Treadmill exercise exerts a synergistic effect with bone marrow mesenchymal stem cell-derived exosomes on neuronal apoptosis and synaptic-axonal remodeling
Xin-Hong Jiang,Hang-Feng Li,Man-Li Chen,Yi-Xian ZhangHong-Bin Chen,Rong-Hua Chen,Ying-Chun Xiao,Nan Liu,
Abstract Treadmill exercise and mesenchymal stem cell transplantation are both practical and effective methods for the treatment of cerebral ischemia.However,whether there is a synergistic effect between the two remains unclear.In this study,we established rat models of ischemia/reperfusion injury by occlusion of the middle cerebral artery for 2 hours and reperfusion for 24 hours.Rat models were perfused with bone marrow mesenchymal stem cell-derived exosomes(MSC-exos) via the tail vein and underwent 14 successive days of treadmill exercise.Neurological assessment,histopathology,and immunohistochemistry results revealed decreased neuronal apoptosis and cerebral infarct volume,evident synaptic formation and axonal regeneration,and remarkably recovered neurological function in rats subjected to treadmill exercise and MSC-exos treatment.These effects were superior to those in rats subjected to treadmill exercise or MSC-exos treatment alone.Mechanistically,further investigation revealed that the activation of JNK1/c-Jun signaling pathways regulated neuronal apoptosis and synaptic-axonal remodeling.These findings suggest that treadmill exercise may exhibit a synergistic effect with MSC-exos treatment,which may be related to activation of the JNK1/c-Jun signaling pathway.This study provides novel theoretical evidence for the clinical application of treadmill exercise combined with MSC-exos treatment for ischemic cerebrovascular disease.
Key Words: apoptosis;axonal regeneration;c-Jun;exosomes;functional remodeling;ischemic stroke;JNK1;mesenchymal stem cells;synaptic formation;treadmill exercise
From the Contents
Introduction 1293
Methods 1294
Results 1295
Discussion 1297
Introduction
Stroke is a leading cause of death and disability (Hankey,2017;Wang et al.,2019),and > 70% of strokes are ischemic (Phipps and Cronin,2020).During the early period of focal brain infarction,axonal injury and synaptic failure(structural destruction to synaptic elements by hypoxic damage) are always followed by irreversible neurotransmission damage in the ischemic penumbra(IP) without timely blood flow restoration (Hofmeijer and van Putten,2012;Zheng et al.,2018;Muzzi et al.,2019).Salvaging penumbra neurons is an active research focus (Puig et al.,2018);however,available therapeutic options for ischemic stroke (IS) are currently limited.Despite being the only pharmacological treatment licensed by the U.S.Food and Drug Administration,recombinant tissue plasminogen activator has a narrow therapeutic window for IS reversion (Hacke et al.,2008;Powers et al.,2019).Hence,only a few patients can benefit from this agent (Zerna et al.,2018;Yang et al.,2020).Identifying new therapeutic targets for efficient and effective IS treatment is of great significance.Cell therapy is emerging as a promising IS treatment option,especially at delayed time points (Sarmah et al.,2018;Venkat et al.,2020).Previous studies have suggested that mesenchymal stem cells (MSCs) exert their therapeutic effects by robustly releasing exosomes to improve post-IS neurological outcomes (Xin et al.,2013,2017;Tian et al.,2018;Kim et al.,2020).Numerous recent studies have confirmed that MSC-derived exosomes (MSCexos) mediate cell-to-cell communication by transmitting RNA,DNA,proteins,and lipids to activate signaling pathways through regulating the bioactivity of recipient cells within the nervous system.MSC-exos have been used as therapeutic agents in IS treatment (Xin et al.,2013;van Niel et al.,2018;Lin et al.,2020;Khan et al.,2021).Similarly,many studies have demonstrated that exercise is conducive to functional neural recovery and recommended it as an effective IS treatment option.Its beneficial effects further include the inhibition of neuronal apoptosis,attenuation of infarct volume and neuronal damage,acceleration of neurogenesis and angiogenesis,and strengthening of cerebral neuroplasticity (Chen et al.,2001;Liu et al.,2011;Zhang et al.,2013,2015;Wang et al.,2014;Sun et al.,2019;Xie et al.,2019).
The c-Jun N-terminal kinase (JNK) family of mitogen-activated protein kinases participates in diverse pathological and physiological processes in the developing and adult brain.These include neuron survival,migration,polarity,regeneration,and developmental apoptosis (Kuan et al.,1999;Qu et al.,2013;Coffey,2014;Zeke et al.,2016;Zheng et al.,2018;Ugbode et al.,2020).As one of its most predominant members,the activation of JNK1 is associated with the phosphorylation of several downstream transcription factors,including c-Jun,Ets-like protein-1,activator transcription factor-2(ATF-2),and ATF-3 (Herdegen and Leah,1998;Chang et al.,2003).Among these,the phosphorylation of c-Jun plays the most important role in poststroke neural plasticity and functional remodeling,including the mediation of neuronal apoptosis and promotion of synaptic formation and axonal regeneration (Ratajczak et al.,2006;Antoniou and Borsello,2012).During neurogenesis and in the adult brain,c-Jun is overexpressed in response to neuronal injury (Herdegen and Leah,1998;Raivich et al.,2004).Our previous study found that treadmill exercise increased Netrin-1 and deleted in colorectal cancer expression,subsequently inducing neuroplasticity in the peri-infarct lesion in rats with cerebral ischemia (CI) (Liu et al.,2011).Netrin-1 stimulated post-IS neurological recovery by facilitating axonal regeneration and synaptogenesis via activating the JNK1/c-Jun signaling pathway (Zheng et al.,2018).Murata et al.(2012) also found that delayed inhibition of JNK/c-Jun signaling aggravated neurological results,with larger infarct volumes after CI.Kim et al.(2020) found that MSCs activate the JNK/c-Jun signal transduction pathway to increase the levels of growth factors,neuroprotective factors,and anti-apoptotic factors,suggesting that activating the JNK/c-Jun pathway may promote neuroprotection.However,further investigation is needed to elucidate the exact mechanism of treadmill exercise combined with MSC-exos treatment in patients with IS and determine whether these treatments share a common signaling pathway.
The present study investigated the role of treadmill exercise+MSC-exos in functional recovery and the potential regulatory mechanism by establishing a middle cerebral artery occlusion (MCAO) model.
Methods
Cell culture
MSCs derived from the bone marrow of male Sprague-Dawley rats were purchased from ScienCell Research Laboratories (San Diego,CA,USA,Cat#R7500).Cells were identified by immunofluorescence with antibodies to CD73,CD90,and CD105,and lipid staining after differentiation by ScienCell Research Laboratories.The cells were then cultured in a Dulbecco’s modified Eagle’s medium (Sigma-Aldrich,St.Louis,MO,USA) solution containing 10%fetal bovine serum,100 U/mL penicillin G,and 100 mg/mL streptomycin.The solution was incubated at 37°C with humidified 5% CO2for MSC cultivation.The culture media were replaced every 3 days,and MSCs were passaged using a 0.05% trypsin-ethylene diamine tetraacetic acid solution (Gibco,Grand Island,NY,USA) (Soleimani and Nadri,2009;Zhang et al.,2015;Meng et al.,2018;Xu et al.,2020).MSCs at passages 3–5 from the same batch were prepared for all experiments.
Exosome isolation
To isolate exosomes,ExoQuickTMprecipitation was carried out in accordance with the manufacturer’s instructions (System Biosciences,Embarcadero Way Palo Alto,CA,USA;Lobb et al.,2015;Venkat et al.,2020).Briefly,to remove any particulate matter,a 0.22-μm syringe filter (Merck Millipore,Darmstadt,Germany) was used to filter 500 μL of clarified culture media.Next,at a ratio of 1 mL Exoquick/5 mL,the ExoQuick-TCTMsolution (System Biosciences) was added to phosphate-buffered saline (PBS).The sample was stored overnight at 4°C and then centrifuged at 1500 ×gfor 30 minutes to remove the supernatant.The supernatant was discarded,and the pellet was resuspended in PBS and stored at–80°C until use.The MSC-exos protein concentration was determined using a bicinchoninic acid (BCA) Protein Assay Kit (Beyotime,Shanghai,China).
Exosome characterization
The MSC-exos suspension was applied to a formvar carbon-coated copper grid(Electron Microscopy Sciences,Hatfield,PA,USA) for transmission electron microscopy observation,as previously described (Xin et al.,2012;Xu et al.,2020).The grid was dried with filter papers and stained with uranyl acetate.The shape and size of the MSC-exos were then observed under a Philips EM208 transmission electron microscope (Philips,Eindhoven,Noord-Brabant,Netherlands).Moreover,as specific markers of MSC-exos,the expression of tetraspanins CD9 and CD63 was confirmed by western blot analysis.
Animals
A total of 68 clean adult male Sprague-Dawley rats (weighing 250–280 g and aged 2 months) purchased from the Experimental Animal Center of Fujian Medical University (license No.SYXK (Min) 2020-0005) were used in the present study.All experimental protocols were conducted in accordance with The National Institute of Health’s Guide for the Care and Use of Laboratory Animals (8thed,2011).All experimental procedures were approved by the Fujian Medical University Institutional Animal Care and Use Committee.Rats were housed in cages (less than five animals per cage) under a 12-hour light/dark cycle withad libitumaccess to food and water,a room temperature set at 22 ± 1°C,and a humidity of 60–70%.The rats were first anesthetized with isoflurane (containing 3% for induction or 1.5% for maintenance in 30% O2and 70% N2O,Sigma-Aldrich) during modeling and sampling.The rats were randomly divided into four groups: vehicle (MCAO+vehicle),exercise (MCAO+exercise+PBS),MSC-exos (MCAO+MSC-exos),and exercise+MSC-exos(MCAO+exercise+MSC-exos) (n=17/group).The study flow chart is shown inFigure 1.
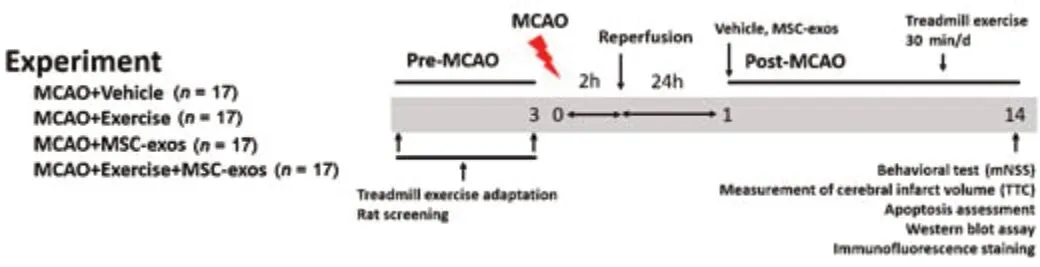
Figure 1|The flow chart of the in vivo study.
Rat models of focal CI established by MCAO
Rats were subjected to MCAO to induce focal CI as previously described (Longa et al.,1989).The right common carotid artery,external carotid artery,and internal carotid artery were exposed through a midline neck incision.With its tip rounded by heating near a flame,a 3.0 monofilament nylon suture(18.5 mm) was advanced from the external carotid artery into the lumen of the internal carotid artery until the origin of the middle cerebral artery was blocked.At 2 hours after MCAO,the rats were re-anesthetized,and reperfusion was accomplished via suture withdrawal until the tip cleared the external carotid artery lumen.Rectal temperature was maintained at 37.0 ±0.5°C during the operation and recovery from the operation.
MSC-exos treatment
At 2 hours after MCAO and 24 hours after stroke induction,the MCAO rats were injected with 100 μg of total MSC-exos protein (equivalent to~3 × 1011exosomes) in 0.5 mL PBS via the tail vein.Rats in the vehicle and exercise groups were injected with 0.5 mL PBS via the tail vein (Xin et al.,2013).
Treadmill exercise training
Exercise in the present study was performed using a motor-driven treadmill(Treadmill Simplex II,Columbus Instruments,Columbus,OH,USA) to force the rats to run with an electric stimulation system installed on the rear floors.
A 3-day adaptation period was applied before the MCAO procedure.During this period,all animals were placed on a moving belt and underwent treadmill exercise adaptation.The belt was moved towards an electrified grid to induce running in the direction opposite to the movement of the belt,thus avoiding foot shocks (Yang et al.,2003;Liu et al.,2011).Only the rats that learned to run during the adaptation period were included in the present study.
A gradual treadmill exercise schedule was followed in all eligible rats:4 m/min on day 1,8 m/min on day 2,and 12 m/min on the remaining days with an incline of 0°.
Starting at 24 hours after MCAO,the rats in exercise and exercise+MSCexos groups underwent treadmill exercise at an intensity of 30 minutes/day for 14 consecutive days.The rats in vehicle and MSC-exos groups were not forced to run and were left to freely use the treadmill for 30 minutes (Liu et al.,2011;Zhang et al.,2015).After completing the treadmill exercise,the rats underwent behavioral test and sampling.
Behavioral test
Prior to MCAO and on day 14 after MCAO,all rats underwent behavioral testing via modified neurological severity scores (mNSS),as described previously (Chen et al.,2001).Briefly,the mNSS consisted of motor (abnormal movement and muscle status),sensory (proprioceptive,tactile,and visual),reflex (pinna,corneal,and startle),and balance tests and was used to evaluate the severity of neurological deficits.Neurological function was graded on a scale of 0–18 (normal score: 0;maximal deficit score: 18),and one point was awarded for the inability to perform the tasks or for the lack of a tested reflex.
Measurement of cerebral infarct volume
To measure brain infarct volume in the experimental groups on day 14,rats were perfused transcardially with PBS and subsequently with PBS containing 4% paraformaldehyde.The brains were removed quickly and sectioned into six 2-mm-thick coronal slices using a Leica CM1850 cryostat (Leica Microsystems GmbH,Wetzlar,Hesse-Darmstadt,Germany).The fresh brain slices were incubated in a 2% solution of 2,3,5-triphenyltetrazolium chloride and fixed by immersion in PBS containing 4% paraformaldehyde for 30 minutes at 37°C.The unstained area in each brain slice was defined as the cerebral infarction area.The volumes of the infarcted tissue and both hemispheres in each brain slice were calculated using ImageJ analysis software (1.46r,National Institutes of Health,Bethesda,MD,USA) (Schneider et al.,2012).
Apoptosis assessment using TdT-mediated dUTP nick end labeling assay
On day 14 after MCAO,neuronal apoptosis in the peri-ischemic cortex was estimated using TdT-mediated dUTP nick end labeling (TUNEL) staining following the manufacturer’s instructions (n=3 in each group).TUNEL staining was conducted with a commercially available kit (Roche Applied Science,Penzberg,Germany).Briefly,fresh coronal brain sections were subjected to fixing,incubation,enzyme reaction,and counterstaining,followed by observation.The number of TUNEL-positive cells per field in six randomly selected fields was counted under a Nikon Eclipse Ti–U fluorescence microscope (Nikon,Tokyo,Japan),as previously described (Hu et al.,2017;Lai et al.,2020).The number of TUNEL-positive cells was presented as the mean percentage in each group.
Western blot assay
To identify MSC-exos,the expression levels of CD9 and CD63 were analyzed by western blot assays.Isolated MSC-exos were prepared in PBS to detect protein concentration.To evaluate JNK1/c-Jun signaling pathway activation,neuronal apoptosis,and synaptic-axonal remodeling in the peri-infarct cortex,the expression levels of JNK1,c-Jun,phosphorylated JNK1 (p-JNK1),phosphorylated c-Jun (p-c-Jun),Bax,cleaved caspase-3 (c-caspase-3),Bcl-2,post-synaptic density protein-95 (PSD-95),synaptophysin,microtubuleassociated protein-2 (MAP-2),growth-associated protein of 43-kDa (GAP-43),and neurofilament-200 (NF-200) were analyzed by western blot assays (n=3 in each group).At 14 days after MCAO,rats in each group were anesthetized with isoflurane (containing 3% for induction or 1.5% for maintenance in 30%O2and 70% N2O,Sigma-Aldrich) and perfused transcardially with PBS (pH 7.4),and then brain tissues from the peri-infarct cortex were extracted.A BCA Protein Assay Kit (Beyotime,Shanghai,China) was used to measure the protein concentration,as previously described (Zheng et al.,2018).
Equal amounts (30 μg) of proteins were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore,Bedford,MA,USA).The membrane was subsequently incubated with the following primary antibodies at 4°C overnight: mouse anti-JNK1 (1:800,Santa Cruz Biotechnology,Dallas,TX,USA,Cat# sc-1648,RRID: AB_675868),mouse anti-c-Jun (1:600,Santa Cruz Biotechnology,Cat# sc-74543,RRID: AB_1121646),mouse anti-p-JNK1 (1:800,Santa Cruz Biotechnology,Cat# sc-6254,RRID: AB_628232),mouse antip-c-Jun (1:600,Santa Cruz Biotechnology,Cat# sc-822,RRID: AB_627262),rabbit anti-CD63 (1:1000,Abcam,Cambridge,UK,Cat# ab134045,RRID:AB_2800495),rabbit anti-CD9 (1:1000,Abcam,Cat# ab236630),rabbit anti-Bcl-2 (1:1000,Abcam,Cat# ab59348,RRID: AB_ 2064155),rabbit anti-Bax (1:5000,Abcam,Cat# ab32503,RRID: AB_725631),mouse anticaspase-3 (1:3000,Proteintech Group,Wuhan,China,Cat# 66470-2-Ig,RRID:AB_2876892),rabbit anti-PSD-95 (1:1000,Abcam,Cat# ab238135,RRID:AB_2895158),rabbit anti-synaptophysin (1:1000,Abcam,Cat# ab32127,RRID: AB_2286949),chicken anti-MAP-2 (1:1000,Abcam,Cat# ab5392,RRID:AB_2138153),rabbit anti-GAP-43 (1:800,Abcam,Cat #ab75810,RRID:AB_1310252),rabbit anti-β-actin (1:400,Boster Biological Technology,Wuhan,China,Cat# BM3873),rabbit anti-NF-200 (1:500,Boster Biological Technology,Cat# A05307),and mouse anti-glycerol 3-phosphate dehydrogenase (GAPDH)(1:1000,Boster Biological Technology,Cat# BM3876).Membranes were then incubated with goat anti-rabbit IgG-horseradish peroxidase secondary antibodies (1:8000,Abcam,Cat# ab97080,RRID: AB_ 10679808) at room temperature (25 ± 1°C) for 2 hours.The optical density of target proteins(CD63,CD9,JNK1,p-JNK1,c-Jun,p-c-Jun,Bcl-2,Bax,c-caspase-3,PSD-95,synaptophysin,NF-200,MAP-2,and GAP-43) was detected using ImageJ software and normalized to that of the internal control (β-actin and GAPDH).
Immunofluorescence staining
To address axonal remodeling in the peri-infarct cortex,immunofluorescence staining for NF-200,MAP-2,and GAP-43 was performed as previously described (Zheng et al.,2018;Lai et al.,2020).Briefly,at 14 days after MCAO,under deep anesthesia with isoflurane,rats (n=3 in each group) were transcardially perfused with PBS (pH 7.4) and then with PBS containing 4%paraformaldehyde at 4°C (pH 7.4).Brain tissues were obtained,kept in the same fixative for 6 hours at 4°C,and dehydrated in a gradient of 20% and 30% sucrose at 4°C before sectioning on a cryostat.Frozen coronal sections(8 μm) were subjected to rewarming,incubation with 0.25% Triton X-100(Amresco,Solon,OH,USA),blocking with 5% donkey serum (Abcam),and incubation with primary antibody at 4°C overnight.The primary antibodies were as follows: rabbit anti-NF-200 (1:100,Boster Biological Technology,Cat# A05307),chicken anti-MAP-2 (1:800,Abcam,Cat# ab5392,RRID:AB_2138153),and rabbit anti-GAP-43 (1:1000,Abcam,Cat# ab75810,RRID:AB_1310252).After being rinsed three times,the sections were incubated in Alexa Fluor 488 donkey anti-rabbit secondary antibody (1:1000,Thermo Fisher Scientific,Waltham,MA,USA) at room temperature for 2 hours and then 4′,6-diamidino-2-phenylindole (DAPI;5 mg/mL,Beyotime,Shanghai,China) for 15 minutes.Finally,fluorescence images of the sections were collected using a Zeiss LSM 750 confocal microscope (Zeiss,Gottingen,Niedersachsen,Germany) and processed by ImageJ software.
Statistical analysis
No statistical methods were used to predetermine sample sizes;however,our sample sizes were similar to those reported in previous publications (Xin et al.,2013;Zhang et al.,2013,2015).Rats in which MCAO was not successfully induced and those that died during the experiment were excluded.Finally,17 eligible rats were included in each group to ensure the accuracy and completeness of the experimental results.All data were statistically analyzed with SPSS 25.0 software (IBM,Armonk,NY,USA) and expressed as the mean± standard error of the mean.All evaluators were blinded to the assignments.Multiple statistical analysis comparisons were performed by one-way analysis of variance (ANOVA).The Bonferronipost hoctest for data of equal variance was performed after analysis of variance.One-way analysis of variance with Dunnett’s T3post hoctest was performed when the data variance was unequal.AP-value < 0.05 was considered statistically significant.
Results
MSC-exos characterization
Two conventional methods,transmission electron microscopy and western blot analysis,were performed for MSC-exos characterization.Transmission electron microscopy showed that MSC-exos exhibited a typical cup-shape morphology (Figure 2A),and western blot assays verified the expression of tetraspanins CD63 and CD9 (specific exosome markers) (Figure 2B).
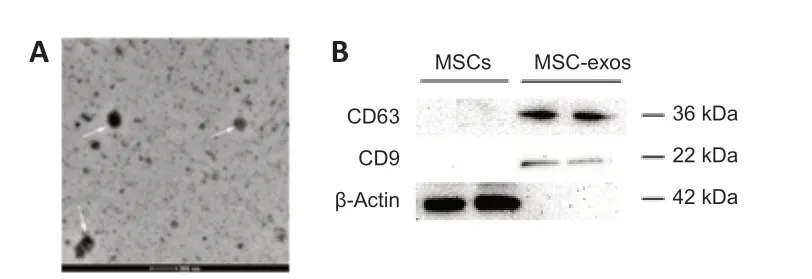
Figure 2|Characterization of MSC-exos collected from MSC-conditioned medium.
Treadmill exercise combined with MSC-exos treatment stimulates JNK1/c-Jun signal activation in the peri-infarct brain area after IS
To explore whether treadmill exercise combined with MSC-exos activated JNK1/c-Jun after MCAO,p-JNK1 and p-c-Jun levels were analyzed using western blot analysis (Figure 3A).p-JNK1 (exercise:P< 0.01,MSC-exos:P<0.001) and p-c-Jun (exercise:P< 0.05,MSC-exos:P< 0.05) expression were significantly higher in the exercise and MSC-exos groups than in the vehicle group.Compared with those in the exercise and MSC-exos groups,p-JNK1(exercise:P< 0.01,MSC-exos:P< 0.01) and p-c-Jun (exercise:P< 0.001,MSCexos:P< 0.001) expression in the IP in the exercise+MSC-exos group were significantly higher (Figure 3BandC).These findings indicate that treadmill exercise combined with MSC-exos stimulates JNK1/c-Jun signal activation in the IP after IS.
Treadmill exercise combined with MSC-exos treatment attenuates infarct volume and facilitates functional neurological recovery
The pale area stained with 2% 2,3,5-triphenyltetrazolium chloride was determined to be the infarct area (Figure 4A).The brain infarct volume was significantly smaller in exercise and MSC-exos groups than in the vehicle group (bothP< 0.001).The infarct volume was even smaller in the exercise+MSC-exos group compared with that in the exercise (P< 0.001) and MSCexos groups (P< 0.001;Figure 4B).To assess the effects of treadmill exercise+MSC-exos and further verify the role of JNK1/c-Jun in neurofunctional recovery at the macro level,the mNSS was determined to examine neural impairments in the motor,sensor,equilibrium,and reflex senses of contralateral limbs in rats that underwent MCAO.
The mNSS in all four groups,estimated on day 14 after MCAO,are summarized inFigure 4C.The mNSS were significantly lower in the exercise and MSC-exos groups than in the vehicle group (bothP< 0.05) and even lower in the exercise+MSC-exos group compared with those in the exercise(P< 0.05) and MSC-exos groups (P< 0.05;Figure 4C).These findings indicate that treadmill exercise+MSC-exos attenuates infarct volume and promotes the recovery of neurological function in the sub-acute phase of IS (> 72 hours;Bernardo-Castro et al.,2020).
Treadmill exercise combined with MSC-exos treatment inhibits neuronal apoptosis
To determine the effects of treadmill exercise combined with MSC-exos on neuronal apoptosis,the expression levels of Bax,Bcl-2,and c-caspase-3 in the cortex (IP) were analyzed in different experimental groups at 14 days after MCAO (Figure 5A).
Western blot assays showed that the expression of Bcl-2 in the cortex (IP)was significantly higher in the exercise+MSC-exos group than in the exercise(P< 0.05) and MSC-exos groups (P< 0.05).In addition,the expression was significantly higher in exercise and MSC-exos groups than in the vehicle group(bothP< 0.01;Figure 5B).The expression levels of c-caspase-3 and Bax in the cortex (IP) in the exercise+MSC-exos group were significantly lower compared with those in the exercise (Bax:P< 0.001,c-caspase-3:P< 0.05)and MSC-exos groups (Bax:P< 0.001,c-caspase-3:P< 0.05).Moreover,the expression levels of c-caspase-3 (exercise:P< 0.01,MSC-exos:P< 0.001) and Bax (bothP< 0.01) in the exercise and MSC-exos groups were significantly lower compared with those in the vehicle group (Figure 5CandD).
Furthermore,CI-induced TUNEL-positive cells were observed in the cortex (IP)on day 14 after MCAO (Figure 5E).The percentage of TUNEL-positive cells in the exercise+MSC-exos group was significantly lower than that in the exercise(P< 0.01) and MSC-exos groups (P< 0.01),and the proportion of TUNELpositive cells in the exercise and MSC-exos groups was significantly lower than that in the vehicle group (bothP< 0.05) (Figure 5F).These findings indicate that treadmill exercise combined with MSC-exos exhibits protective effects on the inhibition of neuronal apoptosis.
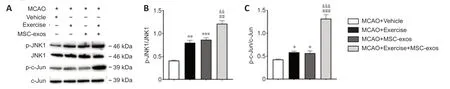
Figure 3|Effects of treadmill exercise combined with MSC-exos on the JNK1/c-Jun signaling pathway in peri-infarct brain tissue on day 14 after MCAO.
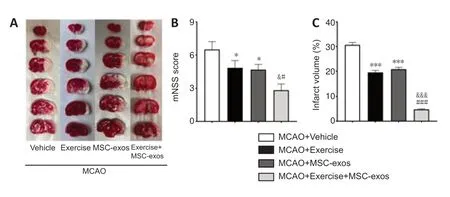
Figure 4|Effects of treadmill exercise combined with MSC-exos on infarct volume and behavioral testing (mNSS) on day 14 after MCAO.
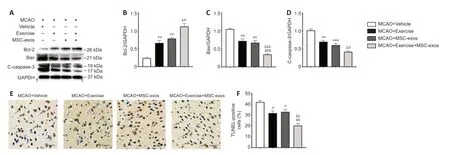
Figure 5|Effects of treadmill exercise combined with MSC-exos on neuronal apoptosis in the cortex (IP) on day 14 after MCAO.
Treadmill exercise combined with MSC-exos treatment facilitates synaptogenesis
To further determine the effect of treadmill exercise combined with MSC-exos on synaptogenesis and its possible mechanism after MCAO,the expression levels of the presynaptic protein synaptophysin and postsynaptic protein PSD-95 were analyzed (Figure 6A).Western blot analysis showed that the expression levels of PSD-95 and synaptophysin in the exercise+MSCexos group were significantly higher than those in the exercise (PSD-95:P< 0.05,synaptophysin:P< 0.01) and MSC-exos groups (PSD-95:P< 0.01,synaptophysin:P< 0.05).Furthermore,the expression levels in both exercise(PSD-95:P< 0.001,synaptophysin:P< 0.001) and MSC-exos groups (PSD-95:P< 0.001,synaptophysin:P< 0.01) were significantly higher compared with those in the vehicle group (Figure 6BandC).These findings indicate that treadmill exercise combined with MSC-exos treatment after MCAO facilitates both presynaptic and postsynaptic formation.
Treadmill exercise combined with MSC-exos treatment promotes axonogenesis
To explore the role of treadmill exercise+MSC-exos in axonal regeneration after MCAO,the expression levels of GAP-43,MAP-2,and NF-200 were analyzed using western blot assays (Figure 7A).The results showed that the expression of these axonal structure-associated proteins in the exercise +MSC-exos group was higher compared with those in the exercise (NF-200:P<0.001,MAP-2:P< 0.001,GAP-43:P< 0.01) and MSC-exos groups (NF-200:P<0.001,MAP-2:P< 0.001,GAP-43:P< 0.05) (Figure 7B–D).
Concurrently,the expression levels of NF-200 in both exercise (P< 0.01) and MSC-exos groups (P< 0.001),MAP-2 in the MSC-exos group (P< 0.05),and GAP-2 in the exercise group (P< 0.05) were significantly higher compared with those in the vehicle group (Figure 7B–D).
Interestingly,there was no evident difference in MAP-2 expression between exercise and vehicle groups or in GAP-43 expression between MSC-exos and vehicle groups (Figure 7CandD).
Additionally,the immunopositivities of NF-200,MAP-2,and GAP-43 were analyzed in different experimental groups after MCAO via immunofluorescence (Figure 8A).The results showed that the immunopositivities of these axonal structure-associated proteins in the exercise+MSC-exos group were significantly higher compared with those in the exercise (NF-200:P< 0.05,MAP-2:P< 0.05,GAP-43:P< 0.05) and MSC-exos groups (NF-200:P< 0.05,MAP-2:P< 0.05,GAP-43:P< 0.05)(Figure 8B–D).Similarly,the immunopositivity of NF-200 was significantly higher in the exercise group than that in the vehicle group (P< 0.05),and the immunopositivities of MAP-2 and GAP-2 in both exercise and MSC-exos groups were significantly higher compared with those in the vehicle group(MAP-2: bothP< 0.05,GAP-43: bothP< 0.05) (Figure 8B–D).There was no significant difference in the immunopositivity of NF-200 between MSC-exos and vehicle groups (Figure 8B).These findings indicate that treadmill exercise combined with MSC-exos promotes axonal regeneration.
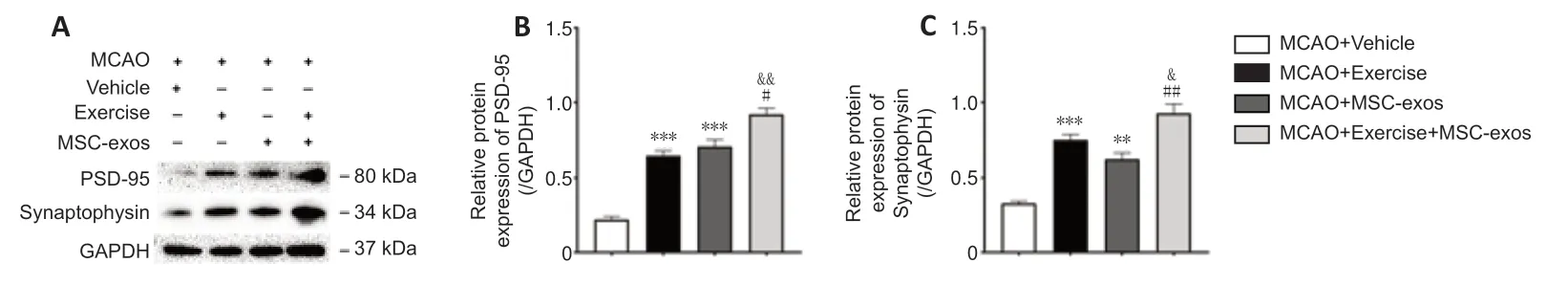
Figure 6|Effects of treadmill exercise+MSC-exos on synaptic formation in peri-infarct brain tissue on day 14 after MCAO.
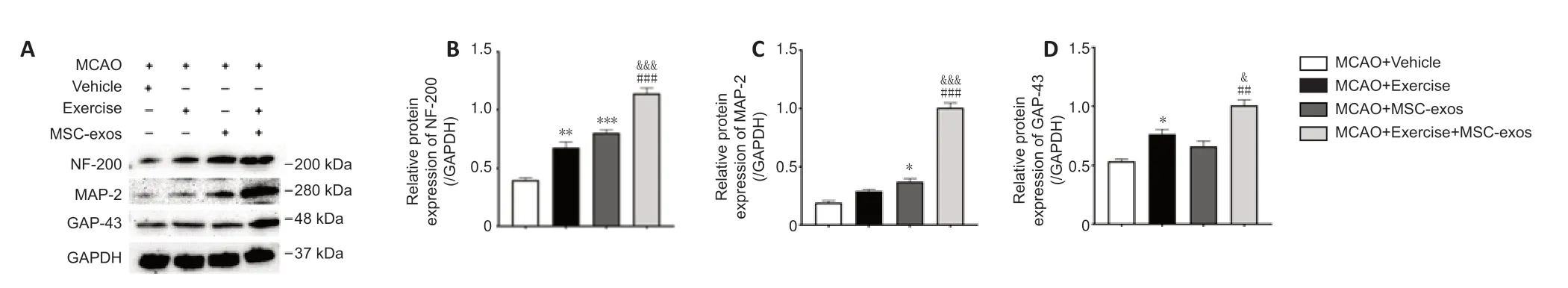
Figure 7|Effects of treadmill exercise+MSC-exos on axonal regeneration in peri-infarct brain tissue on day 14 after MCAO.
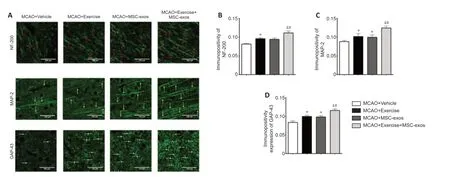
Figure 8|Effects of treadmill exercise+MSC-exos on the immunopositivities of NF-200,MAP-2,and GAP-43 in peri-infarct brain tissue on day 14 after the MCAO.
Discussion
Currently,neurorestorative therapies,which are the predominant CI treatment methods,aim to promote neurofunctional recovery by improving post-IS brain neuroplasticity and neurite remodeling.MSC-exos treatment is well known to augment neurological outcomes by inhibiting neuronal apoptosis and promoting neurogenesis and neurite remodeling in rats with CI(Chopp and Li,2002;Chen et al.,2003;Honmou et al.,2012;Xin et al.,2013;Kim et al.,2020).
On the one hand,exosomes do not cause immunological rejection or elicit vaso-occlusive damage and have both a negligible toxicity and a low risk of triggering malignant transformation or tumors (Shi et al.,2014).Because they easily cross the blood-brain barrier,exosomes are effectively transported to remote somatic cells,specifically brain cells (Bang and Kim,2019;Khan et al.,2021).On the other hand,previous clinical trials and preclinical experiments have suggested that rehabilitation exercise can ameliorate the neural outcomes after CI by inhibiting neuronal apoptosis,decreasing neuronal damage and infarct volume,promoting neurogenesis,and enhancing brain plasticity (Liu et al.,2011;Zhang et al.,2015;Sun et al.,2019;Wang et al.,2019;Tollár et al.,2021).
In addition,many researchers encourage early treadmill exercise for the restoration of neurological functions after CI (Liu et al.,2011;Zhang et al.,2013,2015).Current guidelines recommend that early rehabilitation for hospitalized patients with stroke is provided in environments with organized,interprofessional stroke care;furthermore,stroke survivors should receive rehabilitation at an intensity consistent with the patient’s anticipated benefit and tolerance (Winstein et al.,2016;Gittler and Davis,2018;Powers et al.,2019).
However,the underlying mechanisms and whether both MSC-exos and treadmill exercise mediate JNK1/c-Jun signal activity remain unclear.The present study aimed to determine the synergistic effects of treadmill exercise and MSC-exos and investigate whether the JNK1/c-Jun pathway is involved in the influence of both MSC-exos and treadmill exercise on neural remodeling after IS.Apparent activation of Bcl-2 and marked decreases in Bax and caspase-3 (along with higher phosphorylation levels of JNK1 and c-Jun),accompanied by fewer TUNEL-positive cells and a prominent reduction in apoptotic rates in the IP,were found on day 14 in the exercise and MSC-exos groups;this result is consistent with finding from previous studies (Liu et al.,2011;Zhang et al.,2015).
In the exercise+MSC-exos group,along with higher phosphorylation expression levels of JNK1 and c-Jun,the activation of Bcl-2,marked reduction in caspase-3 and Bax,and decrease in TUNEL-positive cells exhibited a 2-to 4-fold difference compared with those in the other experimental groups.These findings indicate that both treadmill exercise and MSC-exos inhibited neuronal apoptosis after CI via the JNK1/c-Jun signaling pathway.
Numerous studies have demonstrated that JNK is a key player in diverse physiological and pathophysiological events,including neuronal development,neuronal pathfinding,neuroregeneration,neuroplasticity,neuroinflammation,and neuronal survival and apoptosis (Kuan et al.,1999;Qu et al.,2013;Coffey,2014;Zeke et al.,2016;Zheng et al.,2018;Igarashi et al.,2020;Myers et al.,2020;Ugbode et al.,2020).Our previous data showed that elevated phosphorylation levels of JNK1 and c-Jun in the peri-ischemic cortex soon after CI/reperfusion injury upregulated the levels of synaptophysin(presynaptic protein),PSD-95 (postsynaptic protein),GAP-43,MAP-2,and NF-200 and induced neural microstructural reconstruction (Zheng et al.,2018).
These results indicate that the activation of JNK1/c-jun signaling facilitated synaptogenesis and axonogenesis after IS.The consensus is that synaptophysin,PSD-95,MAP-2,GAP-43,and NF-200 are important for evaluating the capacity of neuroregeneration (Stroemer et al.,1998;Zheng et al.,2018).
The present study found that after stroke,treadmill exercise or MSC-exos alone,along with higher expression levels of p-JNK1 and p-c-Jun,increased the expression levels of synaptophysin and PSD-95.Moreover,treadmill exercise combined with MSC-exos enhanced the above effects.This indicates that treadmill exercise combined with MSC-exos can improve synapse formation in both the presynaptic and postsynaptic regions after stroke via the JNK1/c-Jun pathway.
Furthermore,western blot analysis found that after stroke,both treadmill exercise and MSC-exos treatment improved axonal regeneration and outgrowth by upregulating NF-200 expression.Treadmill exercise also upregulated the expression level of GAP-43,while MSC-exos upregulated the expression level of MAP-2.Treadmill exercise combined with MSC-exos enhanced these effects.
Immunofluorescence analysis found that both treadmill exercise and MSCexos facilitated axonal regeneration and outgrowth by upregulating the levels of MAP-2 and GAP-43;treadmill exercise also upregulated the expression of NF-200,but MSC-exos did not affect this measure.Treadmill exercise combined with MSC-exos remarkably enhanced these effects.This again suggests that treadmill exercise+MSC-exos promotes axonogenesis through the JNK1/c-Jun pathway.
This research explored the therapeutic efficacies of treadmill exercise combined with MSC-exos treatment on functional outcomes after MCAO by evaluating the mNSS.Along with higher phosphorylation levels of JNK1 and c-Jun,rats in the exercise+MSC-exos group displayed fewer neurologic impairments than those in both exercise and MSC-exos groups.Furthermore,exercise and MSC-exos groups exhibited fewer neurologic dysfunctions than the vehicle group.The data in the present study show a more remarkable reduction in the infarct volume after treatment with treadmill exercise and MSC-exos administration.
Several limitations are worth mentioning in our study.First,we established MCAO model as CI model in rats.Though previous studies have showed that it was the most classic and common model,and could generate a stable CI model,it has some significant disadvantages (Chen et al.,2003;Xin et al.,2017).As usually very sudden reperfusion,rather than the progressive one seen in IS in clinical,it can not perfectly simulate IS in humans (Barthels and Das,2020).And it also inevitably has some shortcomings including potential risks of incomplete artery occlusion and vascular hemorrhage,and possible induced hyperthermia in animals (Sommer,2017;Barthels and Das,2020).Due to limitations of current experimental conditions,we will use CI models and methods that are closer to the human in future studies,such as live imaging of isolated neurons.Second,we mainly investigated the effects of treadmill exercise+MSC-exos on neuronal apoptosis and synaptic-axonal remodeling on day 14 after MCAO,but we did not examine them at other time points,such as during the acute phase after CI.Further studies are required to explore the effects of treadmill exercise+MSC-exos at other time points.Third,the quantitative analysis of cerebral infarct volume on day 14 after MCAO was performed using 2,3,5-triphenyltetrazolium chloride staining.However,magnetic resonance imaging,which is a noninvasive and visual way to assess the severity of CI,was not conducted to quantitatively estimate the infarct volume.Moreover,previous studies (Yang et al.,2010;Zhang et al.,2011) have shown that estrogen has neuroprotection effects in rats after CIreperfusion injury,and male rats are more stable and tolerant than female rats;therefore,only male rats were selected as experimental animals in this study,which is also a limitation.
In conclusion,treadmill exercise+MSC-exos treatment inhibits neuronal apoptosis,attenuates infarct volume,and facilitates axonal regeneration and synaptic formation.Treadmill exercise may (1) exert its synergistic effect with MSC-exos to facilitate synaptic formation and axonal regeneration and(2) reduce neuronal apoptosis to re-establish neural circuits through the JNK1/c-Jun pathway post-CI.These findings shed new light on the effects of treadmill exercise+MSC-exos and provide key targets in the clinical treatment of post-acute-phase IS.
Author contributions:Study conception and design: XHJ,HFL,NL;data acquisition,analysis and interpretation: MLC,HBC;statistical analysis: MLC,YCX;funding acquisition: XHJ;manuscript draft: XHJ,HFL;manuscript revision:YXZ,HBC,RHC.All authors read and approved the final manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update