Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update
Joana Sofia Correia,Sara Duarte-Silva,António José Salgado,Patrícia Maciel,
Abstract Spinocerebellar ataxias are heritable neurodegenerative diseases caused by a cytosine-adenineguanine expansion,which encodes a long glutamine tract (polyglutamine) in the respective wild-type protein causing misfolding and protein aggregation.Clinical features of polyglutamine spinocerebellar ataxias include neuronal aggregation,mitochondrial dysfunction,decreased proteasomal activity,and autophagy impairment.Mutant polyglutamine protein aggregates accumulate within neurons and cause neural dysfunction and death in specific regions of the central nervous system.Spinocerebellar ataxias are mostly characterized by progressive ataxia,speech and swallowing problems,loss of coordination and gait deficits.Over the past decade,efforts have been made to ameliorate disease symptoms in patients,yet no cure is available.Previous studies have been proposing the use of stem cells as promising tools for central nervous system tissue regeneration.So far,pre-clinical trials have shown improvement in various models of neurodegenerative diseases following stem cell transplantation,including animal models of spinocerebellar ataxia types 1,2,and 3.However,contrasting results can be found in the literature,depending on the animal model,cell type,and route of administration used.Nonetheless,clinical trials using cellular implants into degenerated brain regions have already been applied,with the expectation that these cells would be able to differentiate into the specific neuronal subtypes and re-populate these regions,reconstructing the affected neural network.Meanwhile,the question of how feasible it is to continue such treatments remains unanswered,with long-lasting effects being still unknown.To establish the value of these advanced therapeutic tools,it is important to predict the actions of the transplanted cells as well as to understand which cell type can induce the best outcomes for each disease.Further studies are needed to determine the best route of administration,without neglecting the possible risks of repetitive transplantation that these approaches so far appear to demand.Despite the challenges ahead of us,cell-transplantation therapies are reported to have transient but beneficial outcomes in spinocerebellar ataxias,which encourages efforts towards their improvement in the future.
Key Words: cell transplantation;engraftment;induced pluripotent stem cells;mesenchymal stem cells;neural progenitor cells;neuroprotection;polyglutamine spinocerebellar ataxias;secretome;spinocerebellar ataxia;stem cell therapy
From the Contents
Introduction 1203
Search Strategy 1203
Clinical,Pathological,and Etiological Aspects ofSpinocerebellar Ataxias 1204
Cell-Based Neural Regeneration Approaches 1204
Where Are We Going? 1209
Conclusion 1209
Introduction
Spinocerebellar ataxias (SCAs) are monogenic dominantly inherited,and progressive disorders of the central nervous system (CNS),mostly characterized by motor impairments,namely a progressive loss of movement coordination that affects most aspects of daily living.The worldwide prevalence for dominant hereditary ataxias is~2.7 cases per 100,000 individuals (Ruano et al.,2014).Several genetic etiologies for SCAs were identified in recent years,using massive parallel sequencing approaches.The most common subtypes,types 1,2,3,6,7,and 17,are included in the polyglutamine (polyQ) group of disorders.Although polyQ SCAs have common symptomatology,they also have specific pathological mechanisms and manifestations,causing significant clinical heterogeneity among this family of ataxias.The age of symptom onset can vary within SCA subtypes and genotypes,mid-adulthood being the most common (Klockgether et al.,2019).Like in other types of ataxias,the loss of motor skills and muscular weakening strongly impact the quality of life of SCA patients,leading to reduced daily life activities,including social life (strongly affected by the dysarthric component),and increased risk of falls and immobility (Zeigelboim et al.,2013;Lo et al.,2016).These devastating disorders strongly affect the life span of SCA patients with a tendency for disease symptoms to worsen over time and from generation to generation.Moreover,feelings of morbidity,anxiety,and consequently depression are described for 17–26% of SCA patients which also negatively impact their functional status as well as their families,relatives,and caretaker’s quality of life [reviewed in Klockgether et al.(2019)].Therefore,it is urgent to find novel therapies that may cure or halt SCA progression in these patients.
The discovery and deep knowledge of the molecular mechanisms involved in these diseases are key for therapy development.However,no cure is yet available for SCAs and most of the applied treatments are based on pharmacological approaches that achieve only symptomatology relief.Current preclinical studies are either focused on drug-based approaches targeting the altered pathways in disease,usually related to protein homeostasis deregulation or dedicated to silencing or reducing the expression of the implicated gene for each specific SCA as a disease-modifying approach[reviewed in Klockgether et al.(2019);Neves-Carvalho et al.(2020);Karwacka and Olejniczak (2022)].Recent developments in stem-cell technology,however,promote new possibilities for cellular-based approaches for the treatment of neurodegenerative disorders.Previous clinical studies have shown that mesenchymal stem cell (MSC) grafts can restore motor impairments in polyQ SCA patients but with limited sustained effects (Dongmei et al.,2011;Zhang et al.,2011;Tsai et al.,2017),possibly requiring for protocol adaptation,regarding dosage,type or time of administration.In fact,better outcomes were observed in pre-clinical models of SCAs where a multi-intravenous injection regimen was applied in contrast to single intrathecal or intracerebral administration (Chang et al.,2011;Suto et al.,2016;Oliveira Miranda et al.,2018).Whether the disease-modifying action of stem cells occurs through cellular replacement or neuronal trophic support is still not fully established.Furthermore,the lack of cell source and procedures standardization may be delaying the establishment of these therapies.In this review,we aim to discuss the cellular approaches used so far in the context of polyQ SCAs in the pre-clinical and clinical studies,compare the results obtained with different treatment regimens,and what could be the future challenges for the development of such therapies.
Search Strategy
An extensive literature search was performed electronically on PubMed database using the following keywords: spinocerebellar ataxia epidemiology;neural progenitor cells terminology;stem cell therapy;spinocerebellar ataxia;cell therapy challenges;neurodegenerative diseases;mesenchymal stem cells;neural stem cells;induced pluripotent stem cells.Using the term “cell therapy AND spinocerebellar ataxia”,482 studies were obtained from which only 32 were selected referring to polyQ SCAs.Only English-language articles were considered.Information on clinical studies was assessed between April 24 and May 9,2022,in the ClinicalTrials.gov database using the condition term;“ataxia”;other terms: “stem cell”;12 studies were found but 5 could be confirmed using cell therapies in SCA patients.Autosomal recessive ataxias and X-linked ataxia were excluded.With this search strategy,no studies were found using cell-based approaches in non-polyQ spinocerebellar ataxias.One study using cell grafts on SCA7 was excluded as it was directed to treat nonmotor symptoms.
Clinical,Pathological,and Etiological Aspects of Spinocerebellar Ataxias
SCAs are a group of heterogeneous diseases,including 49 genetically distinct subtypes recognized so far (Ashizawa et al.,2018;Genis et al.,2018;Gennarino et al.,2018;Corral-Juan et al.,2022).Generally,these diseases affect the spinocerebellar tracts,i.e.they show an involvement of the spinal cord and cerebellum in the disease (Ashizawa et al.,2018).The clinical hallmark of SCAs is progressive ataxia,affecting balance,and overall motor coordination,with an impact on gait,speech,and swallowing.In addition,SCA patients often present heterogeneous manifestations that include altered muscle tone,impaired proprioception that further contributes to poor postural control,ophthalmoplegia,dystonia,progressive involuntary muscle contraction and wasting due to fasciculations,decreased sensitivity,eyelid retraction,weight loss,sleep disorders and fatigue (Moro et al.,2014;GARD,2017;Appelt et al.,2021).
According to genotype,there are two main groups of SCAs: repeat expansion and non-repeat mutation-associated SCAs.In the repeat expansion SCAs,it is known that longer repeats tend to cause disease with greater severity and an earlier onset than shorter repeats.Furthermore,as it occurs in many other repeat expansion disorders [reviewed in Paulson (2018)],there is a tendency for future generations to inherit higher repeat expansions accompanied by worse symptomatology– the genetic anticipation phenomenon.
A subgroup of repeat expansion SCAs is the family of polyQ diseases,which are caused by cytosine-adenosine-guanidine (CAG) triplet expansion in the coding region of different disease-causative genes.Nowadays,six polyQ SCAs constitute the most genetically recognized forms of SCAs– types 1,2,3,6,7,and 17.Of notice,the most common type is SCA3,also known as Machado-Joseph disease (MJD),representing 20% to 50% of the families living with SCA worldwide [reviewed in Klockgether et al.(2019)].While SCA7 and SCA17 have the lowest prevalence worldwide,representing 0–3% of families with SCA (Hersheson et al.,2012),basic research work on SCA7 has illuminated relevant aspects of polyQ SCAs pathogenesis and therapeutics (La Spada,1993;In: GeneReviews® [update July 23,2020]).
The different polyQ SCAs have distinct clinical and neuropathological manifestations,which may be related to the specificity of the affected protein and its cellular location [reviewed in Paulson et al.(2017);Buijsen et al.(2019);Klockgether et al.(2019)].In SCA1,SCA2 and SCA3,the cerebellum and brainstem are more vulnerable to degeneration,but a variable degree of damage is found in the anterior and posterior regions of the brain.However,SCA3 differs from SCA1 and SCA2,with less involvement of cerebellar cortex and olivary nuclei,but higher disturbance of the deep cerebellar nuclei circuitry,as well as spinal,vestibular,and pontine afferents (Sequeiros and Coutinho,1993;Rub et al.,2008,2013;Scherzed et al.,2012).Moreover,SCA6 is mostly described as a pure cerebellar disorder,which does not seem to reduce lifespan.This aspect may be related to a lower number of repeats in the affected protein ([20–33]CAG) in comparison to SCA1,2,and 3 ([38–85]CAG,[36–100]CAG,[60–87]CAG,respectively) (Klockgether et al.,2019).Particularly,SCA7 is a retinal-cerebellar degenerative disorder characterized by initial visual loss due to progressive retinal dystrophy (Stevanin et al.,1997;Paulson et al.,2017).
The expansion of polyQ tracts leads to abnormally large proteins causing their misfolding and aggregation in neurons (Saute et al.,2015;Nethisinghe et al.,2018;Klockgether et al.,2019).Undeniably,neuronal aggregation is a defining neuropathological feature of polyQ SCAs.But whether the large insoluble protein aggregates are neurotoxic or neuroprotective is still under debate.Nonetheless,for most polyQ SCAs the soluble oligomers/fragments seem to present a toxic gain of function (Takahashi et al.,2010;Paulson et al.,2017;Da Silva et al.,2019).Thus,it is discussed that an interrelationship between the disruption of the native protein and the toxic gain of function could continuously trigger the dysfunction of multiple cellular processes,perturbing intercellular homeostasis and leading to neuronal cell loss,and progressively resulting in neurodegenerative symptoms.Several molecular mechanisms are known to be affected in polyQ SCAs [reviewed in Paulson et al.(2017);Neves-Carvalho et al.(2020)] namely transcriptional regulation(Crespo-Barreto et al.,2010),mitochondrial function (Cornelius et al.,2017;Ramos et al.,2019;Tichanek et al.,2020;Harmuth et al.,2022),ubiquitinproteasome system (Burnett et al.,2003;Donaldson et al.,2003;Chen et al.,2019),RNA metabolism (Evers et al.,2014),and autophagy (Menzies et al.,2010;Nascimento-Ferreira et al.,2011;Alves et al.,2014;Duarte-Silva et al.,2016;Ashkenazi et al.,2017).Recently,the toxicity of repeat-associated non-ATG (RAN) translation peptides was described to be relevant for some polyQ SCAs (Scoles et al.,2015;Rudich et al.,2020).Pharmacological development and drug repurposing approaches targeting the different mechanisms involved in disease led to the advancement of a variety of candidate drugs to treat SCAs,some of them with translational value (Ashizawa et al.,2018;Duarte-Silva and Maciel,2018;Klockgether et al.,2019).To date,the available treatments rely on reducing or relieving the severity of symptoms of patients suffering from one of these highly debilitating diseases,yet with limited effects on slowing or stabilizing disease progression.Despite researchers’ best efforts,rigorous pre-clinical studies are still needed,as no drug was found to halt neuronal degeneration in SCA patients.To overcome this scientific and clinical challenge,stem cellbased regeneration techniques have emerged in the last years as promising therapeutic strategies for SCAs.
Cell-Based Neural Regeneration Approaches
The low regenerative potential of the CNS represents a challenge for the development of new therapeutic strategies for neurodegenerative diseases,including SCAs.From this perspective,regenerative medicine presents today promising tools to overcome this challenge through the use of cell-based therapies (Pereira et al.,2019;Vasanthan et al.,2020).In the latter years,the central focus of regenerative medicine for the nervous system has been the restoration of cellular circuitries.Consequently,various therapies have been employed to regenerate or replace cells and tissues in a damaged or nonfunctional state.Currently,at least four types of regenerative treatments are available: stem cell therapies,platelet-rich plasma-based therapies,lipogems,and prolotherapy.Traditionally,prolotherapy and lipogem-based therapies consist of non-surgical interventions where dextrose or a fatderived solution,respectively,is injected to promote ligament-tendon healing through immunomodulatory responses [reviewed in Vasanthan et al.(2020)].Prolotherapy can also be applied to subcutaneous nerves in the treatment of neurogenic inflammatory pain (Han et al.,2022).More recent studies,however,suggest the capacity of the transplanted cells to provide environmental enrichment through secretion of neurotrophic factors or to promote the development of auxiliary neural networks [reviewed in De Luca et al.(2019)].Examples of stem cell-based approaches used for clinical application in SCAs are reviewed in the following sections.
Stem cells: types,sources,and properties
Types of stem cells used for clinical application include pluripotent stem cells(PSCs),and multipotent stem cells,such as neural progenitor cells (NPCs) and mesenchymal stem cells (MSCs).
Pluripotent stem cells
Human PSCs such as induced PSCs (iPSCs) and embryonic stem cells (ESCs) are a powerful cell source in biomedical research,allowing the study of molecular mechanisms of diseasein vitro.ESCs are obtained from the inner cell mass of the mammalian blastocyst,having unlimited self-renewal potential and the ability to differentiate into the three germ layers: endoderm,ectoderm,and mesoderm (Figure 1).Back in 1981,the successful isolation and maintenance of normal diploid ESC line from the inner cell mass of mouse embryos were made possible by culturing ESCs on feeder cell layers under conditioned media(Martin,1981).In 1984,Thompson and colleagues could isolate and derive genetically identical clone cells from human teratocarcinoma that were highly adaptable to culture overgrowth,maintaining their differentiation potential.From this work,the differentiation of ESCs into neuron-like cells was possible using retinoic acid (Thompson et al.,1984).In the following years,efforts were made to ensure successful derivation and establishment of human ESCs,maintaining a normal karyotype and stem cell-like morphology [reviewed in Pereira et al.(2019)].Adaptation of protocols to promote efficiency in culture maintenance was made throughout the subsequent years.However,the low availability of human embryos and the ethical and religious disapproval surrounding ESC research application (see the section “Main challenges and limiting factors to be addressed”),triggered the development of new methodologies for the generation of PSCsin vitro.
Beginning in 2006,Takahashi and Yamanaka could successfully reprogram adult mouse fibroblasts,and later adult human fibroblasts to the pluripotent state,generating the so-called iPSCs (Takahashi and Yamanaka,2006;Takahashi et al.,2007).Their method allowed the direct reprogramming of committed adult cells to pluripotency via retroviral vector induction and overexpression of OCT3/4,c-MYC,SOX2,and KLF4 transcription factors.Besides,presenting the essential characteristics for the biomedical application referred to above,iPSCs are a powerful tool for disease modeling as they carry the genotypic profile of the original somatic cell.In basic research,the successful differentiation of iPSCs into specific neuronal subtypes benefits the correlation between phenotype and genotype in a patient-specific manner.Given this fact,iPSCs may also be used in autologous transplantation,minimizing the possibility of graft rejection by the host immune system.Despite the benefits,some controversy regarding the use of iPSCs in the clinic persists in the literature (Moradi et al.,2019).The initial method using viral systems that could integrate the host genome and cause random insertion mutations hampered their clinical use.However,with the advances in gene transduction techniques several alternatives using integrationfree reprogramming methods to derive iPSCs from somatic cells were already tested: episomal plasmids (Drozd et al.,2015),Sendai virus vectors(Zhou et al.,2021),certain transposon systems (Sebe and Ivics,2016),and synthetically modified mRNA transfection (Bailly et al.,2022).Thus,although iPSCs obtained using integrative methods can be effectively used to conduct basic biomedical research,drug screening,and model diseasesin vitro,for cell-based therapies non-integrative methods brought significant advantages regarding safety,as they minimize the potential for secondary mutations to occur.At this point,it is accepted that the efficiency of iPSC induction can vary between reprogramming methods and somatic cell sources (urine,skin fibroblasts,plasma,etc.) (Aydin and Mazzoni,2019;Liu et al.,2020a;Wang et al.,2021).Consequently,further studies are needed to determine the most suitable cell sources and reprogramming techniques for clinical application.Unfortunately,the direct use of PSCs for treatment purposes is not possible due to their tumorigenic potential.Nonetheless,PSCs can be differentiated into desired cell types,which confers them autologous therapeutic potential.Furthermore,PSCs are excellent tools to study phenotypic aspects of disease through the generation of specific neuronal subtypesin vitro[reviewed in Mendonca et al.(2018)].
Neural progenitor cells
Starting at embryonic layers,each specific tissue,including the CNS,derives from cellular divisions of specific multipotent stem cells also referred to as progenitor cells.NPCs arise from the ectoderm layer and give rise to neural cells in the CNS.NPCs generate neuronal and glial cell populations (astrocytes and oligodendrocytes),but not the immune system cells that populate the CNS.NPCs are not strictly embryonic cells though,they are also present in the neonatal brain and develop different features in the adult brain (Merkle et al.,2004).Therefore,NPCs are classified according to their brain location,morphology,gene expression profile,temporal distribution,and function[reviewed in Martinez-Cerdeno and Noctor (2018)].In the adult mammalian brain,NPCs reside in the main neurogenic niches,such as the subventricular zone,surrounding the lateral ventricles of the mature cerebral cortex,and the subgranular zone of the dentate gyrus of the hippocampus.Other less abundant cell populations expressing neuronal progenitor markers were also found in the adult hypothalamus and amygdala (Goncalves et al.,2008;Pellegrino et al.,2018;Roeder et al.,2022),and NPC-like subtypes such as the“Bergmann glia” are present in the cerebellum (Gonzalez-Perez et al.,2012;Guo et al.,2013;Mendonca et al.,2015).Interestingly,NPCs can be obtained from (1) direct isolation from primary CNS tissue or (2) through ESCs or iPSCsin vitrodifferentiation (Figure 1),using chemical compounds or growth factors [reviewed in Pereira et al.(2019)].Embryonic NPCs can multiply at a sufficient rate to be used in cellular therapies in human patients of various pathologies.In vitro,both rodent and human NPCs are prone to differentiate into neurons (with detectable physiological electrical activity),astrocytes,and oligodendrocytes (Gritti et al.,1999;Vescovi et al.,1999;Bottai et al.,2003).NPC-based treatments of different neural defects have been widely investigated in animal models,namely Huntington’s disease (Reidling et al.,2018),multiple sclerosis (Sullivan et al.,2020;Smith et al.,2021),and spinal cord injury (Bottai et al.,2008;Pereira et al.,2019).Depending on the pathologic environment the therapeutic effect of NPCs could happen through cell replacement,differentiating into oligodendrocytes progenitors (Sullivan et al.,2020) and allowing remyelination,through trophic support,and/or reducing inflammation (Bottai et al.,2008;Pereira et al.,2019).In patients with Parkinson’s disease,the transplantation of NPCs was proven to be safe and effective,with no obvious side effects,such as tumor formation or noticeable inflammatory responses upon transplantation (Lige and Zengmin,2016).
Mesenchymal stem cells
While very elegant work is being developed using NPCs,MSCs have also been put forward as biological therapy agents for neurodegenerative diseases.MSCs are multipotent cells that reside in several tissues (mainly the bone marrow and fat).Besides their primary role to support hematopoiesis and produce cells of the mesodermal lineage (Pittenger et al.,1999;Lennon and Caplan,2006),previous studies have described the immunomodulatory and neurotrophic properties of these cells [reviewed in Dabrowska et al.(2020);Miceli et al.(2021);Li et al.(2022)].At the pre-clinical stage,MSC administration was shown to suppress autoimmune responses (Grigoriadis et al.,2011;Harris et al.,2012) and to support remyelination of lesioned sites in animal models of nerve injury or neurodegenerative disorders (Dabrowska et al.,2020;Esmaeilizade et al.,2021;Clark et al.,2022;López-Muguruza et al.,2022;Miyano et al.,2022;Ortiz et al.,2022;Zhang et al.,2022).Additionally,allow increased reproducibility in comparison to adult NPCs,because they are easy to isolate and expandin vitrowhile maintaining their multipotency and low tumorigenicity (Lu et al.,2006;Cofano et al.,2019;Johnson et al.,2021;Miceli et al.,2021).Although autologous transplantation is preferable,MSCs can also avoid host immune responses by directly mediating cell-tocell interactions through adhesion molecules,integrins and lymphocytes,thus allowing allogenic engraftments (Masgutov et al.,2018;Wang et al.,2018;Jiang and Xu,2020;Johnson et al.,2021),which increases their translation potential.Moreover,these cells can migrate to the lesioned sites upon transplantation,possibly attracted by chemokines secreted by degenerating neurons (Weiss and Dahlke,2019;Li et al.,2022),directly offering neurotrophic effects,and rescuing them from degeneration possibly by regulating tissue homeostasis.In fact,MSCs were described to secrete several bioactive factors while repopulating the lesioned site (Teixeira et al.,2015;Tsai et al.,2019),which supports their promise for clinical application.Despite their great differentiation potentialin vitro,the benefits of MSC transplantation do not depend solely on their neuron replacement potential.To avoid the small numbers of viable cells and differentiated cells found at the end of pre-clinical trials,some repeated administration approaches have been tested with successful results in rodent models of SCA3 (Oliveira Miranda et al.,2018).Additionally,the potential ofin vitrocollected MSC secreted factors,known as secretome,has been extensively studied to try to overcome safety aspects and to prosper for more effective therapeutics (Pires et al.,2016).In fact,the immunomodulatory effects of MSCs may be mediated by paracrine factors,rather than a direct cellular action (Mendes-Pinheiro et al.,2019;Krawczenko et al.,2020),and their anti-inflammatory properties can be enhanced by preconditioning of MSCs with hypoxia and cytokines treatments,to promote the efficacy of immunomodulation as a therapeutic strategy in the field of regenerative medicine [reviewed in Miceli et al.(2021)].A recent comparative analysis determined two types of factors that may induce variations in the paracrine secretion and differentiation properties of MSCs: (i) innate factors of the tissue source (developmental stage,niche organization,and functional predisposition),and (ii) acquired factors from the isolation and culture conditions (Petrenko et al.,2020).Nonetheless,in the previous study,the presence of neuroprotective factors was detected in all secretomes,independently of the MSC origin.As described above,the value of secretome-based therapies has been extensively demonstrated in the context of neurodegenerative diseases,mainly in Parkinson’s disease animal models,with potential clinical translation ability (Mendes-Pinheiro et al.,2019).Therefore,similar effects could be found for polyQ SCAs (reviewed in the following sections).
Stem cell therapy for polyQ SCAs
In a recent systematic search,Chen and colleagues registered a total of 1209 research studies using stem cell interventions in human trials,excluding neoplasm and hematological diseases (Chen et al.,2021).From this total,17.34% of the studies were related to CNS disorders,and the top 4 diseases being addressed using cell-based approaches were related to motor system dysfunction (19.28% of all the studies analyzed).The authors also found that MSCs are among the most widely used cell types,possibly due to their availability,but mainly,to their paracrine and immunosuppressive actions.In fact,from the literature,it is spotted that the difficulties in isolating pure populations of adult NPCs limit the study of their neural stem regulation and secretion,which consequently constrain their use.
Although the effective transplantation of dopaminergic neurons in the striatum of patients with Parkinson’s disease (Lindvall et al.,1990),back in 1990,paved the way for the initiation of cell-based clinical trials for polyQ disorders (Kopyov et al.,1998),the use of stem cell therapy in polyQ SCAs is still mostly in a pre-clinical research phase (He et al.,2021a).Few clinical studies have been conducted to dissect the safety and efficacy of stem cell therapies in SCA patients (Figure 2).While clinical studies in other polyQ diseases are using human fetal NPCs (Bachoud-Levi et al.,2021),in the case of SCAs the preferred cell type seems to be human MSCs derived from the umbilical cord or adipose tissues (Tables 1and2).In the following sections,we will review the pre-clinical (Table 1) and clinical trials (Table 2) performed so far for cell therapy applied to polyQ SCAs.

Figure 1|Origin and lineage differentiation of different stem cells.
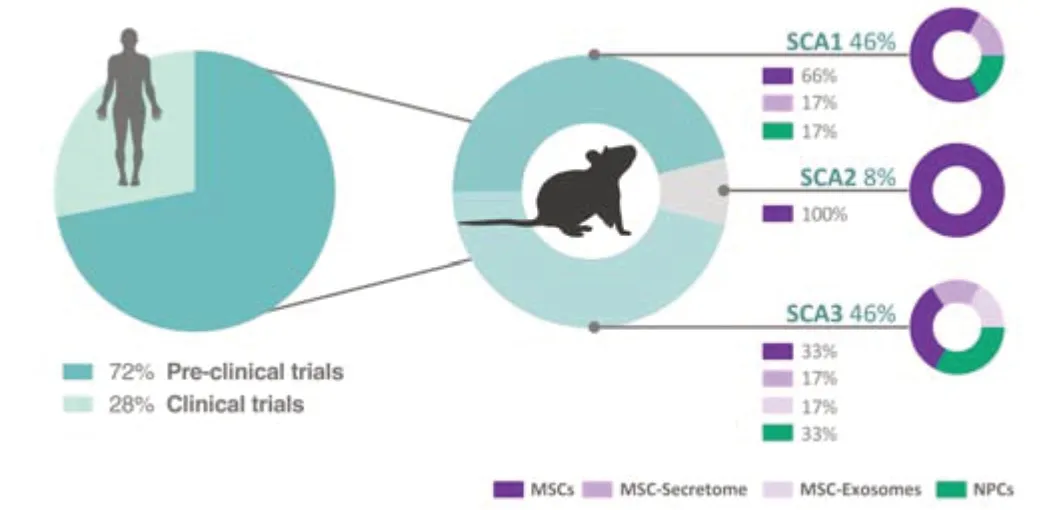
Figure 2|Percentage of pre-clinical and clinical studies using cell-based therapies in SCAs.
Preclinical studies in polyQ SCAs
In the past years,research has been developed to assess the effects of cellular therapies in animal models of polyQ SCAs (Table 1).Different cellular approaches were tested,differing in origin,tissue source,injection site,and method of administration,in the attempt to mitigate the motor impairments and neuropathology present in these disorders.
Within the SCA1 context,one initial study using the B05/+transgenic mouse(expressing human mutated ataxin 1 protein with 82Q) transplanted with cerebellum primordium NPCs,at early symptomatic ages have shown a longlasting improved phenotype (Kaemmerer and Low,1999).Contrarily,the transplant of mouse NPCs derived from the adult subventricular zone into the cerebellar white matter of the same mouse model at the symptomatic stage of 24 weeks,allowed the functional recovery of motor phenotype with increased Purkinje cell (PC) survival,morphological improvement,and normalization of the membrane polarization (Chintawar et al.,2009).However,similar treatment was not effective for younger SCA1 mice treated at 5 or 13 weeks of age with absent or initial PC degeneration,respectively.In this study,no cellular replacement has occurred,but migration of NPC grafts was observed towards substantially degenerated PCs in 24-week-old SCA1 mice.Because the authors aimed to initiate stem cell therapy before any significant sign of degeneration and cellular loss,they performed another preclinical trial where mouse bone marrow MSCs (KUM10 line) were intrathecally injected in 5-week-old SCA1 mice in a single administration regimen;in this case,they observed the complete suppression of progressive motor deficits(Matsuura et al.,2014).This pre-clinical trial confirmed that MSCs,but not NPCs,could halt disease progression and prevent PC loss in the cerebellum of SCA1 mice and even be used in a preventive approach.Additionally,intrathecal grafts of MSCs of the same source effectively suppressed peripheral nervous system degeneration in a SCA1 knock-in mouse model(Mieda et al.,2016).Similar results were observed after single and/or multiple intravenous injections of mouse MSC conditioned medium (MSC-CM;Table1) through the orbital vein of the SCA1 knock-in model (Suto et al.,2016).Interestingly,results show that MSC-CM attenuated the degeneration of axons and myelin of spinal motor neurons in both approaches.Consequently,treatment with MSC-CM ameliorated nerve conduction velocity in spinal motor neurons and reduced motor incoordination of SCA1 mice.Together,the results of these pre-clinical trials suggest that MSC paracrine signaling could be responsible for the neuroprotective action of these cellular therapies in SCA1 mouse models.Furthermore,a recent study developed in the B05/+transgenic mouse model,using human umbilical cord MSCs (HUCMSCs) bilaterally transplanted into the cerebellum Lobules IV (Tsai et al.,2019),showed that this cellular treatment partially ameliorated balance and exploratory behavior.This treatment regimen alleviated cerebellar atrophy,reverted PC death,and increased neuronal-muscular response strength to stimuli 6 months after stem cell transplantation.Previous studies using mouse-derived MSCs showed that these cells adopted a glia-like phenotype.In contrast,human-derived MSCs transplanted into the mouse cerebellum showed a long-lasting survival (for 20 weeks),being able to produce cytokines even in an undifferentiated state (Tsai et al.,2019).Other particularities were described for MSC grafts in late symptomatic stages of neurodegeneration,for which binucleated PCs containing a human MSC-derived marker protein were found in the cerebellar cortex of mice,thus suggesting a fusion event (Kemp et al.,2011).This finding was then confirmed by Huda and colleagues,using GFP-expressing AAV9 vectors,where GFP-positive cells were only detected on PCs and interneurons upon treatment of symptomatic SCA1 mice,but not of their non-symptomatic counterparts,2 weeks after human fetal MSC transplantation (Huda et al.,2016).
To the best of our knowledge,only one study was dedicated to cellular therapeutic approaches in a SCA2 mouse model (Chang et al.,2011).In this study,intravenous or cerebellar intracranial serial administration of human bone marrow MSCs (HBM-MSCs) was performed before and after installation of the motor phenotype,at different stages of the disease.Intravenous delivery of HBM-MSCs favored prolonged cell engraftments,which were more efficient in delaying the onset of motor impairments and PC loss in SCA2 animals,comparing to those achieved through intrathecal injection (Chang et al.,2011).
Regarding SCA3,one study reported that mouse cerebellar NPCs,when transplanted into the cerebellum of transgenic mice expressing human mutant ataxin-3 (ATXN3) bearing 69Q,could significantly overcome motor symptoms and neuropathology,with reduction of PC loss,cerebellar neuroinflammation and increased secretion of neurotrophic factors (Mendonca et al.,2015).Similar results were obtained in the SCA3-YAC-84Q transgenic mouse,which received an intracranial injection of human NPCs into the dorsal raphe nucleus after disease onset (Hsieh et al.,2017).Hsieh and colleagues also observed increased levels of tryptophan hydroxylase 2 enzyme,which is involved in serotonin synthesis,arguing for potentiation of the serotonin-dependent PC maturation after NPC transplantation in the SCA3 mouse cerebellum.
The therapeutic potential of MSCs has also been tested in SCA3.In fact,both human and mouse-derived MSCs have been applied in different mouse models of the disease (Li et al.,2018;Oliveira Miranda et al.,2018;You et al.,2020;Correia et al.,2021).Efficacy of such cells appears to depend on route of engraftment,number of administrations (multiple or single),and extent of longitudinal assessment.As an example,when comparing HBM-MSC grafts to HBM-MSC secretome administration in a 25-week long longitudinal assessment,a single MSC secretome administration was more beneficial than a unique MSC transplantation,particularly when administered in the cerebellum and basal ganglia of CMVMJD135 transgenic mouse (Correia et al.,2021).Immunostaining for human nuclear antigen also confirmed possible migration or fusion of MSCs into PC layers 8 weeks post-transplantation in the cerebellum [supplementary data from Correia et al.(2021)].Human MSCderived exosomes were also tested in the SCA3-YAC-84Q mice,with treatment leading to a better rotarod performance that was maintained for 6 weeks post-injection (You et al.,2020).All these pre-clinical trials share conclusions on some neuroprotective potentials of MSCs in SCA3 mouse models,namely through elevation of neurotrophic factors levels in the cerebellum (Li et al.,2018) or GABA and glutamate in the striatum (Oliveira Miranda et al.,2018).
Clinical studies in polyQ SCAs
Following the observed results of the potential therapeutic effects of MSCs in pre-clinical studies,this cellular therapy approach has been translated to humans in clinical trials dedicated to the development of cell-based treatments for polyQ SCAs (Table 2).The safety,tolerability,and efficacy of allogeneic adult adipose-derived MSCs (adipose tissue-derived stem cells– ASCs) [http://www.clinicaltrials.gov,NCT01649687 (completed);accessed on April 24,2022;Tsai et al.(2017)] and HUC-MSCs were evaluated in phase I/II clinical trials in SCA patients from China (Dongmei et al.,2011;Yang et al.,2011;Jin et al.,2013).Evidence pointed out that allogeneic intravenous injection of ASCs from healthy donors seemed well tolerated in patients with SCA3 and no biological-related adverse events were observed in the 12-month follow-up,measured by the Scale for the Assessment and Rating of Ataxia and the Sensory Orientation Test (Tsai et al.,2017).The ASCs formulation used in this first clinical trial has been patented and a phase II clinical trial is now being conducted to further assess its efficacy in a randomized,double-blinded,and placebo-controlled fashion for SCA2 and SCA3 patients (Stemchymal®,http://www.clinicaltrials.gov,NCT02 540655;accessed on April 24,2022).In another trial,intrathecal injection of HUC-MSCs could significantly decrease motor deficits for 1 month,though the disease still progressed in some SCA patients (Dongmei et al.,2011).Importantly,to further understand the best route of administration in MSCbased therapeutics,a random,open-label,and parallel controlled experiment is currently being conducted to compare the safety and efficacy between intravenous and intrathecal injections of HUC-MSCs in SCA1,2,3,and 6 patients(http://www.clinicaltrials.gov,NCT03 378414;accessed on April 24,2022).
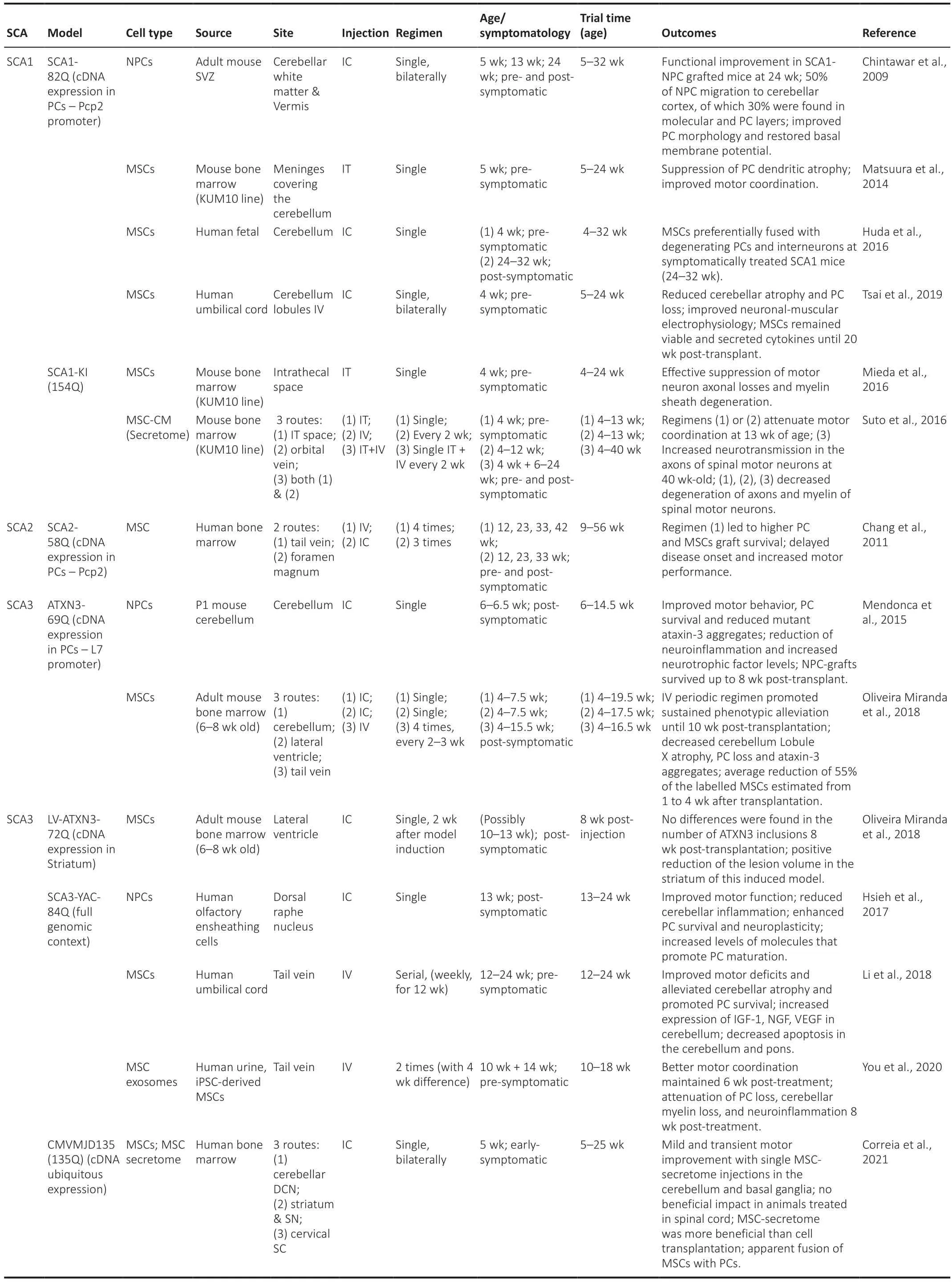
Table 1|Pre-clinical studies using cellular-based therapies in SCA mouse models

Table 2|Clinical application of cell-based therapies in polyQ SCA patients
Meanwhile,a systematic review and meta-analysis of MSC-based clinical trials in SCAs revealed low evidence and no-statistical difference to support the functional recovery of SCA patients (Appelt et al.,2021).However,there is a common agreement on the need for larger-sized,randomized,doubleblinded,placebo-controlled clinical trials to confirm the long-term safety profile and therapeutic potential of allogeneic cell therapy in SCAs.The establishment of experimental designs dedicated to exploring the dosage and frequency of treatment strategies could also help to overcome relapse episodes.Other clinical trials are underway to try to clarify such aspects of MSC treatment,readjusting the protocols in the attempt to achieve higher efficacy (http://www.clinicaltrials.gov,NCT01360164,NCT01489267,NCT01958177,NCT02 540655;accessed on April 24,2022).
Main challenges and limiting factors to be addressed
While stem cells can be an exciting therapeutic alternative for neurodegenerative diseases there are possible side effects of cell transplantation to consider.Tumor formation is a major concern in cell therapy using PSCs due to their high proliferative potential.Specifically,the major drawbacks for ESC clinical application are risks associated with (1)teratoma or neuroepithelial tumor formation,(2) aneuploidy karyotype,and (3) ethical concerns regarding their acquisition from human embryos(Mendonca et al.,2018;Charitos et al.,2021;Clark et al.,2021).Here,iPSCs could provide a useful alternative for differentiation into NPCs or specific neuronal subtypes both from healthy donors or patient-derived.Protocols have been developed to generate cerebellar neuronsin vitro,particularly Purkinje cells (Watson et al.,2018),which are mostly affected in severe SCAs.The production of a sufficient number of cells for successful transplantation may be limited by the low efficiency of these protocols,however,the relevance of transplanting fully differentiated PCs into a formed circuit can also be questioned [reviewed in Watson et al.(2015);Nayler and Becker(2018)].Moreover,iPSC genomic and epigenomic signature may also limit their engraftment,as iPSC-derived neurons may be ineffective in reverting degeneration Imm et al.(2021).Nevertheless,the main challenge is to achieve high fidelity differentiation into the appropriate neuronal subtype,which then needs to connect to target neurons and establish functional synaptic transmission in the host brain,without being rejected.Further studies are needed to fully understand these mechanisms.Additionally,there are important aspects to consider regarding protocol quality control that ultimately influence their efficiency and reproducibility [reviewed in Steeg et al.(2021)].To achieve quality attributes for clinical-grade iPSC lines analytical tests are recommended for the characterization of iPSC lines in terms of their microbiological sterility,genetic fidelity and stability,viability,and differentiation potential.Guidelines for parameter implementation have been recently summarized by Sullivan et al.(2018),and can comprise the use of: single tandem repeat genotyping;mycoplasma,bacterial,viral,and residual vector testing;single nucleotide polymorphism arrays;whole genome sequencing and other genetic and disease marker analyses.The authors also strike the importance of periodic criteria revision,according to developments in scientific knowledge,technology,and best practice.
Many studies have demonstrated that the donor’s age negatively affects many characteristics of stem cells such as differentiation,expansion,immunogenicity,and reprogramming efficiency [reviewed in Nguyen et al.(2019)].Additionally,donor-to-donor and cell-to-cell heterogeneities within the cultured population are important factors to be addressed,as they may affect the therapeutic potential and reproducibility of outcomes (Li et al.,2021).For example,regardless of donor age or autologous/allogeneic source,MSCs usually enter senescence with prolongedin vitroexpansion(Gao et al.,2018),which can hinder the secretion of neurotrophic or antiinflammatory factors.So,for clinical purposes,it is important to monitor senescence and find strategies to avoid or reverse this process [reviewed in Liu et al.(2020b)].Still,it is accepted that autologous MSCs are easy to obtain and present no immune rejection upon transplant.Nevertheless,culture isolation and expansion of autologous MSCs require weeks to release,delaying transplantation.On the other hand,allogeneic MSCs enable the selection of healthy donors,presenting low immunogenicity,and can be collected ahead of time from various tissue sources,allowing biobank storage.Interestingly,a previous study dedicated to comparing repeated intra-articular injection of autologous and allogeneic MSCs in the equine joint,found a significant adverse response to the second injection of allogeneic MSCs but not autologous MSCs,suggesting an adaptive anti-donor immune response (Joswig et al.,2017).Therefore,aspects of autologous vs allogeneic transplantation call for longitudinal clinical evaluation.A recent systematic review by Lohan and colleagues discusses new ways to minimize antidonor immune responses in allogeneic transplantation,including the use of immunosuppressive drugs (Lohan et al.,2017).
Previous pre-clinical studies have observed different grades of efficacy depending on site and route of administration.Despite some authors discuss that multiple systemic injections may lead to better outcomes,this route of administration is often associated with adverse events,namely an increased risk for pulmonary and cerebral embolism (Jung et al.,2013;Cui et al.,2015).Combining the lessons learned from animal models and previous clinical studies it is expected that readjustments to protocols occur to favor less invasive and more effective approaches.In fact,most of the clinical studies already performed in SCA patients used single intravenous administration of human MSCs (Jin et al.,2013;Tsai et al.,2017),but limited effects were yet reported.Here,MSC secretome systemic administration could be both an effective and safe alternative.However,we still need to further understand the secretion profile of MSCs in the context of different diseases and to improve the production methods to obtain larger amounts of MSC-secretome for clinical application.
In the last decade,no major adverse events were reported when cellbased strategies were applied in several clinical trials,including for SCAs(Appelt et al.,2021).The most common side effects reported so far included dizziness,back pain,and headache,sustained within 1–3 days without special treatment,but excluded microorganism infections or fevers (Dongmei et al.,2011).However,the logistic difficulties in obtaining and maintaining donor cells and the poorly defined subject selection criteria will delay the translation of cell therapy to a clinical set.Moreover,recent pre-clinical studies have stressed the need for careful cell phenotyping before transplantation and to address long-term monitoring of cell graft integration and survival (Heo et al.,2016;Suto et al.,2016;Oliveira Miranda et al.,2018;Yang et al.,2018;Bachoud-Levi et al.,2021;Rosser et al.,2022).This lack of standardization of key readouts in animal models and of limited predictive outcomes in human trials,plus the extensive costs of running clinical trials,significantly affect celltherapy development.Thus,it would be important to define principles that could guide clinical translation in a more standardized manner.A detailed set of challenges associated with the clinical translation of cell therapies have very recently been reviewed by an international consortium of experts dedicated to providing guidance for stem cell therapy in the context of Huntington’s disease,another polyQ disorder (Rosser et al.,2022).The highlighted difficulties are pertinent and common to other neurodegenerative conditions.The authors support that the next step is towards standardization but without neglecting disease and patient specificities.In addition,it is urgent to define procedures for toxicity testing to ensure validation,robustness,and homogeneity,promoting optimal manufacturing practice for cell scaling and stability.
Where Are We Going?
The advances in cellular therapy based on the therapeutic use of MSCs make this a potential approach for SCA treatment.As revised above,the potential of MSCs is mainly provided through paracrine secretion,which can act in a multi-target fashion.This unique characteristic,already encouraged scientists to explore cell-free strategies in SCAs,such as MSC-secretome(Correia et al.,2021),but also through MSC-exosomes (You et al.,2020).Promising tools for large-scale production processes were developed over the last years,through the use of bioreactor systems [reviewed in Marques et al.(2018)].These 3D culturing systems stand out for being dynamic in recreating the interactions of cells with microenvironment,enabling the spatial distribution of macromolecules and mechanical stimulationin vitro,which can also potentiate standardized secretion profiles.Biomaterials are also being applied to mimic native extracellular matrix,providing increased structural support upon transplantation (Marques et al.,2018).Indeed,regenerative medicine could benefit from the conjugation of cells,paracrine factors,and biomaterials,to further enhance its therapeutic potential for neurodegenerative disorders.
Other methodologies are being discussed in the literature to potentiate the therapeutic effect of MSCs,namely through culture pre-conditioning.This strategy aims to increase the presence of growth factors and cytokines,promoting cell survival,regulation of immune responses,and goal-directed cell migration and differentiationin vivo(Barros et al.,2020).Alternatively,as previously observed in Huntington’s disease mouse models,pre-treatment of MSCs with pharmacological agents or genetically engineering these cells to overexpress neurotrophic factors can help improve their efficacy in rescuing behavioral dysfunction and neuropathological defects [reviewed in Barros et al.(2020)].Therefore,combined therapies using MSCs for drug delivery,also taking advantage of their neurotrophic secretion and immunoregulatory effects,will possibly strengthen the development of novel cell-based therapeutic strategies for polyQ SCAs.
Patient-derived iPSCs provide disease-relevant models to study the molecular mechanisms behind neuroprotection and neuro-regeneration processes in cell therapy.Consequently,the generation of iPSCs from SCA patients has emerged (Maguire et al.,2019;Wei et al.,2020;Yang et al.,2020;He et al.,2021b;Shuvalova et al.,2021).Exciting results were found in SCA3 patientderived iPSCs after CRISPR/Cas9 mediated genetic correction.This genetic strategy was able to precisely repair and reverse the abnormal disease phenotypes (He et al.,2021c).Moreover,the corrected SCA3-iPSCs retained a normal karyotype and pluripotent characteristics,being successfully differentiated into NPCs and maintaining the appropriate electrophysiological characteristics in iPSC-derived cerebral cortical neurons (He et al.,2021c).Unfortunately,to date,it is still very challenging to recapitulate the complexity of cerebellar development.For iPSC differentiation into Purkinje cells,the authors used mouse fibroblasts as supporting matrix,which is not a satisfactory scenario for clinical translation where xeno-free conditions are preferred.Nonetheless,iPS-derived cells offer several advantages including near-unlimited availability,and the potential for standardized manufacturing,cryopreservation,and use of cell purification strategies (using cell-sorting techniques) to diminish possible PSCs in culture and decrease tumorigenicity,facilitating surgery,dosing,and distribution [reviewed in Parmar et al.(2020)].Similar to the above described for MSCs,interesting results were obtained for rodent models of spinal cord injury using combined approaches,either by pretreating iPS-derived NPCs with neuronal differentiation promotors or through biomaterials to support their survival [reviewed in Pereira et al.(2019)].Lastly,engineered 3D bioprinting of tissues,through organoid formationin vitro,that can mimic the natural architecture of the damaged networks offers an interesting alternative that could allow for autologous tissue replacement and more complexin vitromodeling (Ong et al.,2018).
Conclusion
As we move forward to the clinical translation of stem cell-based therapeutic strategies,there are crucial aspects to address in terms of safety,delivery routes,dosage,source,and culture conditions to effectively determine their application and long-term effects.While choosing the best cellular type as a therapeutic option for SCAs,their regenerative potential must also be taken into consideration.The literature available so far in the field points towards the use of MSCs as the first choice to treat this type of disorders,based on their general safety profile as well as their ability to secrete important neuronal feeding factors.The promise of iPSC auto-transplantation is also valued but requires further study regarding safety.Therefore,it is still important to investigate the biological features of each cell type as a critical aspect for the success of cell-based therapy.Moreover,the complex arrays of factors that impact their development are still challenging to monitor and standardize,and quality control measures are essential in this perspective.More work needs to be developed to find alternatives that could ultimately facilitate progress and future directions towards their therapeutic benefit for SCAs.
Acknowledgments:The authors would like to thank Maciel Lab and Salgado team members for relevant scientific discussions.Additionally,the authors acknowledge Bruna Ferreira-Lomba (ICVS;ICVS/3B’S,Braga,Portugal) for her help with figure illustrations.
Author contributions:Conception,design,and manuscript editing: JSC,SDS,and PM;drafting the article and manuscript preparation: JSC;critical manuscript revision: SDS,AJS,and PM.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Do tau-synaptic long-term depression interactions in the hippocampus play a pivotal role in the progression of Alzheimer’s disease?