硅基纳米探针用于眼部疾病的成像检测与治疗
储彬彬,何 耀
(苏州大学功能纳米与软物质研究院,苏州市纳米技术与生物医学重点实验室,苏州 215123)
1 Introduction
The eyes are one of the most important sensory organs of human beings,which connect to cerebral sensory nervous system,providing above 80%of the knowledge in the brain.Anatomically,the eyeball is mainly composed of eyeball wall,eye cavity and contents,nerves and blood vessels.It is well recognized that the eyes possess several particular physiological barriers,covering anterior segment barriers and posterior segment barriers,potential for preventing the pathogen invasion[1].In detail,the anterior segment barriers comprise of conjunctival barriers,cornea,and tear film;the posterior segment barriers are constitutive of blood-retinal barrier(BRB)and inner limiting membrane[1—3].Despite these unique barriers,millions of individuals worldwide suffer from ocular diseases,including but not limited to corneal diseases,vitreous/retinal diseases,glaucoma,dry eye syndrome,myopia and ocular tumors[4—6].Most of ophthalmic diseases can lead to serious visual impairment and even blindness,thus substantially affecting the quality of life.The last decade has witnessed a surge in pathogenic investigations to elucidate various pharmacological interventions for diverse ocular diseases[6,7].For example,many types of nanomedicine(e.g.,the functional nanomaterials-based theranostic systems)have been developed,aiming at effective analysis and treatment of ocular diseases[1,4—8].However,the existing modes of drug delivery for treating eye diseases,such as eye drops,intravitreal or intravenous administration have some intrinsic deficiencies(e.g.,poor permeation,penetrating the BRB with difficulty,ineffective distribution and insufficient bioavailability)due to the presence of ocular barriers[9,10].Additionally,most of the theranostic strategies are unable to rapidly and specifically diagnose ocular diseases in the early stage,and can not accurately monitor the visual recovery after the administration[11].Therefore,there is an urgent need to design safer and more efficient alternatives to combat ophthalmic diseases.
Owing to favorable biocompatibility[12—15],various kinds of silicon-based nanomaterials have receive widespread concerns in biomedicine[16—22].We have witnessed the rapid advancements in developments of various silicon-based nanostructures,including porous silicon(pSi)particles[23],mesoporous silica nano-particles(MSNs)[24],silicon/silica nanoparticles(SiNPs)[21,22,25],silicon nanorods(SiNRs)[26,27],silicon nanowires(SiNWs)[28],and the silicon nanoneedles(SiNNs)array[29—31]viatop-down(i.e.,electrochemical/chemical etching method,laser ablation or pyrolysis,thermal decomposition,microwave-assisted synthetic method)or bottom-up(i.e.,hydrothermal/solvent heating,microwave-assistant method,photochemical approach,room temperature-related synthesis)synthetic methods[14,32].Furthermore,it has been previously demonstrated that silicon nanomaterials additionally modified with functional molecules/groups or loaded with therapeutic drugs/agents are suitable for diagnosing and treating different diseases[22].Particularly,the SiNPs or SiNWs functionalized with therapeutic drugs or nanoagents[e.g.,doxorubicin(DOX),curcumin,short interfering RNA(siRNA),iron oxide nanoparticles(IONPs),gold nanoparticles(AuNPs),etc.]can be employed for synergistic cancer therapy[33—37];the SiNPs modified with dopamine(DA)and rhodamine B isothiocyanate(RBITC)or SiNRs doped with europium(Eu)can be used for long-term and real-time tracing of pH changes in live cells[27,38];the fluorescent SiNPs conjugated with targeting groups[e.g.,cyclo-(Arg-Gly-Asp-D-Tyr-Cys)(c-RGDyC),folic acid,antibodies,etc.]are able to be utilized in accurate imaging analysis and treatment of different diseases(e.g.,ocular diseases)[39—41].Beyond the above synthesis and bioapplications of silicon nanomaterials,organosilane and herbal(e.g.,Panax notoginseng,Plumbago indica,etc.)or green tea extracts have been exploited as reaction precursors under the microwave treatments to develop silicon-based nanomaterials featuring strong and stable fluorescence,as well as intrinsic anticancer,antibacterial and/or wound healing activity,suitable for multimodal therapy of diverse diseases[42—46].In addition to the above-mentioned achievement,intensive efforts have been made to achieve exciting progressions in the field of detection and therapy of eye-related diseases by using silicon-based nanoprobes,which will be representatively illustrated in this review.
Herein,this review will particularly focus on recent advancements in the preparation of silicon-based nanoprobes(made of MSNs,SiNPs and SiNNs modified with other functional molecules or groups)and their applications in the detection and treatment of ocular diseases,including corneal diseases[e.g.,corneal neovascularization(NV),bacterial keratitis],retinal diseases(e.g.,retinitis pigmentosa,retinal NV),glaucoma and so forth(Fig.1).In the first section of this review,we mainly give an account of the design and construction of silicon-based nanoprobes for analyzing and treating corneal diseases,such as corneal NV and bacterial keratitis,in the early stage.In the next section,we introduce the functionalization of silicon nanomaterials for long-term monitoring of intraocular drug delivery and real-time detection of retinal diseases,such as retinitis pigmentosa(RP)and retinal NV,in different living models.Following that,we illustrate the typical examples of silicon-based nanoprobes for real-time imaging analysis and high-efficacy therapy of glaucoma and other eyerelated diseases.Finally,we further discuss the future perspective of silicon-based nanoprobes for the imaging detection and therapy of ocular diseases.
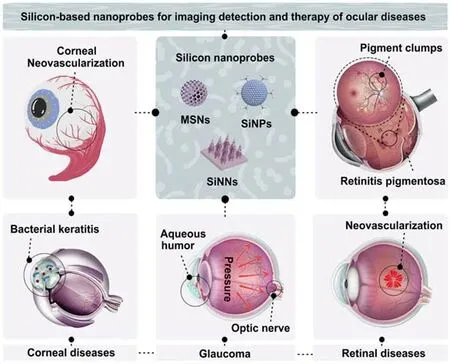
Fig.1 Silicon-based nanoprobes for imaging detection and therapy of ocular diseases
2 Corneal Diseases
Corneal disease is one of the most severe ocular diseases,leading to vision loss and even blindness.It is well known that the normal cornea of the anterior eye is transparent,avascular and immune-privileged structure,whose avascularity can be maintained by balancing angiogenetic and antiangiogenetic factors[47,48].Each keratopathy is seen in association with different clinical scenarios.Hence,many types of silicon-based nanosystems(e.g.,hybrid silicon-lipid-based delivery system,silicon contact lens,silica-thermogel nanohybrids,etc.)have been developed to achieve the goals of therapying corneal diseases and promoting corneal epithelial healing,without showing obvious cytotoxicity to the corneal epithelial cells[49—51].
2.1 Corneal Neovascularization
The corneal NV,meaning the unusual ingrowth of new blood vessels from the limbal plexus,is capable of affecting the corneal avascularity and further threatening the vision[52,53].Hence,it is important to develop the diagnostic technology with reliability and accuracy for detecting corneal NV in clinical trials.To date,several imaging and diagnostic technologies[e.g.,slit lamp microscopy,ultrasound biomicroscope,optical coherence tomography(OCT),etc.]have been exploited for clinical imaging analysis of corneal NV[52,54].Nevertheless,it is still need to develop new imaging methods,suitable for identifying blood vessels in corneal scaring and monitoring the treatment response of corneal NV.In this regard,fluorescent and photoacoustic imaging have drawn extensive attention in the visual detection of corneal NV[52,54—56].The previously reported fluorescein angiography was firstly employed to visually observe the new blood vessels on cornea.Then,various types of antibody-based inhibitors of the vascular endothelial growth factor(VEGF)were intravitreally injected to treat corneal NV[57].Recently,growing efforts have focused on designing safer and more effective ocular nanomedicines based on various silicon nanomaterials[58,59].Particularly,Tanget al.[60]designed a novel kind of the c-(RGDyC)-conjugated SiNPs(SiNPs-RGD),possessing strong fluorescence coupled with good photostability,as well as the specific targeting capability to angiogenic endothelial cells,capable of labeling angiogenic blood vessels on cornea.As presented in Fig.2(A)and(B),the slit-lamp images of corneal NV indicated that the unusual blood vessels began to obviously invade into the cornea from the limbus toward the suture stitches at day 1 and further grew to reach the stitches after the 7 d alkali-burn injury.These imaging results demonstrate the successful construction of corneal NV models.As further exhibited in Fig.2(C),compared with negligible fluorescence signals in SiNPs-treated groups,obvious green fluorescence was observed in the SiNPs-RGD-labeled corneal NV,showing a similar distribution pattern to the Alexa Fluor 568 conjugated-isolectin IB4 coupled with the good co-localization[Fig.2(C)].This study hence provides the imaging evidence that SiNPs-RGD indeed have the ability to selectively and visually identify the new blood vessels on corneain vivo.Furthermore,Fig.2(D)presented that the constructed SiNPs-RGD could also inhibit the growth of corneal NV after 14 d.In 2021,Lyu and coworkers[53]utilized the MSNs modified with the bevacizumab(one of anti-VEGF drugs)and cyclosporine A(one of anti-inflammation drugs)to inhibit corneal NV after the subconjunctival injection.Recently,to help address the challenges and limitations in ocular delivery(e.g.,large dose or frequent topical administration,unavoidable side effects,ocular barriers,etc.),Parket al.[61]developed a tear-soluble contact lens modified with the biodegradable SiNNs,featuring minimal corneal damage,painless injection,precise dosage adjustment and leakage-free drug loading ability[29—31],suitable for the painless and sustained delivery of ocular drugs.The SiNNs-based contact lens could be firstly inserted into the corneal epithelial layer and then they were dissolved by tear to release ocular drugs in a long-term and sustained manner.The fluorescence imaging analysis obtained from confocal laser scanning microscopy(CLSM)indicated that most of IgG 647(red fluorescence signals)remained at the surface of SiNNs-based contact lens in cornea over 48 h whereas eye drops(ca.1 to 30 min)and ointments(ca.1 to 8 h)have the shorter residence time[61],suggesting the long-term sustained delivery features of SiNNs-based systems.Based on these above advantages,the constructed SiNNs-based systems were demonstrated to show obvious therapeutic efficacy to inhibit the growth of corneal NVviathe multi-model imaging analysis.The crosssectional OCT images of the rabbit eye under therapy could provide the imaging evidence that SiNNs-based systems had the ability to promote the corneal recovery,also suggesting distinct therapeutic effects of SiNNs-based systems for corneal NV.
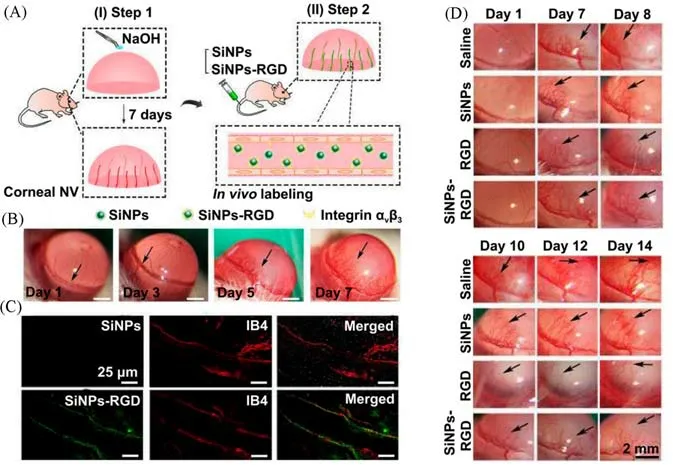
Fig.2 Silicon-based nanoprobes for imaging analysis and treatment of corneal NV[60]
2.2 Bacterial Keratitis
Bacterial keratitis,known as a typical class of infectious keratitis,produces serious threat to the visual system[22,62,63].In a medical emergency,most bacterial keratitis,such asStaphylococcus aureus(S.aureus)-induced keratitis,are able to cause blindness within days when the corneal stroma is completely destroyed.However,established diagnostic techniques(e.g.,Gram staining,bacterial culture and so on)need to take hours to days due to the tedious manipulation[64].More significantly,the early and effective treatment would greatly inhibit progress of the disease and hinder the visual impairment[65].Therefore,it is challenging to design novel technologies,which is capable of effective detection and therapy of bacterial keratitis in the early stage.The past decade has witnessed that many types of silicon-based nanoprobes are fabricated for the imaging and therapy of bacterial keratitis[22,62—65].For instance,Jeonget al.[66]developed the SiNPs-based nitric oxide(NO)delivery system,which was constituted of branched polyethylene imine(BPEI)-modified SiNPs loaded with NO[Fig.3(A)],effective for killing bacteria in cornea rather than normal corneal cells by the release of NO gas[Fig.3(B)].As shown in Fig.3(C),the designed SiNPs delivery systems could be used to combat bacterial keratitis.Due to the unique fluorescent property and easy modification of SiNPs,Zhanget al.[67]designed a novel SiNPs-based nanoagents[Fig.3(D)],which are made of classic vancomycin(Van)molecules-modified fluorescent SiNPs(SiNPs-Van),to rapidly,effectively and sensitively diagnose and therapy the bacterial keratitis.The existence of Van molecules could lead the SiNPs-Van to quickly target and image bacterial infections on cornea.The fluorescence images in Fig.3(E)presented that the SiNPs-Vantreated infected cornea exhibited obvious green fluorescence signals while ignorable fluorescence signals were observed in normal cornea,indicating the specific imaging ability of SiNPs-Van for bacterial keratitis.It was important to note that the constructed probes of SiNPs-Van could be exploited to detect theS.aureus-induced keratitisviarapid fluorescence imaging technology in real-time manners,which could exhibit obvious fluorescent signal on the infected corneas duringca.10 min.Moreover,the slit-lamp images[Fig.3(F)]demonstrated that the SiNPs-Van could also therapyS.aureus-induced keratitis.Overall,these new findings would offer new opportunities for developing novel silicon-based theranostic nanoagents for high-efficacy treatment of corneal diseases.

Fig.3 Silicon-based nanoagents for rapid detection and effective treatment of bacterial keratitis
3 Retinal Diseases
Retinal disease,known as a typical kind of ocular diseases,includes retinitis pigmentosa(RP),retinal vein occlusions(RVOs),age-related macular degeneration(AMD),diabetic retinopathy(DR)and tumours(e.g.,retinal retinoblastoma)[68—70].In clinical practice,both fluorescein angiography(FA)and indocyanine green angiography(ICGA)are recognized as the standard diagnostic method to visually assess vascular abnormalities in retinal disease[71].Particularly,the indocyanine green(ICG),as one of the near infrared(NIR)contrast agents featuring relatively high tissue depth penetration,is suitable for imaging choroidal vasculature.However,the weak fluorescence signals of ICG agents would limit their imaging sensitivity.In order to systematically and accurately identify diagnostic information in chorioretinal diseases,the combination imaging of fluorescein sodium(FS)and ICG is employed to analyze fundus diseases[22,72].Throughout the diagnosis,the detailed long-term and dynamic information of fundus diseases is inevitably lost owing to their short blood residency and the deficient targeting capacity of small-molecule organic dyes in the eye.Taking advantages of unique optical properties and facile surface modification,silicon-based nanoprobes,such as MSNs,SiNPs and silica-metal-organic framework(SMOF),have been developed for the diagnosis and treatment of retinal disease[72—74].
3.1 Retinitis Pigmentosa
Retinitis pigmentosa(RP),as one kind of the retinal diseases,is able to affect the degeneration of retina and further lead to the inherited blindness in adults[75—77].Many important researches have revealed that mutations in more than 80 genes are associated with non-syndromic RP[77].As thus,gene therapy is considered as an effective and potential strategy for treating RP.However,the existing systems[e.g.,adenoassociated viral vectors(AAVs),etc.]are difficult to simultaneously deliver coding sequences and silencers to retinal lesions[77].Therefore,in order to meet the needs of delivery of multiple functional drugs,including hydrophilic drugs,nucleic acids,proteins,and any combination thereof,the silicon-based nanosystems have been designed and developed[75—77].In 2020,Wang and co-workers[75]designed a new type of silicon-based nanohybrids which constitutes of silica nanoparticle(SNP)and metal-organic framework nanoparticles,capable of being acted as drug delivery nanosystems.The prepared SMOF is demonstrated to effectively deliver Cas9-sgRNA ribonucleoprotein(RNP)to retinal pigment epithelium(RPE)of mouse after the subretinal injection.After that,the same group further designed a stimulus-responsive SNP for effectively delivering RNP and/or nucleic acid into the RPE tissue of mouse[76].It is worth noting that nucleic acid delivery/genome editing efficiency of SNP were investigated in the transgenic Ai14 mice.More recently,Valdés-Sánchezet al.[77]utilized the MSiNPs which contain amino groups on their surface(namely N-MSiNPs)as a novel kind of delivery systems to effectively transport gene for curing the RP diseases.In brief,N-MSiNPs prepared through the sol-gel strategy were demonstrated to have the ability to load and deliver plasmid DNA,such as exogenous PRPF31-GFP transgene,to the RPE cell layer of mouse retina after the subretinal injection.Fig.4(A)and(B)showed that the constructed PRPF31-GFP plasmid-loading N-MSiNPs could be transfected into HEK-293T cellsin vitroto induce the significant expression of foreign genes in nucleus.In addition,all of the fundus images,fluorescent fundus and immunostaining of retinal sections provide imaging evidence that the constructed N-MSiNPs can deliver the gene of PRPF31-GFP plasmid to RPE cells of experimental animals,potential for imaging analysis and treatments of RP[Fig.4(C)—(F)].These results suggest that N-MSiNPs is able to be utilized as a safe vector to deliver the potentially therapeutic genes into retinal lesions for treating the RP diseases.
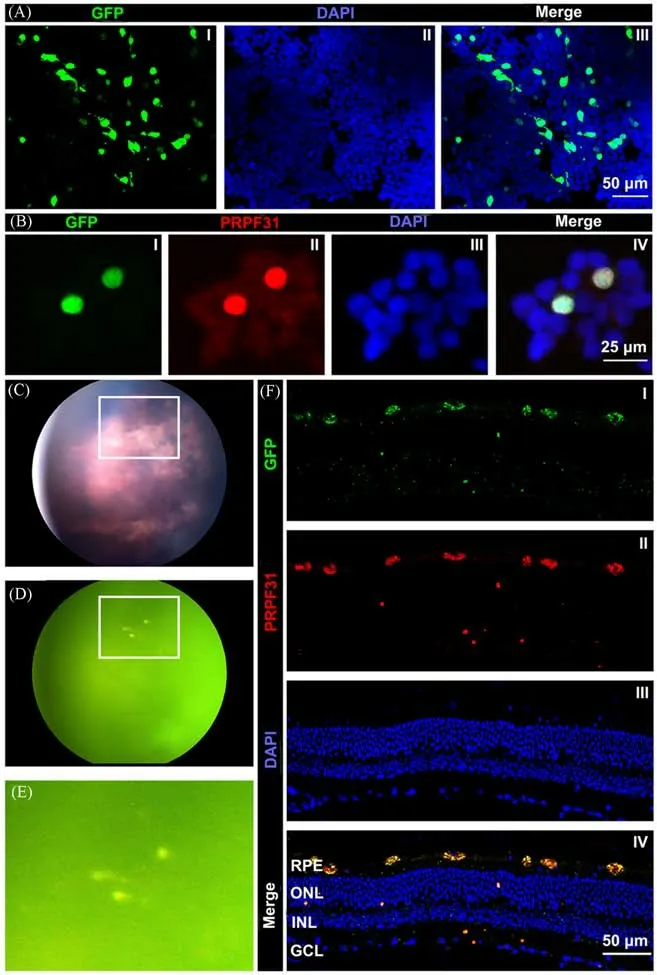
Fig.4 SiNPs-based nanoplatform for the delivery of gene and treatment in RP diseases[77]
3.2 Retinal Neovascularization
Retinal neovascularization(NV),mainly involving DR,RVOs,AMD and retinopathy of prematurity(ROP),could lead to serious vision loss,and thus threaten healthy lives of human worldwide[78].It has been mentioned in previous studies that retina contains abnormally high levels of VEGF in these diseases,which can be utilized to develop novel kinds of anti-VEGF agents for treating retinal NV[79—81].Many types of nanomaterials-based delivery systems thus have been synthesized to inhibit the retinal NV[80].In addition,many other therapeutic strategies,such as vitrectomy,panretinal photocoagulation and cryotherapy,have been utilized to therapy retinal NV[81].Despite these achievements in therapying the retinal NV,without timely and effective treatment,the retinal NV can seriously lead to vitreous hemorrhage and even induce the happening of tractional retinal detachments[81].Hence,early and accurate diagnosis of retinal NV is of significance to overcome difficulties in treating visual damages.Intriguingly,silicon-based nanoprobes have their own unique merits,particularly in visual imaging analysis of retinal vessels in long-term and real-time manners[72].Tanget al.[72]developed a novel kind of fluorescent SiNPs prepared through the microwave-assisted synthetic strategy,simultaneously featuring negligible toxicity,strong fluorescence as well as ultra-high photostability[Fig.5(A)].Their research highlighted that the prepared SiNPs offered the ability to be utilized for the longterm visualization of retinal vessels in rat models thanks to their strong and stable fluorescence[Fig.5(B)].Of particular note,the authors further employed cynomolgus macaque for constructing animal models,in which the real-time and dynamic changes of choroidal NV and drusen could be readily monitored[Fig.5(C)].Fig.5(D)further showed that the FS-labeled retinal capillaries were hardly observed when the imaging time was up to 1 min and disappeared completely after 5 min;in sharp contrast,the fluorescent SiNPs-labeled retinal vessels could be clearly detected even after the 10-min observation.These results revealed that the constructed silicon-based nanoprobes possess good photostability,potential for long-term and real-time imaging analysis of ocular diseases.Furthermore,as exhibited in Fig.5(E)and(F),the congenital tortuous retinal vessels could be vividly observed in a cynomolgus macaque model with choroidal NV induced by the laser irradiation.In wet AMD,the increased microvascular permeability ultimately resulted in the bleeding,and thus the constructed SiNPs probes could leak in choroidal NV lesions over time[Fig.5(F),Ⅰ,Ⅲ].This study provides the visual imaging evidence to support the potential applications for diagnosing retinal diseases in different models,ranging from small animal to non-human primate-animal models.

Fig.5 Fluorescent SiNPs-based nanoagents for long-term and real-time imaging detection of retinal diseases in animal models[72]
4 Glaucoma and Other Eye-related Diseases
Glaucoma,one of the progressive optic neuropathies,can induce the concomitant pattern of vision loss through slowly degenerating the retinal ganglion cells(RGCs)and their axons[68,82].According to the statistics,approximately 76 million people are threatened by the glaucoma disease around the world during the last few decades[82].Hence,treatment of glaucoma diseases in an early stage is of significance and necessity.Although the pathogeny of glaucoma is generally believed to be multifactorial,intraocular pressure(IOP)is still one of the significant indicative factors in the diagnosis and treatment of glaucoma[83].It is therefore believed that,lowering IOP can effectively relieve the visual deterioration or partially restore vision,and it thus becomes an important concern for detecting and therapying glaucoma.So far,numerous types of noninvasive IOP sensors have been designed and developed for the diagnosis of glaucoma disease[83].In addition,currently existing medications,including prostaglandin derivatives,latanoprost,carbonic anhydrase inhibitors and brinzolamide,can be exploited to improve the outflow of the uveoscleral pathway or reduce aqueous humor formation to finally therapy glaucoma[84].For example,the gelatin/amino-functional MSNs loaded with glaucoma-related drugs,such as pilocarpine and/or brimonidine,are demonstrated to have the ability to effectively cure the glaucoma disease,by simultaneously increasing the bioavailability of drugs and decreasing the IOP[85—87].Moreover,recent reports provide the evidence that the nitric oxide can effectively target the conventional outflow pathway and further reduce IOP,which is approved by the Food and Drug Administration(FDA).However,it is notable that the corresponding ocular drugs are hardly transported to the anterior chamber(AC)of the eyeviathe popular oral or intravenous administration routes,because the human eye have unique physiological barriers,such as blood-ocular barriers.Moreover,several serious limitations,including the blinking,tear turnover,and naso-lachrymal drainage,can hinder the bioavailability of ocular drugs as the eye drops.Therefore,with an aim to improve the therapeutic efficiency of glaucoma diseases,the nanomaterials-based drug delivery has been designed to address the challenge of bioavailability,barriers penetration,lesion targeting and long-lasting treatment[85—88].Given the significance of barriers penetration and lesion targeting in glaucoma therapy,it is conceivable that the direct and visual analysis of the delivery route of drugs is much meaningful.Recently,Hu and co-workers[84]presented the detailed information of fluoresce-labeled MSNs moving from the ocular surface to the target AC through the cornea tissues[Fig.6(A)],providing direct imaging evidence for effective penetration and targeting of glaucoma.In this system,the constructed FITC-MSNs were utilized as an eye drop for imaging analysis and therapy of glaucoma.As further exhibited in Fig.6(B),obvious fluorescence signals could be observed in the cornea after the 1 h treatment of FITC-MSNs,suggesting that FITC-MSNs could effectively accumulate in the cornea section;meanwhile,both trabecular meshwork(TM)and Schlemm’s channel(SC),two important parts of the AC,exhibited weak green fluorescence signals,indicating that the as-prepared FITC-MSNs could effectively travel from the corneal surface to the target tissue.After that,the increasing fluorescence signals could be observed in both TM and SC tissues with the time increase,while the decreasing fluorescence signals were detected in the cornea.After the treatment of FITC-MSNs for 12 h,the fluorescence intensity gradually decreased with the time increase,and almost no fluorescence signals could be observed after 56 h.This study provides a detailed understanding of how eye drop penetrates and reaches the target tissues to treat glaucoma,beneficial for designing highquality drug delivery nanosystems.
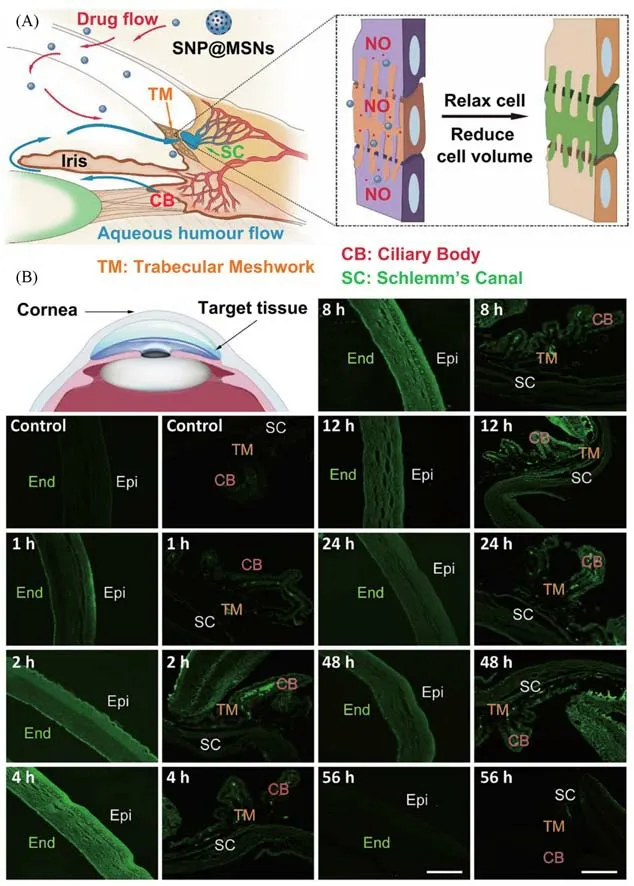
Fig.6 Long-term and real-time visualization of fluorescein-labeled MSNs moving from the ocular surface through the cornea into the anterior chamber[84]
In addition,various functional MSNs and silicon quantum dots(SiQDs)have been designed and constructed to detect and treat other eye-related diseases,such as pterygium and dry eye disease[89,90].In 2020,Wu and co-workers[89]developed the low-density lipoprotein(LDL)-conjugated MSNs loaded with mitomycin C(MMC)drugs(MMC@MSNs-LDL)to inhibit the proliferation of pterygium subconjunctival fibroblasts.In detail,the constructed MMC@MSNs-LDL could specifically prevent the proliferation of pterygium subconjunctival fibroblasts,rather than that of normal fibroblasts,potential for administering the pterygium recurrence.In 2022,Sarwatet al.[90]synthesized different metals[e.g.,scandium(Sc),copper(Cu),zinc(Zn),etc.]-doped SiQDs and further took them as the novel biomarker for the imaging analysis of tear film.They indicated that three kinds of metal-doped SiQDs possessed the feasibility for studying tear film dynamics,potential for the future bioimaging detection of dry eye disease.
5 Summary and Outlook
In summary,we have introduced representative studies to highlight the recent advancements and achievements in the development of silicon-based nanoprobes and imaging strategies,capable of high-efficacy diagnosis and treatment of ocular diseases(e.g.,corneal diseases,retinal diseases,glaucoma and other eye-related diseases).Typically,in the virtue of unique optical features and favorable biocompatibility,the silicon-based nanoprobes have received intensive concerns in the rapid detection of corneal diseases,long-term tracking of retinal diseases and real-time labeling of glaucoma.
Despite the above-mentioned progresses in silicon-based nanoprobes for the detection and treatment of ocular diseases,there are several inescapable limitations and/or major challenges that emerge.An important aspect of limitations is that the fluorescence signals of silicon-based nanoprobes mainly locate in visible range(e.g.,from blue and green to pink colors),which is adverse to the imaging sensitivity because of the limited tissue penetration depth.As hence,it is crucial to develop novel type of silicon-based nanoprobes featuring truly red-and near infrared(NIR)-emitting fluorescence or phosphorescence signals,beneficial to further improve imaging effects and accuracy in the detection of eye-related diseases.The use of specific surface ligands or doping elements(e.g.,rare earth or metal elements,etc.)is expected to improve the photoluminescent quantum yield and develop the truly NIR-emitting fluorescent or phosphorescent silicon-based nanoprobes.The second aspect is that most existing silicon-based nanoprobes lack the capability of barriers penetration and lesions targeting,which can reduce the therapeutic efficacy and increase potential side effects.As thus,it is of significance to develop the high-quality silicon-based nanoprobes with barriers penetration and lesions targeting ability,thus capable of improving the imaging detection and therapy of ocular diseases.Another important aspect of limitations is that the existing silicon-based nanoprobes are limited in the laboratorial environments,mainly including small animal(e.g.,mouse and rabbit)and non-human primate-animal models(e.g.,cynomolgus macaque models),in which seldom imaging applications involve in the actual clinical cases.Therefore,it is an emerged need to design the higher-performance silicon-based nanoprobes simultaneously featuring excellent optical property,good biocompatibility,ocular barriers penetration and lesions targeting ability to promote their translation in clinic applications.The utilization of functional ligands(e.g.,the RGD peptides,saccharides,etc.)or cell membrane(e.g.,retinoblastoma cell membrane,erythrocyte membrane,etc.)would provide possibility for endowing silicon-based nanoprobes with the targeting and barriers penetration ability,potentially capable of penetrating and targeting the ocular lesions.We thus expect that accompanied by the deepening understanding of above challenges,the silicon-based nanoprobes would raise new perspectives for precise imaging detection and effective therapy of different ophthalmic diseases in biomedical and clinical studies.

