014).circRNAs usually do not encode proteins but can regulate gene expression through the circRNA-miRNA-mRNA axis,which is a commonly identified mechanism.Specifically,circRNA can competi tively bind miRNA and weaken the inhibition of miRNA on target mRNA.This process is called miRNA sponging and is the most important mechanism of circRNA development(Hansen et al.,2013;Zhou and Kuang,2021).circRNA can also interact with lncRNA or RNA-binding proteins to participate in transcription regulation.
It has been widely reported that changes in the expression of circRNAs are closely associated with many pathological events involved in DR (Figure 2).For example,increased circRNA circHIPK3 promoted endothelial proliferation and vascular dysfunction in DR by sequestering and inhibiting miR-30a-3p activity,and downregulation of miR-30a-3p relieved the inhibition of downstream target genes,including VEGFC,Wnt2,and frizzled-4 (Shan et al.,2017).cZNF532 expression was upregulated in the vitreous of patients with PDR or diabetic macular edema and retinal vessels of diabetic mice.cZNF532 silencing ameliorated diabetes-induced retinal inflammation,pericyte degeneration,and vascular dysfunction through inhibiting miR-29a-3p and upregulating candidate genes neural/glial anti gen-2,cyclin-dependent kinase 2,and lysyl oxidase-like 2 (Jiang et al.,2020).circDNMT3B was decreased in the diabetic retina,which promoted human retinal microvascular endothelial cell (HRMEC) proliferation,migration,and tube formation through regulating miR-20b-5p expression and targeting bone morphogenetic protein and acti vin membrane-bound inhibitor (BAMBI) (Zhu et al.,2019).Highly expressed circCOL1A2 sponged miR-29b during the pathological progression of DR,then enhanced VEGF expression and facilitated angiogenesis of HRMECs (Zou et al.,2020).Furthermore,the role of the circ_0002570-miR-1243-angiomotin axis was reported as a potenti al regulating effect on glucose-induced angiogenesis and inflammation of HRMECs (Liu et al.,2020a).Diabetes-related stress increased cPWWP2A expression in pericytes,and the cPWWP2A in exosomes indirectly regulated ECs biology,such as inducing retinal vascular dysfunction via sequestering and inhibiting miR-579 acti vity,then increased target gene expression (angiopoietin 1,occludin,and Sirt1);thus,this work discovered a mechanism for the crosstalk between vascular pericytes and ECs (Liu et al.,2019).In HG-induced human RPE cells (ARPE19),the hsa_circ_0041795-miR-646-VEGFC axis contributed to cell inflammation and apoptosis (Sun and Kang,2020).The cZNF609/miR-615-5p/myocyte enhancer factor 2A network was involved in regulating retinal vessel loss and suppressing pathological angiogenesis (Liu et al.,2017).The circDNMT3B-miR-20b-5p-BAMBI axis was inhibited under diabetic conditions,which promoted retinal angiogenesis,increased retinal acellular capillary numbers,and aggravated visual damage in diabetic rats.Changes in the expression of circDNMT3B and miR-20b-5p were confirmed in the proliferative fibrovascular membranes of patients with DR (Zhu et al.,2019).A retrospective cross-sectional study indicated that hsa_circ_0001953 was highest in whole blood of patients with PDR in contrast with healthy subjects,implying that hsa_circ_0001953 might be a potenti al treatment for DR (Wu et al.,2021).
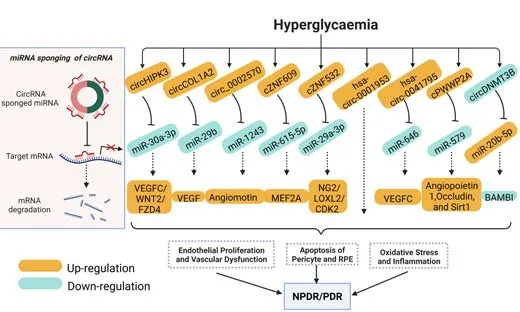
Figure 2|Schematic of hyperglycemia-induced alterations of several circRNAs on retinal function in DR.
Epigenetics and Metabolic Memory in Diabetic Retinopathy
The existence of metabolic memory makes manipulation of hyperglycemic memory a beneficial option for the prevention and treatment of diabetic complications,including DR.For example,some large-scale clinical trials involving patients with type 1 and type 2 diabetes verified that early intensive glycemic control could reduce the incidence and progression of macro-and microvascular complications (Brown et al.,2010;Rodriguez-Guti errez et al.,2019;Taylor et al.,2021).
Since metabolic memory in DR is a complex and dynamic interaction of multiple mechanisms that may persist for a long time,the molecular mechanisms responsible for metabolic memory in DR remain to be clearly elucidated.However,the results from experimental models and human studies support the notion that hyperglycemia-induced persistent epigenetic changes,which generally occur during the beginning stages of disease,are a major driving force underlying metabolic memory (Natarajan,2021).The epigenetic changes under diabetic conditions facilitate the continuous pathogenesis and development of DR by promoting retinal inflammation,oxidative stress,cell apoptosis,autophagy,and angiogenesis (Reddy et al.,2015) (Figure 3).Therefore,therapy based on the reversal of associated epigenetic mechanisms can bring new insight into the area of early diagnosis and treatment mechanisms of DR.
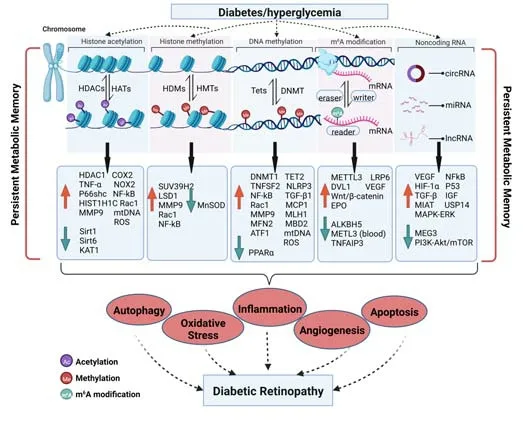
Figure 3|Schematic of epigenetic mechanisms involved in DR and metabolic memory.
Current Progress in Epigenetic Regulation for the Treatment of Diabetic Retinopathy
Since various epigenetic modifications are involved in the pathogenesis of DR,targeted regulation of DR by using specific activators or inhibitors of epigenetic modification enzymes may be an effective method of blocking persistent impairment of metabolic memory.Current progress in the regulation of epigenetics in DR is discussed below and shown inTable 1.
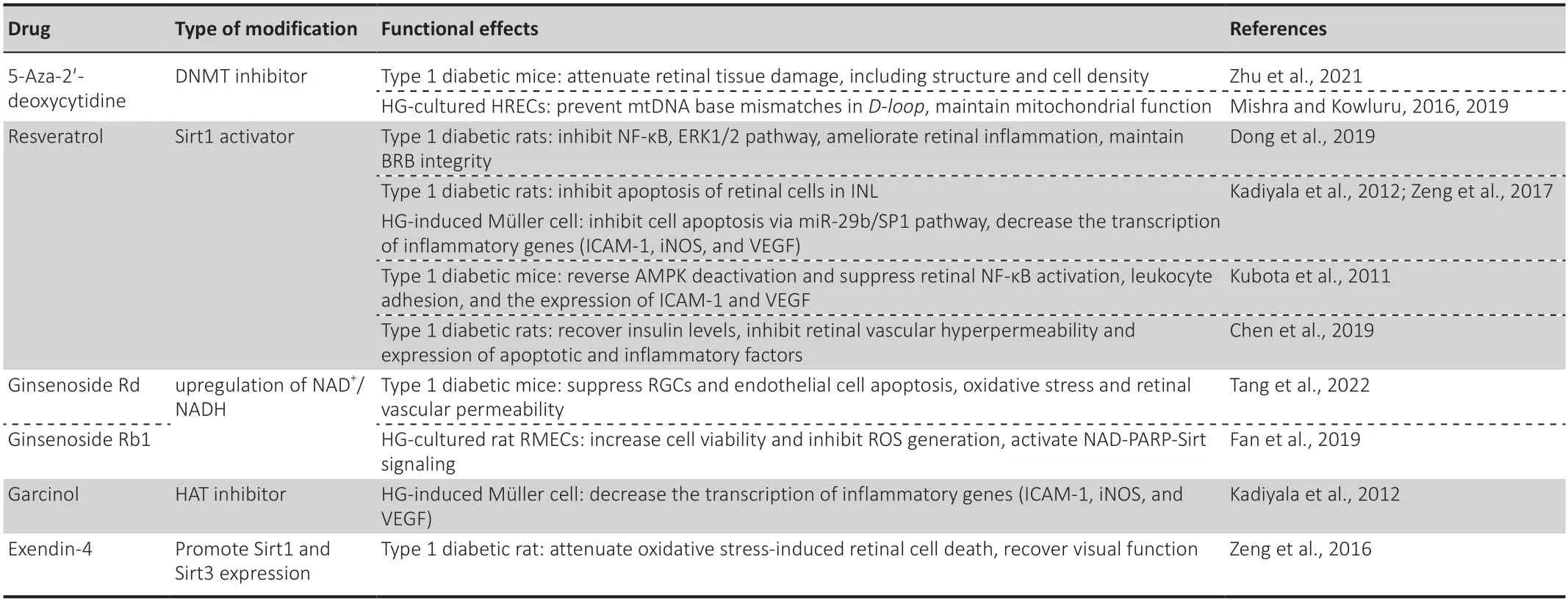
Table 1|Targeting epigenetic modifications for the treatment of diabetic retinopathy
5-Aza-2'-deoxycyti dine,also known as decitabine and an inhibitor of DNMT,acquired Food and Drug Administration approval for the treatment of myelodysplastic syndromes and chronic myelomonocytic leukemia in July 2020 in the United States and Canada (Dhillon,2020).5-Aza-2′-deoxycyti dine effectively inhibited DNMT acti vity and expression under diabetic conditions,maintaining redox homeostasis and downregulating the expression of several inflammatory cytokines (Zhu et al.,2021).In HG-induced HRMECs,5-aza-2′-deoxycytidine supplementation prevented an increase in mitochondria DNA base mismatches at the displacement loop (D-loop) and protected mitochondrial respiration and physiological function (Mishra and Kowluru,2016,2019).
Resveratrol,a Sirt1 activator,has promising anti-inflammatory,antiapoptotic,and vascular-protective properti es in DR.For instance,resveratrol application reduced phosphorylation of NF-κB and ERK 1/2 (Dong et al.,2019) and reversed diabetes-induced retinal AMP-activated protein kinase(AMPK) deactivation in streptozotocin-induced diabetic rats (Kubota et al.,2011;Kadiyala et al.,2012).This resulted in the amelioration of retinal ischemia,inflammation (reduced expression of VEGF-1,TNFα,monocyte chemoattractant protein-1,interleukin-6,interleukin-1β,and intercellular adhesion molecule-1 [ICAM-1]),leukocyte adhesion,endothelial injury,and capillary loss,and maintenance of BRB permeability (Dong et al.,2019).Resveratrol could also recover insulin levels (Chen et al.,2019).Resveratrol administration inhibited apoptosis of inner nuclear layer retinal cells in streptozotocin-induced diabetic rats and HG-induced retinal Müller cells (Zeng et al.,2017).
A multicenter,randomized,observer-blinded trial evaluated the efficacy and safety of adding resveratrol to the age-related eye disease medicinal product’s recommended dose.The results showed that compared with the standard age-related eye disease formula,there was almost no difference on visual acuity after addition of resveratrol,while the latter could improve the fatty acid profile and increase carotenoid serum levels,which may inhibit the proinflammatory and proangiogenic profile of patients with age-related macular degeneration (García-Layana et al.,2021).
Ginsenoside Rdpossesses diverse and powerful anti-inflammatory,anticancer,and neuroprotective pharmacological capacities (Wan et al.,2017;Liu et al.,2020b).Recent studies demonstrated that ginsenoside Rd activated the AMPK pathway and increased Sirt1 expression by the upregulation of NAD+/NADH levels in endothelial cells,whilein vivoginsenoside Rd administration improved retinal neurovascular abnormalities by suppressing the apoptosis of retinal ganglion and endothelial cells and lowered retinal vascular permeability and oxidative stress in diabetic mice (Tang et al.,2022).Another active compound,ginsenoside Rb1,protected REC viability and mitochondrial DNA copy number and reduced ROS generation via enhanced Sirt activity and Sirt1/Sirt3 expression.The protective mechanism of Rb1 is due to modulation of the NAD-poly (ADP-ribose) polymerase-Sirt signaling pathway and maintenance of the cellular reducing power (Fan et al.,2019).
Garcinol,a HAT inhibitor,inhibited the increased histone H3 acetylation in HG-incubated Müller cells and decreased the transcription of inflammatory genes,including ICAM-1,inducible nitric oxide synthase,and VEGF (Kadiyala et al.,2012).
Exendin-4,a glucagon-like peptide 1 analogue,injected intravitreally significantly attenuated oxidative stress-induced retinal cell death and recovered visual function through upregulation of Sirt1 and Sirt3 in early stage of DR (Zeng et al.,2016).
In addition to the ongoing novel therapies regulating epigenetics,targeting the final pathways for metabolic memory might provide clues for further treatment of DR.
Conclusions and Perspectives
In this review,we summarized the evidence from both experimental and clinical epigenetic studies and the mechanisms related to DR to elucidate the importance and contribution of epigenetics and metabolic memory to DR,demonstrating their potenti al roles as biomarkers and therapeutic targets in improving the clinical management of DR and metabolic memory.Metabolic memory is,in fact,epigenetic memory,and the message of abnormal metabolism is carried by epigenetic factors,which are all involved in the pathogenesis of DR,including dysfunction of various retinal cells (e.g.,ECs,RPE,neurons,and glial cells),excessive oxidative stress and inflammation,angiogenesis,and BRB breakdown.These processes lead to activation of complex cellular molecular signaling.If untreated,the disease can culminate in vision loss and eventual blindness (Pitale and Gorbatyuk,2022).
Epigenetic modification is a rapidly developing field and frequently implicated in DR progression (Kowluru and Mishra,2015).Epigenetic modifications of genes,such as DNA methylation,histone modification,m6A modification,and ncRNA activity,play an important role in the occurrence and development of DR.Moreover,epigenetic factors involved in epigenetic modifications can interact with each other and together contribute to the pathogenic process of DR.As evidence of epigenetic modifications in DR accumulates,a better understanding of these modifications has the potenti al to identi fy new targets for inhibiting such a devastating disease.
Hyperglycemia is an essenti al factor that can induce epigenetic modifications in DR.The influence of epigenetics on DR is an emerging but pivotal field,and reversible epigenetic modifications might provide potenti al strategies for the diagnosis,prevention,and treatment of DR.The ongoing preclinical studies targeting epigenetics might provide alternative therapies for the treatment of DR.For example,histone modification inhibitors and simulators of DNA methylation and miRNAs are now being used in the treatment of cancer and other chronic diseases.Thus,in-depth studies of these modifications will provide new ideas for the prevention and treatment of DR.
Author contributions:DDL and JFZ reviewed the literature and contributed to manuscript draft ing;CYZ,JTZ,LMG and GTX reviewed the literature and contributed to discussion;DDL,GTX and JFZ interpreted the figures;JFZ and LMG contributed funding acquisition.JFZ is guarantor of this work,who has full access to all the data in this study and takes responsibility for the integrity and accuracy of the data;all authors approved final approval for the version to be submitted.
Conflicts of interest:The authors have no conflicting financial interests to declare.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

