Glial cell line-derived neurotrophic factor and brainderived neurotrophic factor regulate the interaction between astrocytes and Schwann cells at the trigeminal root entry zone
Madeha Ishag Adam,Ling Lin,Amir Mahmoud Makin,Xiao-Fen Zhang,Lu-Xi Zhou,Xin-Yue Liao,Li Zhao,Feng Wang,,Dao-Shu Luo,
Abstract The trigeminal root entry zone is the zone at which the myelination switches from peripheral Schwann cells to central oligodendrocytes.Its special anatomical and physiological structure renders it susceptible to nerve injury.The etiology of most primary trigeminal neuralgia is closely related to microvascular compression of the trigeminal root entry zone.This study aimed to develop an efficient in vitro model mimicking the glial environment of trigeminal root entry zone as a tool to investigate the effects of glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor on the structural and functional integrity of trigeminal root entry zone and modulation of cellular interactions.Primary astrocytes and Schwann cells isolated from trigeminal root entry zone of postnatal rats were inoculated into a two-well silicon culture insert to mimic the trigeminal root entry zone microenvironment and treated with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor.In monoculture,glial cell line-derived neurotrophic factor promoted the migration of Schwann cells,but it did not have effects on the migration of astrocytes.In the co-culture system,glial cell line-derived neurotrophic factor promoted the bidirectional migration of astrocytes and Schwann cells.Brain-derived neurotrophic factor markedly promoted the activation and migration of astrocytes.However,in the co-culture system,brain-derived neurotrophic factor inhibited the migration of astrocytes and Schwann cells to a certain degree.These findings suggest that glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor are involved in the regulation of the astrocyte-Schwann cell interaction in the co-culture system derived from the trigeminal root entry zone.This system can be used as a cell model to study the mechanism of glial dysregulation associated with trigeminal nerve injury and possible therapeutic interventions.
Key Words: astrocytes;brain-derived neurotrophic factor;cell migration;glial cell line-derived neurotrophic factor;glial interaction;Schwann cells;trigeminal nerve
From the Contents
Introduction 1364
Methods 1365
Results 1366
Discussion 1369
Introduction
The trigeminal root entry zone (TREZ) is a boundary that anatomically connects the peripheral nervous system (PNS) and central nervous system(CNS).This narrow zone is the point at which myelination switches from being mediated by peripheral Schwann cells to myelination mediated by central oligodendrocytes (Toma et al.,2006).The glial environment at the TREZ has an organized architecture;astrocytes and oligodendrocytes occupy the central part of the TREZ,whereas Schwann cells are located at the peripheral part (Fraher,1992,2000).Microvascular compression of the trigeminal nerve at the entry zone results in trigeminal neuralgia,which causes neuropathic pain in most primary trigeminal neuralgia patients (Araya et al.,2020;Yin et al.,2022).The regeneration rate and number of fibers at the CNS/PNS interface are more sluggish and limited,respectively,as axons pass from the peripheral to the central compartment.Astrocytes play a tightly regulated role in the creation of the refractory boundary that limits the movement of growing axons (Dieb and Hafidi,2013).Recently,TREZ has attracted much attention and researchers have begun to unravel the mechanisms and factors that direct glial cells toward and across the transition zone of TREZ.
Neurotrophic factors play key roles in the development and maintenance of both the CNS and PNS (Ji et al.,2020;Idrisova et al.,2022).Glial cell linederived neurotrophic factor (GDNF) was first identified as a dopaminergic neuron survival factor.GDNF was subsequently discovered to have functions extending beyond survival,such as maintaining and promoting proliferation,differentiation,maturation and neurite outgrowth (Cortés et al.,2017).Moreover,GDNF may be a key factor for glial cells that contributes to the development and maintenance of neuropathic pain.Brain-derived neurotrophic factor (BDNF) is expressed widely in neurons and glial cells throughout the brain and spinal cord (Donnerer and Liebmann,2018).BDNF improves neuronal survival and enhances myelinating Schwann cells by interacting with the neurotrophic receptor P75NTR(Angelova and Angelov,2017;Palasz et al.,2020;Beura et al.,2022).Additionally,BDNF has been recognized as a multifunctional factor that regulates multiple functions including cell migration,phenotypic differentiation and axonal growth (Kotla et al.,2021).Both GDNF and BDNF are involved in neuropathic pain and play key roles as pain modulators/mediators (Park and Poo,2013;Luo et al.,2020).During trigeminal nerve injury,the TREZ can be disrupted and the CNS/PNS components are vulnerable to transgression,which is involved in the initiation and maintenance of chronic pain (Coulpier et al.,2010).Overwhelming evidence has demonstrated the effect of neurotrophic factors on axonal regeneration,which may be mediated through their effect on glial cell behavior (Zhu et al.,2020;Kotliarova and Sidorova,2021).However,the role of these neurotrophic factors in the regulation of TREZ glial compartments is unclear.
We hypothesized that cellular interactions at the CNS/PNS interface of the TREZ are regulated by neurotrophic factors that regulate cellular segregation at the boundary of the TREZ.In this study,we used a two-well silicon culture insert as a co-culture system of astrocytes and Schwann cells,mimicking the biological and physiological interactions of the TREZ microenvironmentin vivo,to evaluate the effects of GDNF and BDNF on glial cell interactionsin vitro.
Methods
Animals
Primary astrocytes and Schwann cells were prepared from neonatal Sprague-Dawley rats (3–5 days old;provided by the Experimental Animal Center of Fujian Medical University,license No.SCXK (Min) 2016-0002).All animal experiments were approved by the Animal Care and Use Committee of Fujian Medical University (approval No.SYXK (Min) 2020-0005) on July 17,2020 and carried out in compliance with the National Institute of Health,Guide for the Care and Use of Laboratory Animals (8thed,2011).
Schwann cell culture
A schematic for the harvesting of TREZ and cell purification is shown inFigure1.Schwann cells were cultured following a previously described procedure(Andersen et al.,2016).Briefly,12 postnatal rats (age ranging from postnatal days 3 to 5) were exposed to 3% isoflurane (RWD Life Science,Shenzhen,China) for approximately 1 minute,sterilized with 75% alcohol and sacrificed by cervical dislocation.Trigeminal roots were aseptically harvested under a dissecting microscope.The collected nerve segments were placed in a 35 mm Petri dish containing ice-cold Dulbecco’s modified Eagle’s medium(DMEM;Cat# 11330,Thermo Fisher,Waltham,MA,USA) and chopped into fragments with ophthalmic scissors.The nerve fragments were incubated in a mixture of 1 mL of 0.1% collagenase (Solarbio,Beijing,China) with 1 mL of 0.25% trypsin-ethylenediaminetetraacetic acid (Cat# 25200072,Gibco,Carlsbad,CA,USA) at 37°C for 25 minutes.To inhibit enzymatic reaction,enzyme inhibitor solution containing DMEM-F12 supplemented with 10%fetal bovine serum (FBS,Sigma-Aldrich,St.Louis,MO,USA) and 1% penicillin/streptomycin (Boster Biological Technology,Wuhan,China) was added and the sample was centrifuged at 174.4 ×gfor 5 minutes.The supernatant was discarded;the pellet was resuspended in DMEM containing 10% FBS and triturated using flamed Pasteur pipettes until a homogenous cell suspension was obtained.The cell suspension was plated on flasks pre-coated with poly-L-lysine (PLL;Solarbio) and kept in a humidified incubator (37°C,5% CO2,95%air).On the following day,cytosine arabinoside (AraC,1:1000,Sigma-Aldrich)was added to the medium and cells were cultured for 48 hours.Schwann cells were incubated in DMEM-D-valine (Sigma-Aldrich) supplemented with 2 μM Forskolin (Sciencell,San Diego,CA,USA),20 mg/mL bovine pituitary extract (BPE,Sciencell) and 10% FBS.The cells were grown to confluence and transferred to new dishes twice.The purity of Schwann cells was determined by immunofluorescence for P75NTR(Additional Figure 1).
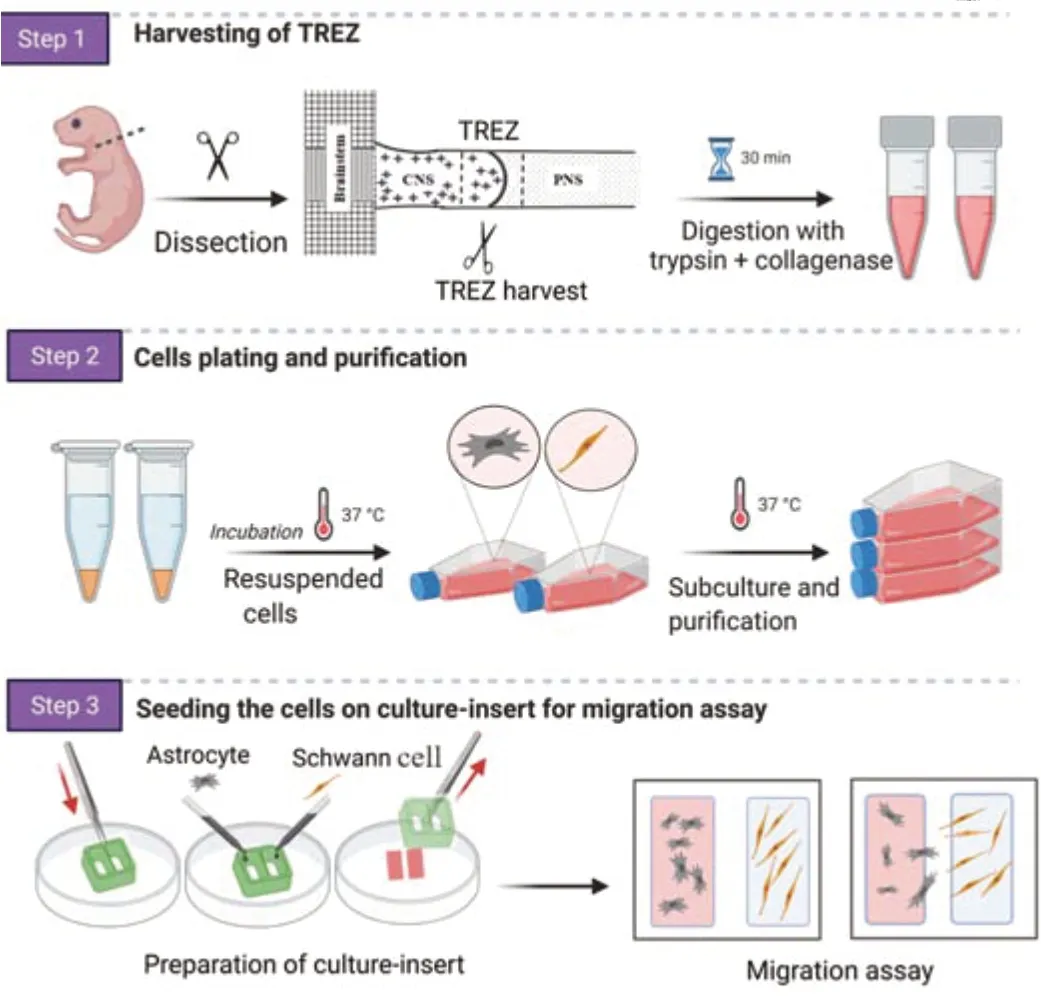
Figure 1|Schematic diagram of the experimental procedure of the preparation of primary cell culture from the trigeminal root entry zone (TREZ) from rats and an overview of the in vitro model.
Astrocyte culture
Astrocytes from the TREZ of postnatal rats were harvested as described above and digested with a combination of 0.1% collagenase and 0.25% trypsinethylenediaminetetraacetic acid for 25 minutes.The enzyme solution was removed and DMEM-F12 with 10% FBS was added.After centrifugation at 174.4 ×gfor 5 minutes,the cells were plated into a T25 flask pre-coated with PLL and allowed to proliferate in DMEM-F12 with 10% FBS and 1% penicillin/streptomycin.Once the cells reached 90% confluence (7–10 days),flasks were shaken overnight at 240 r/min to remove the oligodendrocytes and microglia on top of the astrocyte monolayer (Lian et al.,2016).Floating cells were removed by two washes with calcium/magnesium-free Hanks balance salt solution (HBSS;Thermo Fisher) and fresh medium was added.The purified cells were then subcultured,re-plated in 35 mm Petri dishes and maintained at 37°C in a 5% CO2incubator.Astrocytes were identified with GFAP-positive staining.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
To assess the effect of GDNF and BDNF on cell viability and proliferation,3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed as previously reported (Xiong et al.,2013).In brief,cells were seeded at a density of 5 × 103in 96 well plates and incubated with serumfree medium at 37°C in a 5% CO2incubator for 24 hours.On the following day,cells were treated with recombinant BDNF (10,30 and 50 ng/mL,Cat# B3795,Sigma,Shanghai,China) or recombinant GDNF (10,20,30,50 and 100 ng/mL;Absin,Cat# abs00928,Shanghai,China) for 24 and 48 hours.Untreated cells were included as controls.At the indicated time points,20 μL of 5 mg/mL MTT reagent (Boster Biological Technology,Wuhan,China) was added to each well and cells were incubated for 4 hours in a 5% CO2incubator in the dark.The MTT reagent was removed and 200 μL dimethyl sulfoxide was added for 15 minutes to dissolve the formazan crystal.Cells were evaluated by measuring the absorbance values at 550 nm using 490 nm as a reference wavelength on a microplate reader (Epoch ELX800,BioTek,Houston,TX,USA).The cell viability was calculated using the following formula:

The viability of the cells in the control group was considered as 100%.Each test was conducted in triplicate.
Preparation of the confrontation assay for the in vitro CNS/PNS interface model
Cell migration was examined using two-well silicon culture inserts (Ibidi,Martinsried,Germany) (Cerqueira et al.,2018).A 24-well plate was coated with PLL solution for 1 hour at room temperature 24 ± 2°C,washed with phosphate buffer saline (PBS) and allowed to completely dry under ultraviolet light inside a laminar flow hood.The two-well silicon culture insert was placed at the bottom of each well of the 24-well plate using sterile forceps (Figure1).Before seeding the cells,two parallel straight lines were carefully marked on the outer surface of the culture plate bottom using the position of the silicon culture insert.These lines were used as a reference for the area to be analyzed.
Astrocytes and Schwann cells in culture were rinsed twice with PBS and detached using trypsin-ethylenediaminetetraacetic acid solution 0.25% for 2 minutes.An equal volume of DMEM-F12 with 10% FBS and 1% penicillin/streptomycin was added to each well and cells were collected separately into 15 mL falcon tubes and centrifuged for 5 minutes at 174.4 ×g.The cell densities were adjusted to 9 × 104cell/mL (Schwann cells) and 3 × 104cells/mL(for astrocytes),and 50 μL of each cell suspension was applied separately to each chamber of the two-well culture insert.The cells were cultured until the boundaries between astrocytes and Schwann cells formed.
GDNF and BDNF treatment in the confrontation assay
To assess cellular migration and border formation under GDNF or BDNF treatment,the confrontation assay was established as described above;the medium was aspirated from both chambers and the culture insert was removed from the well.The 500 μm area of the cell free-gap (created by removing the insert) was considered as the initial size at 0 hours.Cells were incubated in freshly prepared serum-free medium (1:1 mixture of Schwann cell medium and astrocyte medium);the serum was removed to eliminate interference from serum proteins on migration.The medium contained recombinant GDNF or recombinant BDNF (50 ng/mL each);this concentration was determined from previous studies on glial cells bothin vivoandin vitro(Santos et al.,2016;Siebert and Osterhout,2021).The plates were cultured for 12,24 and 36 hours and then examined using a light microscope (ERc5S,Carl Zeiss Company,Oberkochen,Germany).Bright field images were taken at the indicated time points and at the same position;exposure time was adjusted and images were captured.Cells that migrated within the free-gap were counted for data analysis and the area of the gap was measured.The following formula was used to calculate the percentage of gap closure as migration rate (Grada et al.,2017):
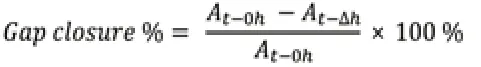
At–0his the area of the gap measured immediately after removing the culture insert (t=0 hour),At–∆his the area of the gap measured h (hours) after the insert was removed.
Time-lapse video microscopy
The confrontation assay was established and astrocytes and Schwann cells were cultured with 50 ng/mL of either GDNF or BDNF as described above.Immediately after GDNF/BDNF-containing medium was administered,cells were transferred to a temperature-and CO2-controlled incubator stage with a confocal laser scanning microscope (Leica SP8,Heidelberger,Germany).Images were taken for 30 hours at 10-minute intervals and video recording was digitized.A representative video showing the migration of cells is shown inAdditional Video 1.
Immunocytochemistry
Astrocytes and Schwann cells in co-culture and monoculture on PLL-coated coverslips under different conditions were fixed with 4% paraformaldehyde(pH 7.4) (Biosharp;Hefei,China) for 20 minutes at room temperature.Samples were washed twice with 0.1 M PBS,followed by permeabilization using 0.25% Triton X100 (Dingguo,Beijing,China) and 1% bovine serum albumin(Beyotime;Shanghai,China) in PBS for 5 minutes at room temperature.After three washes with PBS,cells were blocked in 5% normal goat serum(Moocow,Guangzhou,China) and 3% bovine serum albumin in PBS for 1 hour at room temperature.Samples were then incubated at 4°C overnight with the following primary antibodies: mouse polyclonal anti-GFAP (1:1000,Proteintech,Wuhan,China,Cat# 16825-1-AP,RRID: AB_2109646),rabbit polyclonal anti-P75NTR(1:200,Millipore,Burlington,MA,USA,Cat# 07-476,RRID: AB_310649),and mouse monoclonal anti-GFAP with CY3 conjugation(1:1000,Sigma,St.Louis,MO,USA,Cat# MAB3402C3,RRID: AB_11213580).The next day,cells were washed in PBS at room temperature three times for 5 minutes each.Cells were incubated with donkey anti-rabbit Alexa Fluor Plus 488 (1:1000,Invitrogen,San Diego,CA,USA,Cat# A-21206,RRID:AB_2535792) and goat anti-mouse Alexa Fluor 555 (1:1000,Invitrogen,Cat#A28180,RRID: AB_2536164) secondary antibodies at room temperature for 1 hour in the dark.The samples were washed three times and then incubated for 10 minutes with a 4′,6-diamidino-2-phenylindole (1:1000,Beyotime,Cat#C1002) to stain nuclei.Fluorescence images were visualized using a Leica laser confocal microscope (Leica TCS SP8,Heidelberger,Germany).Astrocytes and Schwann cells were identified with specific glial markers of GFAP and p75NTR,respectively.GFAP-positive astrocyte number,branching profile and primary process lengths were quantified using ImageJ software (v1.8.0,National Institutes of Health,Bethesda,MD,USA;Schneider et al.,2012).
Assessment for astrocyte complexity
To assess whether GDNF or BDNF has a role in morphological alteration of primary astrocytes,immunocytochemical detection for the astrocytic marker GFAP was performed.Astrocytes were cultured from TREZ as described above and cultured for 2 weeks under low-density cell culture.Primary astrocytes were cultured under monoculture and co-culture conditions in the presence or absence of GDNF and BDNF.GFAP Immunostaining for GFAP was then performed.Astrocyte processes were identified by their irregular,concave shape and intermediate filament bundles using the confocal and light microscope.
To analyzein vitromorphological transformation of astrocytes,the area and the perimeter of the astrocyte profile were measured.Any change in astrocyte morphology indicates the cellular response to an altered physiological status induced by administration of GDNF or BDNF,which result in remarkable changes of cell complexity.Thus,the factor that reflects the complexity of a given shape was determined using the shape index equation which is defined as follows (Matsutani and Yamamoto,1997):
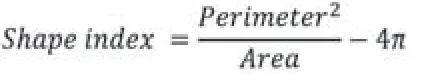
Western blot assay
Astrocytes and Schwann cells were cultured separately at an approximate density of 3 × 104cells,then treated with 50 ng/mL BDNF or GDNF for 24 and 48 hours.Cells were lysed in fresh radio immunoprecipitant assay lysis buffer(Beyotime) containing a cocktail of protease and phosphatase inhibitors.The lysate was incubated on ice for 30 minutes and centrifuged at 12,000 ×gfor 20 minutes at 4°C.Protein concentrations were quantified using a BCA Protein Assay kit (Beyotime,P0011).Protein samples (15 μg) were separated on 10% sodium dodecyl sulphate-polyacrylamide gels and transferred onto polyvinylidene fluoride membrane (Roche,Mannheim,Germany) at 100 V.After washing the membrane in Tris-buffered saline and Tween 20 three times,the membrane was blocked using 5% nonfat dry milk (Yili,Hohhot,Inner Mongolia,China) in Tris-buffered saline and Tween 20 for 1 hour at room temperature.Membranes were then incubated at 4°C overnight with the following primary antibodies: rabbit polyclonal anti glyceraldehyde-3-phosphate dehydrogenase (GAPDH;1:1000,Bioworld,Dublin,OH,USA,Cat# AP0066,RRID: AB_2797448),rabbit polyclonal anti-P75NTR (1:2000,
Millipore,Cat# ABN1655,RRID: AB_2722555) and mouse polyclonal anti-GFAP (1:10,000,Proteintech,Cat# 16825-1-AP,RRID: AB_2109646).After three washes with Tween TBS,membranes were incubated with horseradish peroxidase (HRP) conjugated to goat anti-rabbit IgG (1:10,000,Bioworld,Cat#BS13278,RRID: AB_2773728) and horseradish peroxidase conjugated to goat anti-mouse IgG (1:10,000,Bioworld,Cat# BS12478,RRID: AB_2773727) at room temperature for 1 hour.Membranes were processed with Immobilon Western Chemiluminescent detection reagent (Millipore).Images were captured using the Gel Image system (P&Q Science and Technology,Shanghai,China) and band densities were quantified using ImageJ.GADPH was used as a normalization control.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 8.0.0 for Windows,GraphPad Software,San Diego,CA,USA,www.graphpad.com).Results are expressed as mean ± standard error of the mean (SEM).One-way analysis of variance followed by Tukey’s multiple comparison test was used to compare the data unless stated otherwise.At least three independent experiments were used for each experiment.P< 0.05 was considered statistically significant.
Results
Effects of GDNF and BDNF treatment on the viability of astrocytes and Schwann cells
To investigate the effects of GDNF and BDNF on astrocytes and Schwann cell growth and survival,cells in monoculture were cultured with various concentrations of GDNF (10,20,30,50 and 100 ng/mL) and BDNF (10,30 and 50 ng/mL) for 24 and 48 hours.Cell viability was assessed by MTT assay.Treatment with GDNF at all concentrations for 24 and 48 hours did not result in significant changes in viability compared with the control group (Figure2AandB).In contrast,treatment with increasing doses of BDNF resulted in increased viability at 24 hours (Figure 2C) and 48 hours (Figure 2D).Immunostaining for GFAP (astrocytes) and P75NTR (Schwann cell) confirmed that astrocytes and Schwann cells in monoculture and co-culture grew well with normal morphology in the presence of GDNF (50 ng/mL) or BDNF (50 ng/mL) at 24 and 48 hours (Figure 2E–G).
GDNF and BDNF modulate cell migration and boundary formation in vitro
To determine the effects of GDNF and BDNF on cell migration,boundary formation and cellular intermingling,we established thein vitroconfrontation assay as described in Methods.As shown inFigure 1,astrocytes and Schwann cells were plated separately in each well of a two-well silicon culture insert.After removing the culture insert,the cells were maintained either in serumfree medium (control) or serum-free medium supplemented with GDNF or BDNF.An equal concentration of GDNF (50 ng/mL) and BDNF (50 ng/mL)was used,as reported in earlier studies (Santos et al.,2016;Siebert and Osterhout,2021).All subsequent experiments were performed using this concentration.The results showed that GDNF facilitated cell migration into the cell free-gap and increased the migration rate of both cell populations towards each other,resulting in a higher number of migrating cells compared with the control group (Figure 3AandB).In contrast,BDNF markedly reduced the number of migrating cells compared with the control group (Figure 3AandB).The migration rate of GDNF-treated cells was higher than that in the BDNF and control groups (Figure 3C).This showed that GDNF enhanced cell motility.To assess the influence of GDNF and BDNF on cell boundary formation,the confrontation assay was repeated using higher number of cells for longer periods of time.In the control group,as cells migrated towards one another,a boundary between the two cell types were clearly visible (Figure 4A–C).In contrast,the inhibited cell migration prevented full establishment of boundary formation in the BDNF group.Cells in the GDNF group showed increased migration,and Schwann cells were observed to penetrate into the area of the cultured astrocytes.
The direct effect of GDNF and BDNF on the monoculture system
In the experiments described above,we did not distinguish whether GDNF or BDNF acts directly on astrocytes or Schwann cells to induce migration.To address this question,Schwann cells or astrocytes were each plated in one chamber of the culture insert and the other chamber was left empty.We found that administration of GDNF induced migration of Schwann cells to the cell-free gap (Figure 5AandB).There was no remarkable effect on astrocyte monoculture (data not shown).
Notably,we observed that GDNF remarkably increased the number of migrating Schwann cells and the distance of migration in monoculture.These findings suggest that the presence of astrocytes modulates the effect of GDNF on Schwann cell migration.In addition,GDNF had no effect on astrocyte migration in monoculture but prompted astrocyte migration in co-culture.In contrast,BDNF enhanced astrocyte migration and inhibited the migration of Schwann cells in the monoculture condition.
Modulation of the morphology of astrocytes in the TREZ in the monoculture and co-culture conditions
Staining of primary astrocytes with GFAP revealed that GDNF treatment altered the morphology of astrocytes,with transformation of the ramified stellate shape into a flatten shape,a decreased average size of astrocyte areaand elongated astrocyte processes (Figure 6A).Furthermore,the GDNFtreated cells showed two or three very long processes.The morphometric changes were more commonly observed in co-cultured astrocytes than in monocultured astrocytes.In contrast,administration of BDNF to astrocytes cultured alone or in co-culture induced an increase in the average cell area,enhancement of the number and length of astrocytes processes (Figure 6B–D).The processes of BDNF-treated cells were very fine,straight and relatively longer than those of GDNF-treated cells.This finding indicates that astrocytes cultured with BDNF transform into a more complex shape as assessed by calculating shape index (Figure 6EandF).Notably,the cell soma area was relatively increased together with the number of primary processes emerging from the cell body.These findings indicate that GDNF and BDNF are potent regulators of the morphology of astrocytes in the TREZ.
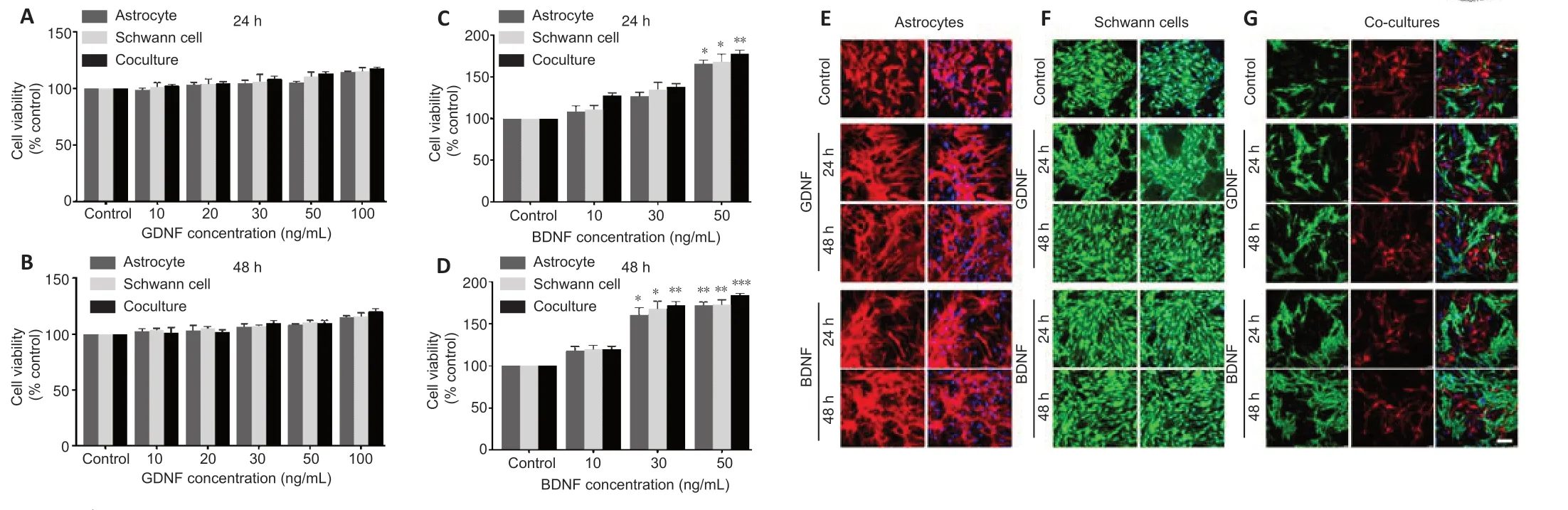
Figure 2|Proliferation of astrocytes and Schwann cells following treatment with GDNF and BDNF.
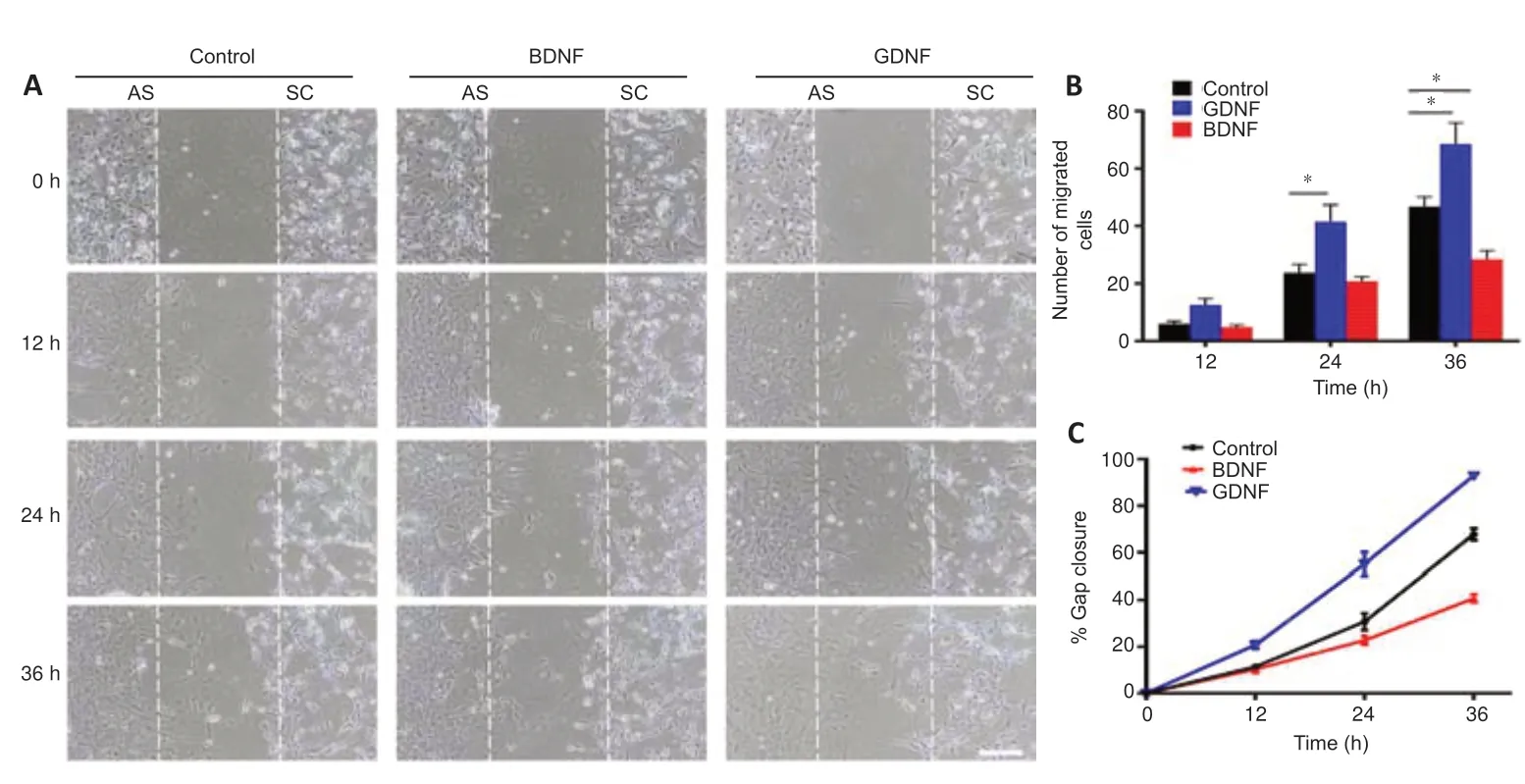
Figure 3|The effect of GDNF and BDNF on the migration of AS and SC in the confrontation assay.
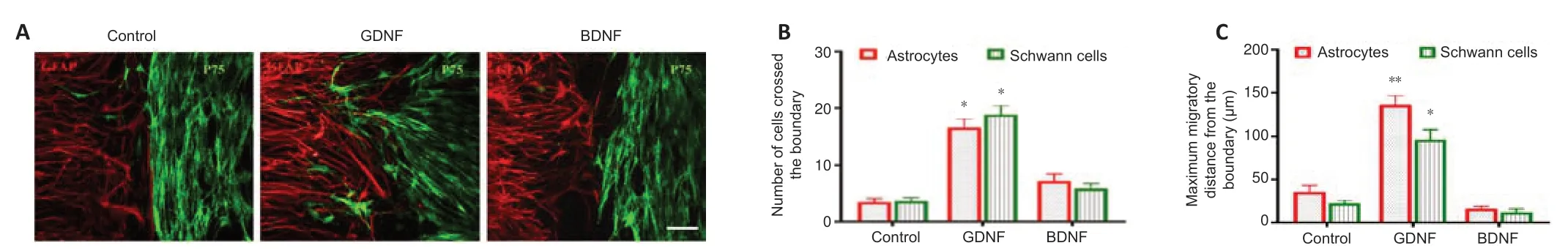
Figure 4|The intermingling between astrocytes and Schwann cells on the boundary assay.
Expression levels of GFAP and P75NTR in astrocytes and Schwann cells cultured in vitro
Immunostaining of GFAP and P75NTRfor astrocytes and Schwann cells showed there was no significant difference in the number of positive immunostained cells among different groups (Figure 7A–D).To verify the effects of GDNF or BDNF on cultured cells from TREZ,western blotting was performed in astrocytes and Schwann cells.The results showed that GFAP expression level in astrocytes treated with BDNF was higher than that of the control group at 24 hours and was much greater at 48 hours (Figure 7E).There was no significant difference in expression level of GFAP in GDNF-treated astrocytes.Schwann cells exhibited less P75NTRexpression in GDNF-treated cells and high P75NTRexpression in BDNF-treated cells (Figure 7F).

Figure 5|Migration assay of Schwann cell monoculture.
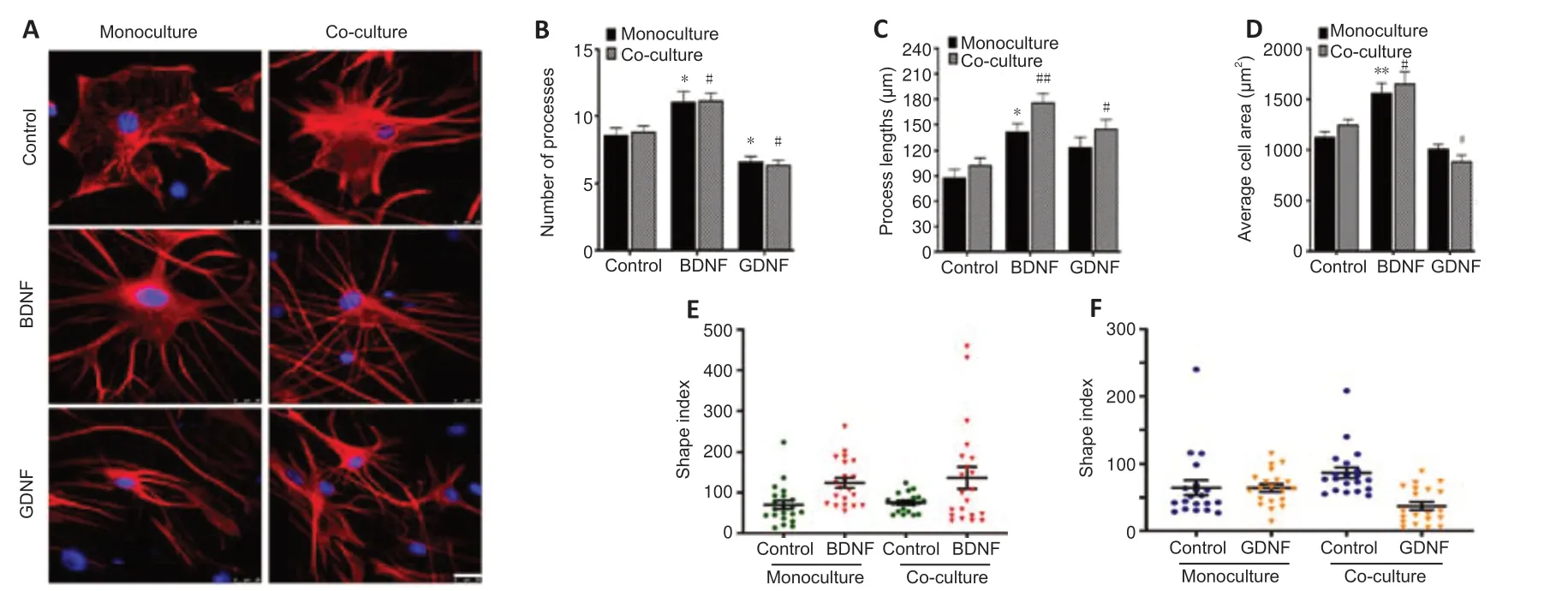
Figure 6|The morphological transformation of primary astrocytes in vitro.

Figure 7|Expression of glial fibrillary acidic protein (GFAP) and P75NTR in astrocytes and Schwann cells,respectively.
Discussion
In vitromodels allow the establishment of systems to examine biological cell-cell interactions and have been considered as convenient and useful predictors that help researchers gain insight intoin vivoresponses.Here we designed a simple co-culture model in an attempt to mimicin vivoglial cell distribution on the TREZ as a means to study cellular interactions in the presence of BDNF and GDNF.This co-culture model may facilitate further study on the mechanism of glial dysregulation associated with trigeminal nerve injury and possible therapeutic interventions.
Astrocytes and Schwann cells are normally present in distinct regions of the CNS and PNS,respectively,and do not interact with each other (Warren et al.,2020).In early CNS-PNS development,the glia limitans is formed by astrocytes,which serves as a barrier to impede Schwann cell migration into the CNS;the glia limitans also functions as a barrier to axonal regeneration following nerve injury (Fraher,1997).Schwann cell migration is key during axon myelination,regeneration and remyelination after nerve injury (Chen et al.,2019;Min et al.,2021).In our co-culture system,GDNF stimulated the cell motility and induced bidirectional migration of astrocytes and Schwann cells toward each other.In monoculture,GDNF enhanced the migration of Schwann cells but had no effects on astrocytes.This indicates that GDNF stimulated the bidirectional movement of co-cultured cells through its direct effect on Schwann cells.Previous findings have reported that GDNF enhances the motility of Schwann cells,suggesting that it could function as a guidance and chemokinetic factor (Blesch and Tuszynski,2003;Mukhamedshina et al.,2016).Moreover,an earlier study reported that administration of a blocking antibody against GDNF into Schwann cell medium resulted in a 75%reduction in neuron survival,indicating another important role of GDNF for Schwann cell-mediated action (Arce et al.,1998).Because GDNF only induced the migration of Schwann cells in monoculture with no effects on astrocytes in monoculture,we concluded that GDNF modulated the migration of astrocytes through an astrocyte-Schwann cell-mediated effect.However,the biological significance of this effect as well as the intracellular mechanism regulating such property remains unclear.A previous study reported sulfatase mediated the Schwann cell-astrocyte interaction by modulating NRG and FGF receptor-linked PI3K/AKT intracellular signaling (O’Neill et al.,2017).We thus propose that GDNF-mediated intracellular signaling may regulate the glial cell interaction at the TREZ.
Our results indicated that culturing astrocytes with BDNF markedly altered cell morphological properties,such as increased average cell area,and astrocytic processes became greater in number and were fully extended.Astrocytes change their morphology in response to a variety of different pharmacological treatmentsin vitro(Cheng et al.,2019;Lee et al.,2022).In addition,the morphological properties of astrocytesin vitromay indicate their functional propertiesin vivo(Bedner et al.,2020).Emerging evidence has indicated that increased GFAP expression associated with cellular hypertrophy and proliferation results in a phenomena referred to as “reactive astrogliosis” (Amalia,2021;Escartin et al.,2021;Patabendige et al.,2021).Such characteristics and changes in morphology are compatible with our observations in astrocytes cultured with BDNF.Western blot analysis confirmed increased GFAP expression in these cells.Thus,our results are in line with other studies reporting that exogenous BDNF evoked astrocytic activationin vivoandin vitro(Zhang et al.,2011;Ding et al.,2020).While exogenous BDNF may be responsible for enhancing cell proliferation and inducing reactive astrocytes,some studies showed that the increased GFAPpositive cells observed in astrogliosis are not from the generation of new cells,but rather are the result of increased GFAP synthesis and condensation of glial filaments in pre-existing cells,resulting in increased detection of GFAP by immunostaining (Sofroniew and Vinters,2010).We also found that astrocyte morphology after treatment with GDNF transformed into a flatten shape and decreased the average astrocyte area (hypotrophy).These findings are in line with previously reported studies,as they indicate that GDNF plays a role in the alleviation of astroglial reactions and modification of the morphological properties of reactive astrocytes (Trok et al.,1996;Iannotti et al.,2003,2004).However,the detailed mechanism by which GDNF mediates astroglial reactions is not fully understood.
Exogenous neurotrophic factors not only provide trophic support for regenerating peripheral axons but also improve the regenerative capacity of Schwann cells when sufficient amounts are supplied (Li et al.,2020).GDNF and BDNF have been intensively studied and shown to have neuroprotective effects,making them a useful therapeutic option for the treatment of some neurological disorders (Allen et al.,2013;Arranz-Romera et al.,2021).Even so,a high concentration of these factors has been shown to have side effects on peripheral nerve regeneration,such as causing axonal entrapment at the site of lesions (Lien et al.,2020;Rakotoarisoa et al.,2022).To the best of our knowledge,there are no studies evaluating the effect of GDNF and BDNF on cultured cells from the TREZ.We evaluated the effects of GDNF and BDNF using MTT assays;while GDNF did not cause a remarkable change in cell viability compared with the control,a remarkable improvement in cellular viability after incubation with BDNF was observed.Both factors did not show toxicity;nevertheless,we cannot fully exclude possible side effects if a higher concentration of these factors was applied.
In the present study,we confirmed that GDNF and BDNF displayed different effects on cellular segregation,boundary formation and phenotypic changes of the cultured cells derived from TREZ using a co-culture model.Coordinated interactions between astrocytes and Schwann cells are required for the regulation and resolution of injured nerves.Our study might provide additional information on the involvement of GDNF and BDNF in the regulation of the interaction between astrocytes and Schwann cells,which may possibly contribute to understanding of the glial cell arrangement in the TREZ.Further functional study of glial cells and their response to the neurotrophic factors at the CNS/PNS interface of the trigeminal nerve is important in the discovery of suitable therapeutic strategies,particularly for the condition associated with TREZ injury.
This study has several limitations.Our results on the trigeminal system were obtainedin vitro,and there are many influencing factors that may change the microenvironment of the TREZ under pathological conditions.Therefore,in vivoadministration of exogenous GDNF or BDNF using animal models of trigeminal neuralgia will be important to validate these findings.In addition,future clinical trials may investigate GDNF or BDNF and their possible therapeutic roles in pain.However,how to deliver these factors accurately at the TREZ to exert their maximum effects will be challenging.
In conclusion,the co-culture system of astrocytes and Schwann cells we designed is a simple and feasible experimental system that mimics glial cell distribution at the TREZ.This co-culture model allows the analysis and observation of glial behavior under exogenous stimuli.We demonstrate that GDNF and BDNF play an important role in modulating the interaction of astrocytes and Schwann cells and their involvement in the TREZ boundary.Our study also provides additional evidence that BDNF and GDNF act as opposing factors in regulating TREZ glial cells.Thein vitromodel described here may be useful as anin vitromodel to illuminate the mechanism underlying glial dysfunction associated with primary trigeminal neuralgia.
Acknowledgments:We thank Shao-Wei Lin (School of Public Health of Fujian Medical University) for providing advices for statistical analysis.
Author contributions:Study design: MIA,FW,DSL;primary cell culture: LZ,MIA;MTT assay: XFZ,MIA;confocal microscope imaging and time lapse video imaging: LL,MIA;morphological data analysis: AMM,MIA;protein concentration measurement: XYL,MIA;western blot assay: LXZ,MIA;data interpretation and manuscript revision: AMM,FW,DSL;manuscript draft: MIA.All authors read and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflict of interests.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:The morphology and identification of primary Schwann cells.
Additional Figure 2:DAPI staining of the boundary formation between astrocytes and Schwann cells.
Additional Video 1:Time-lapse video microscopy for astrocyte-Schwann cell migration.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update

