基于水溶性煤沥青的MnO@C复合材料的制备及储锂性能研究
刘渤,周卫民,陈燕,王坤,张殿浩,张文武,孟祥安,王士戈,安百钢,徐桂英
基于水溶性煤沥青的MnO@C复合材料的制备及储锂性能研究
刘渤1a,周卫民1a,陈燕1b,王坤1a,张殿浩2,张文武2,孟祥安2,王士戈2,安百钢1a,徐桂英1a
(1.辽宁科技大学 a.辽宁省能源材料与电化学重点实验室 b.机械工程与自动化学院,辽宁 鞍山 114051;2.海城申合科技有限公司,辽宁 鞍山 114200)
制备出具有高容量、良好的倍率性能和循环稳定性的MnO@C复合电极材料。使用水溶性煤沥青及KMnO4为原料,通过水热法制备出Mn3O4@C前驱体。然后经过高温碳热还原制备MnO@C复合电极材料。通过SEM、XPS、XRD和Raman等分析技术对MnO@C复合材料的形貌、表面、结构等进行表征,并使用循环伏安、恒流充放电和电化学阻抗等电化学分析技术对其电化学性能进行了评价。TEM和SEM结果表明,制备的水溶性煤沥青表面丰富的含氧官能团与MnSO4溶液中的Mn2+之间有较强的相互作用,提供成核位点,进而促进了后续MnO@C材料中纳米颗粒的形成和均匀生长。这些纳米粒子的形成又起到了提升MnO@C电化学性能的作用。XRD、Raman和XPS结果表明,Mn3O4@C前驱体经过高温碳热还原反应生成MnO@C后,在MnO表面和包覆的碳材料之间生成了大量的Mn—O—C键。电化学结果表明,MnO@C电极在0.1 A/g电流密度下循环100圈后具有606.47 mAh/g较高的储锂容量,即使是在0.5 A/g大电流密度下循环400圈后仍具有293.83 mAh/g的储锂容量。同时,电化学测试也表明,MnO@C复合材料具有非常好的倍率性能。使用鞍钢产的煤沥青根据混酸法制备了水溶性煤沥青。通过使用水溶性煤沥青和KMnO4成功地制备了Mn3O4@C前驱体。以Mn3O4@C前驱为原料,通过高温碳热还原法制备了MnO@C 复合材料。在MnO表面包覆碳层不仅提供活性位点而且起到限制在充放电过程中MnO体积膨胀的作用。特别值得注意的是,Mn—O—C键构筑了MnO和碳层之间的快速导电通道,提升了电极反应动力学。
一氧化锰;水溶性煤沥青;Mn—O—C键;界面阻抗;锂离子电池;负极
锂离子电池因其工作电压高、能量密度大和循环寿命长等特点,已经占据了商用充电电池市场。然而,随着电动汽车、混合电动汽车、便携式电子设备和智能电网等大规模的应用,对锂离子电池提出了更高能量密度的要求。但传统的商用石墨负极因其较低的理论容量(372 mAh/g)已很难满足当今需求[1-3],为了解决这一问题,人们致力于开发新一代锂离子电池负极材料。
过渡金属氧化物(TMOs),如锰基氧化物,其凭借较高的理论容量(>700 mAh/g)和丰富的自然储量,得到了研究者们广泛关注。但是,单纯的锰基氧化物作为电极材料时,容量会发生快速地衰减,这一方面是由于其电导率较低,易产生较大的极化;另一方面是由于锂离子在锰基氧化物晶格内部的嵌入/脱出过程中产生的巨大体积膨胀/收缩效应,不但极易使其从集流体上剥落,无法接触外电路电子,而且还会导致晶格结构的破坏,从而造成容量快速衰减[4-6]。因此,如何构筑稳定且快速的导电网络至关重要。目前,表面碳包覆被认为是提高复合材料电导率和稳定性的有效方法之一。理想情况下,在电化学反应过程中,活性材料产生的电子通过表面碳层传递到外电路,反之亦然。在此过程中,表面碳层既能够保护中心活性材料,缓解其在充放电过程中的体积膨胀,还可以为Li+存储提供更多的电化学活性位点[7-8]。Zhu等[9]为了提升MnO电极材料的结构稳定性,进而提高其电化学性能,设计了一种槟榔状、核壳结构的MnO@C复合材料。MnO核与外部碳壳的协同作用,极大地提升了复合材料的电导率和结构稳定性,在1.0 A/g电流密度下经过900次充放电循环后,MnO@C复合材料仍具有314.3 mAh/g的储锂容量。同样,Sun等[10]为了解决MnO电极材料的循环稳定性和速率性能差等问题,将纳米MnO颗粒负载在石墨烯纳米片上,制备了MnO/graphene复合材料。得益于石墨烯纳米片极高的电导率,MnO/graphene复合材料表现出了优异的速率性能和较长的循环寿命,在2 A/g电流密度下经过400次充放电循环后,其储锂容量高达843.3 mAh/g。然而,传统的碳包覆方法构建的导电网络往往忽略了中心活性材料和表面碳层之间导电通路,二者通常是以物理方式互连而不是化学键共连,这样使得电子需要克服较大的界面阻抗才能够从表面碳网络传递至中心活性材料,难以构建通畅的导电网络。
为了解决这一问题,Hao等[11]受人类神经元结构的启发,设计了一种类神经元结构的Fe3O4@C/RGO复合材料。Fe3O4被表面碳层保护并通过Fe—O—C键共连在一起,反应开始时,电子可以通过氧桥快速转移,使Fe3O4@C/RGO复合材料在2 A/g电流密度下经过1 000次充放电循环后仍具有715 mAh/g的储锂容量。Jia等[12]采用水热和PECVD相结合的方法制备了核壳型MnO2@RGO复合材料,同样发现Mn—O—C键的存在赋予了复合材料超强的导电性和循环稳定性。遗憾的是,到目前为止,如何在MnO@C材料中构筑Mn—O—C键的方法仍然没有被明确提出。此外,MnO@C材料多采用价格昂贵的碳纳米管、氧化石墨烯和高分子化合物等作为表面修饰碳层[13-14],不利于实际应用。
鉴于此,本研究采用价格低廉的煤焦油沥青为碳源,采用水热法制备了Mn3O4@C前驱体,后续经过高温碳热还原,针对性地制备出了富含Mn—O—C键的MnO@C复合材料,相较于单纯的MnO电极,MnO@C复合材料电极表现出良好的倍率性能和优异的循环稳定性。
1 试验
1.1 原料
鞍钢生产的中温煤焦油沥青。高锰酸钾(KMnO4)、硫酸锰一水合物(MnSO4·H2O)、硫酸(98%)、硝酸(98%)、氢氧化钠均购买于阿拉丁工业公司。
1.2 材料的制备
1.2.1 MnO@C复合材料的制备
首先以煤焦油沥青为原料(CP)采用混酸氧化法制备了水溶性煤沥青(WSP)[15]。MnO@C的制备过程如下。
将3 mmol KMnO4与20 mL去离子水混合,并将得到的混合溶液标记为A溶液。将1.0 g WSP分散于30 mL去离子水中并逐滴加入1 mol/L NaOH溶液至pH=10,而后加入4.5 mmol MnSO4·H2O,对得到的混合溶液常温下磁力搅拌30 min,并标记该混合溶液为B溶液。将B溶液缓慢加入到A溶液中,搅拌混合均匀后将该溶液转至100 mL高压反应釜中。将高压反应釜放置于150 ℃恒温干燥箱中,反应12 h。取出后待其冷却至室温,将得到的黑色固体产物用去离子水和无水乙醇分别洗涤3次,并在80 ℃干燥箱中干燥12 h,得到Mn3O4@C材料。将Mn3O4@C材料在氮气气氛下,经过400 ℃炭化5 h得到MnO@C复合材料。在不添加水溶性煤沥青的条件下,采用同样的方法再制备MnO2材料。材料制备流程见图1。
1.2.2 MnO材料的制备
将上述得到的MnO2材料在氮气氛围下,经过700 ℃炭化2 h制备MnO材料。控制不同价态氧化物发生的化学反应方程式见式(1)—(4)[16]。
6Mn2++12OH‒+O2(aq.)→2Mn3O4+6H2O (1)

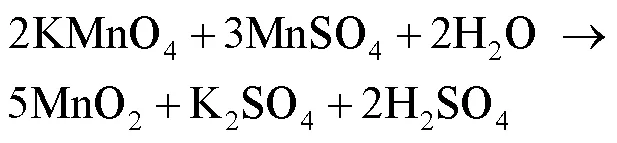
2MnO22MnO+O2 (4)
1.3 电池的组装
将所制备的样品、聚偏氟乙烯(PVDF)和乙炔黑按8∶1∶1的质量比混合均匀,滴加适量的N-甲基吡咯烷酮(NMP),在玛瑙中研磨均匀后涂覆在铜箔上,然后将铜箔置于80 ℃下预干燥1 h以除去绝大部分NMP溶剂,再将其放入真空干燥箱中,120 ℃下干燥12 h。待铜箔冷却至室温,裁剪成直径为11 mm的圆形电极片。最后在充满氩气(O2<0.1%,H2O<0.1%)的手套箱中,按负极壳、锂片、隔膜、电解液、电极片、垫片、弹片、正极壳的顺序组装成CR2032型纽扣半电池。
1.4 样品表征
采用D8 ADVAHCL(德国布鲁克公司)X射线衍射仪(XRD,Cu Kα,=0.154 06 nm)分析样品的物相结构,扫描角度2范围为10°~90°。样品的分子结构采用法国Horiba JobinYvon公司的HR800型激光拉曼光谱仪进行分析测试,激光波长为532 nm。样品表面的化学状态分析采用Thermo Scientific K-AlphaX射线光电子能谱仪(XPS)。样品的表面形貌采用德国卡尔蔡司公司的场发射扫描电镜(ZEISS IGMA/HD)进行观察和分析。
1.5 电化学性能测试
采用武汉蓝电CT 2001A型测试系统对样品进行了倍率性能和循环性能测试,截止电压为0.01~3.0 V。采用上海辰华CHI 660E电化学工作站对样品进行循环伏安性能测试,扫速为0.2 mV/s,电压范围为0.01~ 3.0 V。采用上海辰华CHI 660E电化学工作站对样品进行交流阻抗测试,测试频率为100 kHz~0.01 Hz。
2 结果及分析
2.1 结构分析
首先利用XRD对各个阶段的样品结构进行表征,如图2a所示。在添加水溶性煤沥青的条件下,采用水热法,首先成功制备了Mn3O4@C(ICOD NO.01-089-4837)材料(Mn3O4为四方晶系尖晶石结构),后续经过400 ℃炭化5 h,成功制备了立方紧密堆积结构的MnO@C (ICOD NO.01-077-2363)材料,表明高温炭化过程中,Mn3O4转变为MnO材料。而在不添加水溶性煤沥青的条件下,同样的高温处理,只能制备出价态更高且为隧道结构的α-MnO2材料(ICOD NO.00-044-0141),这表明水溶性煤沥青在高温炭化过程中起到了还原作用,促进了MnO相的生成。为了后续进一步研究MnO表面包覆碳层对复合材料的影响,将MnO2材料在氮气氛围下,进一步经过700 ℃炭化2 h制备了MnO材料,可以看到,与单纯的MnO相比,MnO@C材料在34.9°、40.5°、58.7°、70.18°、73.80°处的特征峰半高宽明显增加,这说明MnO@C材料中的MnO趋向于无定形结构,这利于进一步增加储锂位点,提升电化学性能[17]。
图2b为样品Mn3O4@C、MnO@C和MnO的拉曼光谱图。由图2可知,MnO@C在635.91、1 364、1 607 cm‒1处同时观察到3个振动峰,分别对应着MnO中的Mn—O振动峰以及碳材料中的D峰和G峰,这说明碳成功地包覆在MnO表面。此外,与单纯的MnO相比,样品MnO@C的Mn—O特征峰明显向低波数偏移,表明MnO与表面碳层有较强的相互作用[18]。MnO@C材料的D峰和G峰比值为1.71,明显低于Mn3O4@C材料(D峰和G峰比值为2.44),这说明高温炭化过程提升了锰氧化物表面碳层的石墨化度,这有利于提高复合材料的导电性,从而提升倍率性能。

图2 MnO2、MnO、MnO@C和Mn3O4@C的XRD谱图(a),MnO、MnO@C和 Mn3O4@C的拉曼谱图(b),MnO、Mn3O4和MnO2的晶型结构(c)
为了证实拉曼分析的结果,利用XPS对样品MnO@C和Mn3O4@C进行分析,结果如图3所示。图3a是样品MnO@C的Mn 2p精细谱,在结合能为653.4 eV和641.7 eV处可以明显地发现2个特征峰,分别对应着Mn2+的Mn 2p1/2和Mn 2p3/2轨道,结合能差值为11.7 eV,这说明锰元素是以正二价的形式存在复合材料中[19]。图3b分别是样品Mn3O4@C和MnO@C的C 1s精细谱,从C 1s精细谱中可以看出,样品在结合能284.8 eV和286.3 eV/286.1 eV、288.3 eV和290.4 eV处可以明显地发现4个特征峰,分别对应着C==C、C—O、C==O和C=O—OH[20]。此外,值得注意的是,与样品Mn3O4@C相比,样品MnO@C的C—O键峰位置发生明显偏移,这可能与Mn—O—C键的形成有关[21]。为了进一步讨论Mn—O—C的形成,图3c给出了样品的O 1s精细谱,从图中可以看出,样品在结合能为531.7 eV、533.3 eV/533.2 eV处都可以发现2个明显的特征峰,分别对应着Mn—O—Mn、C==O[22]。但不同的是,样品MnO@C在530.4 eV处出现1个新的特征峰,对应Mn—O—C[23],结合XRD分析可知,高温碳热还原反应有效地促进了Mn—O—C键的生成。
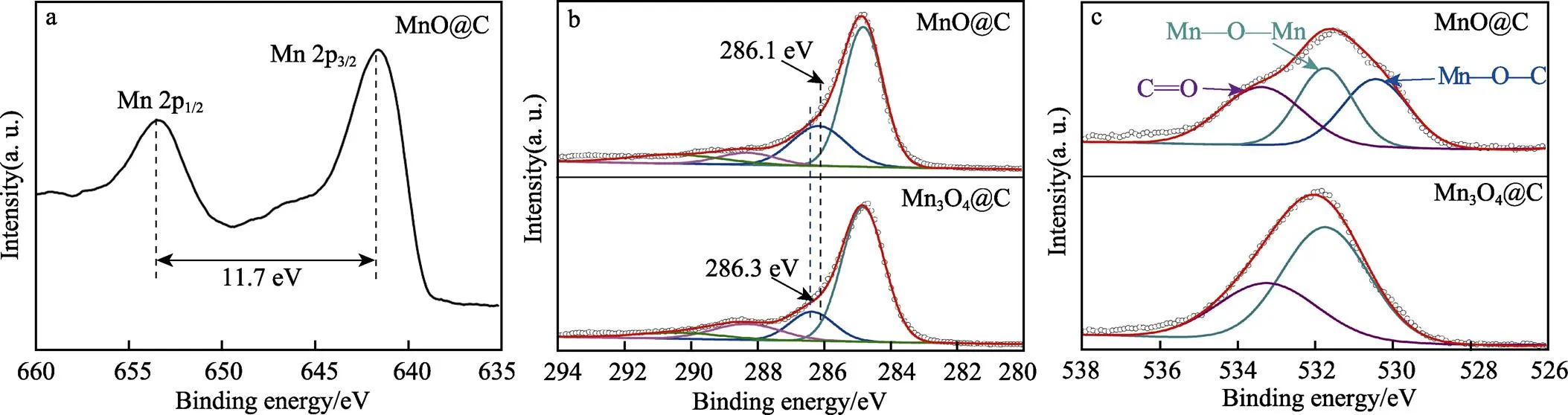
图3 样品MnO@C的Mn 2p精细谱(a),样品Mn3O4@C和MnO@C的C 1s精细谱(b),样品Mn3O4@C和MnO@C的O 1s精细谱(c)
2.2 表面形貌分析
图4为MnO和MnO@C材料的SEM和TEM图。由图可知,单纯的MnO材料尺寸较大,颗粒发生明显团聚,呈不规则块状结构。而MnO@C材料的SEM图可以清晰地看到大小均一的MnO纳米颗粒均匀地分布在碳层中,MnO颗粒尺寸也由1.2 μm下降至21.2 nm,这利于增大电极/电解质界面并缩短Li+的扩散路径,有效促进复合材料氧化/还原反应动力学的提升。
图5为沥青(CP)和水溶性煤沥青(WSP)的红外光谱图。如图5所示,经过强酸氧化后,WSP红外光谱图在1 045、1 345、1 720 cm‒1处出现了新的特征峰,分别对应C—O、C—OH、C==O官能团,在3 420 cm‒1处的—OH峰明显增强,这表明在沥青表面修饰了大量的含氧官能团,这有助于其在水溶液中均匀分散[24-25]。同时,进一步将1.0 g WSP分散于30 mL去离子水,测定其Zeta电位为‒25.7 mV,这不但进一步说明水溶性煤沥青在水中具有良好的分散性,而且可以通过静电相互作用捕获带正电的Mn2+。此外,Zhang等[26]通过理论计算和试验证明了炭材料表面的含氧官能团能够有效地吸附金属离子,进而抑制后续纳米氧化物的团聚,实现纳米氧化物在碳层中的高度分散。基于以上信息,可以推断出水溶性煤沥青表面丰富的含氧官能团与MnSO4溶液中的Mn2+之间有较强的吸附作用,提供成核位点,进而促进了后续纳米颗粒的形成和均匀生长。
2.3 电化学分析
为了证明MnO@C样品锂存储性能的改善,将MnO、Mn3O4@C和MnO@C样品组装成纽扣电池并评估这些样品的电化学性能。图6a为各样品的倍率性能。通过对比可知,MnO@C在0.1、0.2、0.5、1.0 A/g的不同电流密度下循环10圈后,放电比容量分别为500.83、381.64、251.64、136.65 mAh/g。在不同的电流密度下,MnO@C样品具有最高的储锂容量。当电流密度重新回到0.1 A/g时,其仍具有511.68 mAh/g的储锂容量,这都表明MnO@C材料具有较好的倍率性能。
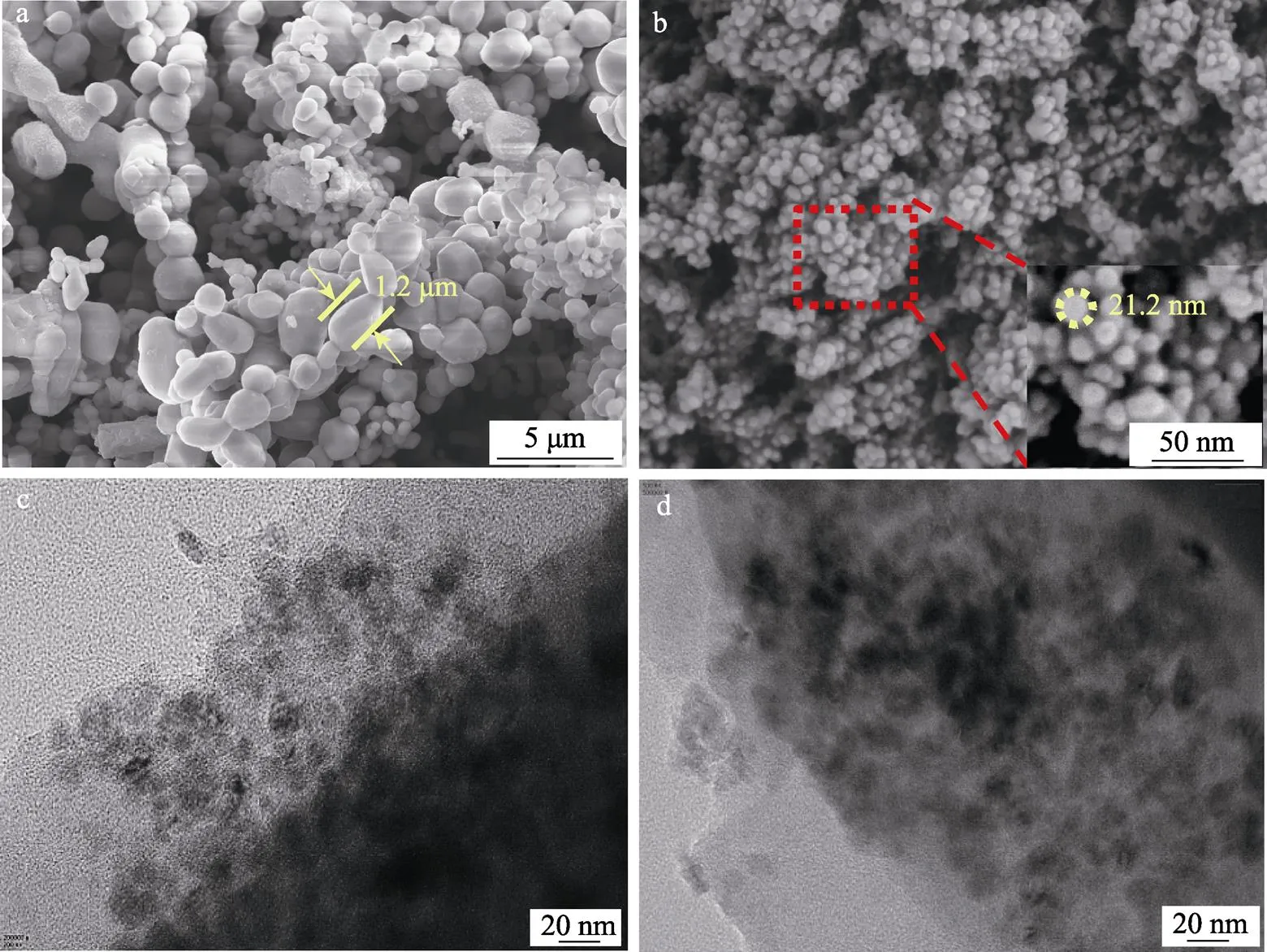
图4 MnO(a)和MnO@C(b)材料的SEM图,MnO@C(c、d)材料的TEM图
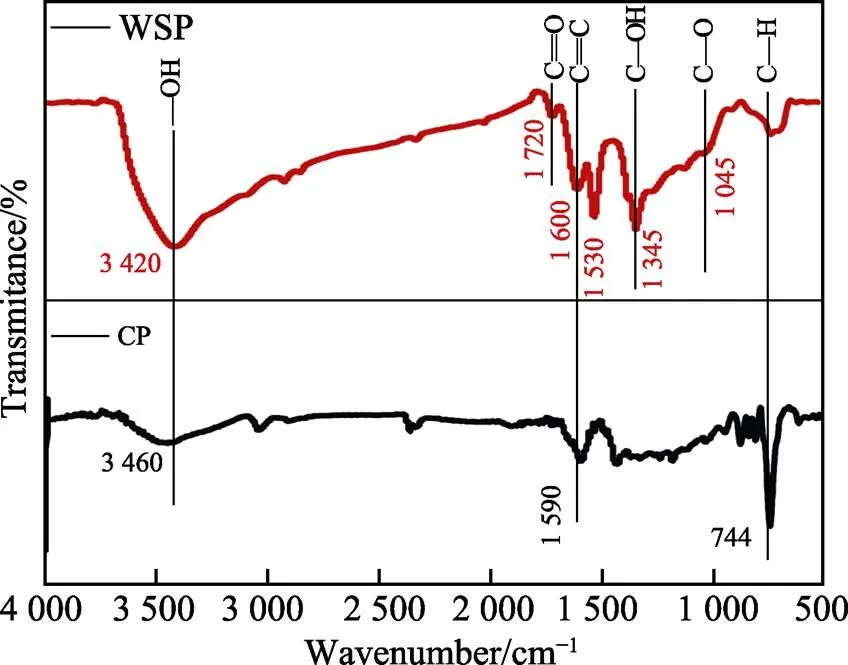
图5 CP和WSP的红外光谱图
图6e给出MnO@C电极在电流密度为0.5 A/g下循环400圈的长循环性能。由图可知,MnO@C电极即使在0.5 A/g大电流密度下循环400圈后仍具有293.83 mAh/g的放电比容量,容量保持率接近100%,这也表明MnO@C具有优异的结构稳定性。
此外,为进一步研究MnO@C样品的电化学性能,对样品进行电化学阻抗分析。图7a为样品MnO、MnO@C在100 kHz至0.01 Hz频率范围内的Nyquist图,主要由高频区代表电荷转移阻抗(ct)的半圆和低频区代表锂离子扩散速率的倾斜直线组成,MnO与MnO@C的ct值分别为261.3、29.15 Ω。通过对比可以得出,MnO@C具有较小的电荷转移电阻,这表明碳包覆后能够明显地改善MnO的电子电导率和电荷转移能力。锂离子的扩散能力与Bode图低频区域的相角有关,相角越小,锂离子扩散能力越强[28]。如图7b所示,MnO@C电极在低于0.1 Hz频率下的相位角更小,具有更强的锂离子扩散能力。此外,MnO与MnO@C样品的锂离子扩散能力可由Warburg系数进一步证实,值越小表明锂离子扩散能力越强[29-30]。值根据公式(5)进行计算。由图7c可知,MnO与MnO@C样品的值分别为820.27、30.08 Ω·s1/2,这说明MnO@C样品具有更佳的锂离子扩散性能。

图6 MnO、MnO@C和Mn3O4@C的倍率性能(a),MnO、MnO@C和Mn3O4@C在0.1 A/g电流密度下的循环性能(b),MnO@C的容量电压曲线(c),MnO@C的循环伏安曲线(d), MnO@C在0.5 A/g电流密度下的循环性能(e)
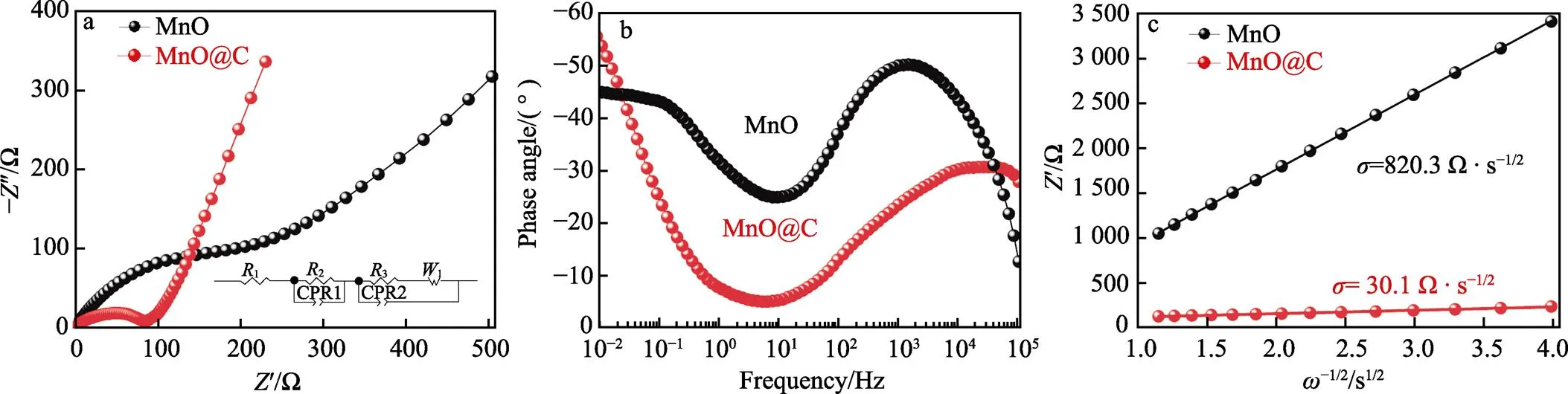
图7 MnO和MnO@C的交流阻抗图(a),MnO和MnO@C的Bode图(b), MnO和MnO@C中的Zʺ与ω‒1/2的线性关系(c)
re=ct+s+·1/2(5)
3 结论
1)以水溶性煤沥青作为MnO纳米颗粒的表面修饰碳层,通过水热和高温碳热还原制备了MnO@C复合材料。Raman、XPS和SEM结果表明,本研究实现了对MnO表面的均匀碳包覆,并且MnO与表面碳层之间存在较强的相互作用,形成了Mn—O—C键。
2)电化学测试表明,MnO表面碳层和Mn—O—C键构筑了MnO和碳层之间的快速导电通道,保证了电荷“自外到内”的快速传输,并能够有效抑制MnO的晶格膨胀。MnO@C在0.1 A/g电流密度下经过100次充放电循环后具有606.47 mAh/g的储锂容量,在0.5 A/g电流密度下经过400次充放电循环后仍具有293.83 mAh/g的储锂容量。
[1] 孙梦璐, 陆萍, 张亦凡, 等. 钛表面硅复合微弧氧化膜负极的制备及其电化学性能研究[J]. 表面技术, 2021, 50(9): 120-127.
SUN Meng-lu, LU Ping, ZHANG Yi-fan, et al. Preparation of Silicon-Containing Micro-Arc Oxidation Film on Titanium and Related Electrochemical Performance Research[J]. Surface Technology, 2021, 50(9): 120-127.
[2] 魏剑, 秦葱敏, 苏欢, 等. 包覆结构Si/C复合负极材料研究进展[J]. 新型炭材料, 2020, 35(2): 97-111.
WEI Jian, QIN Cong-min, SU Huan, et al. A Review of Silicon/Carbon Composite Anode Materials with an Encapsulated Structure for Lithium-Ion Rechargeable Batteries[J]. New Carbon Materials, 2020, 35(2): 97-111.
[3] 马琳, 叶剑波, 黄国创, 等. SnS2-SnO2/石墨烯复合材料的合成及其电化学储锂性能的研究[J]. 表面技术, 2015, 44(1): 8-14.
MA Lin, YE Jian-bo, HUANG Guo-chuang, et al. Synthesis and Electrochemical Li-Storage Performance of SnS2- SnO2/Graphene Composites[J]. Surface Technology, 2015, 44(1): 8-14.
[4] ZHANG Lei, SHEN Lian, LIU Yan-gai, et al. Urchin- Like MnO/C Microspheres as High-Performance Lithium- Ion Battery Anode[J]. Ionics, 2021, 27(4): 1423-1428.
[5] 唐晓宁, 朱绍宽, 宁坚, 等. 二氧化锰基超级电容器的电荷储能机理研究进展[J]. 新型炭材料, 2021, 36(4): 702-710.
TANG Xiao-ning, ZHU Shao-kuan, NING Jian, et al. Charge Storage Mechanisms of Manganese Dioxide- Based Supercapacitors: A Review[J]. New Carbon Materials, 2021, 36(4): 702-710.
[6] LI Se-si, ZHAO Yun-hao, LIU Zheng-wang, et al. Flexible Graphene-Wrapped Carbon Nanotube/Graphene@MnO23D Multilevel Porous Film for High-Performance Lithium-Ion Batteries[J]. Small, 2018, 14(32): e1801007.
[7] 陆地, 郑春满, 陈宇方, 等. 以酚醛树脂为碳源原位合成富锂层状相/尖晶石/碳核壳结构正极材料及其电化学性能[J]. 高等学校化学学报, 2020, 41(7): 1684-1690.
LU Di, ZHENG Chun-man, CHEN Yu-fang, et al. Synthesis of Li-Rich Layers/Spinel/Carbon Composite Cathode Materials with Phenol Formaldehyde Resin and Its Electrochemical Performance[J]. Chemical Journal of Chinese Universities, 2020, 41(7): 1684-1690.
[8] 林伟国, 孙伟航, 曲宗凯, 等. 锂离子电池负极材料纳米多孔硅/石墨/碳复合微球的制备与性能[J]. 高等学校化学学报, 2019, 40(6): 1216-1221.
LIN Wei-guo, SUN Wei-hang, QU Zong-kai, et al. Preparation and Performance of Nano-Porous Si/Graphite/C Composite Microspheres as Anode Material for Li-Ion Batteries[J]. Chemical Journal of Chinese Universities, 2019, 40(6): 1216-1221.
[9] ZHU Ling-feng, WANG Yun, WANG Min-ji, et al. Areca- Inspired Core-Shell Structured MnO@C Composite towards Enhanced Lithium-Ion Storage[J]. Carbon, 2021, 184: 706-713.
[10] SUN Yong-ming, HU Xian-luo, LUO Wei, et al. Reconstruction of Conformal Nanoscale MnO on Graphene as a High-Capacity and Long-Life Anode Material for Lithium Ion Batteries[J]. Advanced Functional Materials, 2013, 23(19): 2436-2444.
[11] HAO Shu-meng, LI Qian-jie, QU Jin, et al. Neuron- Inspired Fe3O4/Conductive Carbon Filament Network for High-Speed and Stable Lithium Storage[J]. ACS Applied Materials & Interfaces, 2018, 10(21): 17923-17932.
[12] JIA He nan, LIN Jing huang, LIU Yu lin, et al. Nanosized Core-Shell Structured Graphene-MnO2Nanosheet Arrays as Stable Electrodes for Superior Supercapacitors[J]. Journal of Materials Chemistry A, 2017, 5(21): 10678-10686.
[13] LI Fen, QIN Teng-teng, SUN Yu-ping, et al. Preparation of a One-Dimensional Hierarchical MnO@CNT@Co- N/C Ternary Nanostructure as a High-Performance Bifunctional Electrocatalyst for Rechargeable Zn-Air Batteries[J]. Journal of Materials Chemistry A, 2021, 9(39): 22533-22543.
[14] CHENG Fang-yan, CHEN Yu-jie, SUN An-tao, et al. Graphene Encapsulated Porous Monodisperse MnO Microspheres as High-Performance Anode Material for Lithium Storage[J]. Ceramics International, 2019, 45(10): 13556-13560.
[15] 郑晓君, 高丽娟, 刘焕, 等. 混酸法制备水溶性沥青的实验条件研究[J]. 辽宁科技大学学报, 2016, 39(5): 376-380.
ZHENG Xiao-jun, GAO Li-juan, LIU Huan, et al. Preparation of Water-Soluble Asphalt by Mixed Acid Method[J]. Journal of University of Science and Technology Liaoning, 2016, 39(5): 376-380.
[16] HUANG Hong-wen, YU Qing, PENG Xin-sheng, et al. Single-Unit-Cell Thick Mn3O4Nanosheets[J]. Chemical Communications, 2011, 47(48): 12831-12833.
[17] PARK J H, CHOI W Y, LEE Sang-min, et al. Graphene Intercalated Free-Standing Carbon Paper Coated with MnO2for Anode Materials of Lithium Ion Batteries[J]. Electrochimica Acta, 2020, 348: 136310.
[18] PANDEY G P, LIU Tao, BROWN E, et al. Mesoporous Hybrids of Reduced Graphene Oxide and Vanadium Pentoxide for Enhanced Performance in Lithium-Ion Batteries and Electrochemical Capacitors[J]. ACS Applied Materials & Interfaces, 2016, 8(14): 9200-9210.
[19] CHEN Jun-jie, YANG Ke, WANG Ji-qi, et al. Peanut- Like Yolk/Core-Shell MnO/C Microspheres for Improved Lithium Storage and the Formation Mechanism of MnCO3Precursors[J]. Journal of Alloys and Compounds, 2020, 849: 156637.
[20] WANG Lu, LI Yu-hong, HAN Zhi-da, et al. Composite Structure and Properties of Mn3O4/Graphene Oxide and Mn3O4/Graphene[J]. Journal of Materials Chemistry A, 2013, 1(29): 8385-8397.
[21] FAN Li-shuang, ZHANG Yu, GUO Zhi-kun, et al. Hierarchical Mn3O4Anchored on 3D Graphene Aerogels via C—O—Mn Linkage with Superior Electrochemical Performance for Flexible Asymmetric Supercapacitor[J]. Chemistry–A European Journal, 2020, 26(42): 9314- 9318.
[22] CAO Li-yun, WANG Rui-yi, XU Zhan-wei, et al. Constructing MnOC Bonds in Mn3O4/Super P Composite for Superior Performance in Li-Ion Battery[J]. Journal of Electroanalytical Chemistry, 2017, 798: 1-8.
[23] LI Shuang, YU Li-li, SHI Yu-ting, et al. Greatly Enhanced Faradic Capacities of 3D Porous Mn3O4/G Composites as Lithium-Ion Anodes and Supercapacitors by C–O–Mn Bonding[J]. ACS Applied Materials & Interfaces, 2019, 11(10): 10178-10188.
[24] BOURLINOS A B, GOURNIS D, PETRIDIS D, et al. Graphite Oxide: Chemical Reduction to Graphite and Surface Modification with Primary Aliphatic Amines and Amino Acids[J]. Langmuir, 2003, 19(15): 6050-6055.
[25] SZABÓ T, BERKESI O, FORGÓ P, et al. Evolution of Surface Functional Groups in a Series of Progressively Oxidized Graphite Oxides[J]. Chemistry of Materials, 2006, 18(11): 2740-2749.
[26] ZHANG Wan-yu, XU Hai, XIE Fei, et al. General Synthesis of Ultrafine Metal Oxide/Reduced Graphene Oxide Nanocomposites for Ultrahigh-Flux Nanofiltration Membrane[J]. Nature Communications, 2022, 13: 471.
[27] FANG Guo-zhao, WU Zhuo-xi, ZHOU Jiang, et al. Sodium- Ion Batteries: Observation of Pseudocapacitive Effect and Fast Ion Diffusion in Bimetallic Sulfides as an Advanced Sodium-Ion Battery Anode [J]. Advanced Energy Materials, 2018, 8(19): 1870092.
[28] ZHAO Rui-zheng, QIAN Zhao, LIU Zhong-yuan, et al. Molecular-Level Heterostructures Assembled from Layered Black Phosphorene and Ti3C2MXene as Superior Anodes for High-Performance Sodium Ion Batteries[J]. Nano Energy, 2019, 65: 104037.
[29] NIU Yu-bin, XU Mao-wen, DAI Chun-long, et al. Electrospun Graphene-Wrapped Na6.24Fe4.88(P2O7)4Nanofibers as a High-Performance Cathode for Sodium-Ion Batteries[J]. Physical Chemistry Chemical Physics, 2017, 19(26): 17270-17277.
[30] HUANG Ying-de, YU Rong-tian, MAO Gao-qiang, et al. Unique FeP@C with Polyhedral Structure In-Situ Coated with Reduced Graphene Oxide as an Anode Material for Lithium Ion Batteries[J]. Journal of Alloys and Compounds, 2020, 841: 155670.
Preparation and Lithium Storage Properties of MnO@C Composites Based on the Water Soluble Pitches
1a,1a,1b,1a,2,2,2,2,1a,1a
(1. a. Key Laboratory of Energy Materials and Electrochemistry Research Liaoning Province, b. School of Mechanical Engineering and Automation, University of Science and Technology Liaoning, Liaoning Anshan 114051, China; 3. Haicheng Shenhe Technology Co., Ltd., Liaoning Anshan 114200, China)
The aim of this research is to realize the constructions of Mn—O—C bonds by covering the coal tar pitch based carbon materials on the surface of MnO. The formations of Mn—O—C bonds play the main role to construct the conductive network so as to diminish the interfacial resistance, which causes that the prepared MnO@C materials possesses the excellent electrochemical performances such as high Li+storage capacity, excellent rate performances and long cycle performances. Based on the hydrothermal method, the Mn3O4@C precursors are synthesized by using the water soluble coal tar pitches (WSP) and KMnO4. The MnO@C materialsaresynthesized by the carbothermic reduction reaction methods using the Mn3O4@C precursors. The morphologies, surface characteristics and detailed structures of MnO@C materials are verified by the TEM, SEM, XPS, XRD and Raman measurements. TEM and SEM results indicate that plenty of oxygen-containing functional groups existing in the WSP possess the interactions with the Mn2+of MnSO4solution, which is able to facilitate the formations of cores which are beneficial to accelerate the formations and uniformly growing of nano particles in MnO@C materials. Formations of nano particles are also play the role to enhance the electrochemical performances of MnO@C materials. The XRD, Raman and XPS results indicate that a lot of Mn—O—C bonds formed between the surface of MnO and carbon materials in the MnO@C materials. The electrochemical performances of MnO@C materials were evaluated by cyclic voltammetry, charge-discharge and electrochemical impedance spectroscopy measurements.As a result, it is found that MnO@C shows the storage capacity at 606.47mAh/g after cycling charge-discharge 100 cycles at a current density of 0.1 A/g. Although the current density was increased to 0.5 A/g, the MnO@C composite materials still show the storage capacity at 293.83 mAh/g after 400 cycles.Additionally, the fact that MnO@C materials have the tremendous rate performances was also determined in these presented studies. In summarization, the WSP were successfully prepared mixed acid methods using the coal tar pitches from ANGANG STEEL. The Mn3O4@C precursors were successfully synthesized by using WSP and KMnO4. The MnO@C composite materials are successfully synthesized by using the Mn3O4@C precursors. The electrochemical evaluations show that covering the carbon materials on the surface of MnO is the effective way to enhance the electrochemical performances of MnO, for covering the carbon materials on the surface of MnO provides the active sides not only, but also can restrain the volume expansion of MnO in charge-discharge process. Especially, the constructions of Mn—O—C bonds between the carbon materials and MnO play the main role to enhance the transfer abilities of electronic and ions between the carbon materials and MnO, which proves the electrode reaction kinetics. Considering the fact that coal tar pitches are the bulk commodities, the Mn3O4@C should have the significant cost advantage in fabrication processes.
manganous oxide;water soluble coal tar pitches; Mn—O—C bond; interface impedance; lithium ion batteries; anodes
tb43
A
1001-3660(2023)01-0298-08
10.16490/j.cnki.issn.1001-3660.2023.01.030
2021–12–28;
2022–04–14
2021-12-28;
2022-04-14
辽宁省教育厅项目(LJKQZ2021126)
The Liaoning Province Education Department of China (LJKQZ2021126)
刘渤(1998—),男,硕士研究生,主要研究方向为电化学储能。
LIU Bo (1998-), Male, Postgraduate, Research focus: electrochemical energy storage.
周卫民(1971—),男,博士,副教授,主要研究方向为纳米材料制备及其电化学能源贮存与转换中的应用。
ZHOU Wei-min (1971-), Male, Doctor, Associate professor, Research focus: nanomaterials for electrochemical energy storage and conversion.
通讯作者:徐桂英(1975—),女,博士,副教授,主要研究方向为煤基功能碳材料的制备及其储能领域的应用。
XU Gui-ying (1975-), Female, Doctor, Associate professor, Research focus: coal-based functional materials for energy storage applications.
刘渤, 周卫民, 陈燕, 等. 基于水溶性煤沥青的MnO@C复合材料的制备及储锂性能研究[J]. 表面技术, 2023, 52(1): 298-305.
LIU Bo, ZHOU WEI-min, CHEN Yan, et al. Preparation and Lithium Storage Properties of MnO@C Composites Based on the Water Soluble Pitches[J]. Surface Technology, 2023, 52(1): 298-305.
责任编辑:万长清

