Translational bioengineering strategies for peripheral nerve regeneration: opportunities,challenges,and novel concepts
Karim A.Sarhane,Chenhu Qiu,Thomas G.W.Harris,Philip J.Hanwright,Hai-Quan Mao,,Sami H.Tuffaha,
Abstract Peripheral nerve injuries remain a challenging problem in need of better treatment strategies.Despite best efforts at surgical reconstruction and postoperative rehabilitation,patients are often left with persistent,debilitating motor and sensory deficits.There are currently no therapeutic strategies proven to enhance the regenerative process in humans.A clinical need exists for the development of technologies to promote nerve regeneration and improve functional outcomes.Recent advances in the fields of tissue engineering and nanotechnology have enabled biomaterial scaffolds to modulate the host response to tissue repair through tailored mechanical,chemical,and conductive cues.New bioengineered approaches have enabled targeted,sustained delivery of protein therapeutics with the capacity to unlock the clinical potential of a myriad of neurotrophic growth factors that have demonstrated promise in enhancing regenerative outcomes.As such,further exploration of combinatory strategies leveraging these technological advances may offer a pathway towards clinically translatable solutions to advance the care of patients with peripheral nerve injuries.This review first presents the various emerging bioengineering strategies that can be applied for the management of nerve gap injuries.We cover the rationale and limitations for their use as an alternative to autografts,focusing on the approaches to increase the number of regenerating axons crossing the repair site,and facilitating their growth towards the distal stump.We also discuss the emerging growth factorbased therapeutic strategies designed to improve functional outcomes in a multimodal fashion,by accelerating axonal growth,improving the distal regenerative environment,and preventing endorgans atrophy.
Key Words: bioengineering;biomaterials;growth hormone;insulin-like growth factor 1;nanotechnology;neurobiology;peripheral nerve regeneration;Schwann cells;translational research
From the Contents
Introduction 1229
Search Strategy 1229
Traditional Approaches for Repairing Nerve Gap Injuries 1230
Bioengineering Strategies to Improve Outcomes Following Repair of Nerve Gap Injuries 1230
Bioengineering Strategies to Improve Outcomes Following Direct Epineural Nerve Repair 1231
Novel Bioengineering Concepts to Promote FunctionalRecovery Using a Multimodal Growth Factors-BasedStrategy1231
Remaining Challenges 1232
Conclusion 1232
Introduction
Peripheral nerve injuries (PNI) continue to be a major source of disability in the USA.Their frequency is reported to be 3% among all trauma patients and up to 5% when brachial plexus or root avulsion cases are counted (Robinson,2000;Scholz et al.,2009).These injuries cause both sensory and motor deficits resulting in partial or complete paralysis/numbness of the injured extremity as well as unrelenting neuropathic pain that is often recalcitrant to medical therapy (Siemionow and Brzezicki,2009).PNI not only impact patients’ quality of life but also affect their productivity and may result in major financial losses to patients and healthcare systems at large.A recent review noted they are associated with $150 billion of health-care expenses per year in the USA alone (Grinsell and Keating,2014).
Despite advances in surgical approaches,recovery of motor and sensory functions following PNI is often poor,especially for proximal injuries and after delayed repair.There are currently no Food and Drug Administrationapproved therapeutic strategies that improve functional recovery despite the considerable research efforts over the past 5 decades directed towards addressing this need (Scheib and Höke,2013;Faroni et al.,2015;Benga et al.,2017).
End-to-end epineurial repair is the current standard surgical treatment for nerve repair (Sunderland,1990;Isaacs,2010).However,when a segmental defect is present such that a tension-free end-to-end repair is not possible,the favored approach to reconstructing the nerve gap involves the use of an expandable nerve autograft.The limited supply of nerve autografts and their associated donor-site morbidity (namely,scarring,numbness,pain) have led to a search for suitable alternatives.The field of tissue engineering has contributed to clinical advances in addressing the nerve gap by introducing various biosynthetic bridges (Gu et al.,2015).More recent advances have provided novel regenerative strategies that are not just limited to mechanically bridging the nerve gap.These include the design of biomaterial scaffolds that interact with the host through tailored mechanical,chemical,and electrical signals,to modulate the tissue response and create a regenerative microenvironment capable of decreasing scarring at the repair site,stimulating axonal growth,improving the distal regenerative environment,and preventing end-organ atrophy.
In this paper,we review the translational bioengineering strategies that carry the potential for improved functional outcomes following repair,highlight their clinical applications in nerve gap and in end-to-end repairs and present their limitations.We also discuss emerging translational concepts in regenerative medicine that are based on a multimodal approach for a controlled and sustained local delivery of growth factors to the regenerating nerve and its target muscle.
Search Strategy
This review analyzes studies found on the PubMed database using the following keywords: nerve regeneration,biomaterials,Schwann cells,axonal growth,material science,and nanotechnology.All studies cited were published between 1972 and 2022,and represent the most relevant articles in the field.
Traditional Approaches for Repairing Nerve Gap Injuries
Current surgical methods for the treatment of PNI include either a direct end-to-end repair or the use of a nerve graft or biosynthetic conduit (when a gap is present).Autologous nerve grafts harvested from the patients’ own expendable nerve tissue have been used since 1972 (Millesi et al.,1972),and are still thought to offer the greatest regenerative support amongst the currently available options for bridging a nerve gap.The primary limitations of nerve autografting arise from the limited availability of expendable nerve tissue and the morbidity associated with their harvest.Due to those shortcomings,synthetic nerve conduits emerged as the first clinically available alternatives to autografts.Synthesized from biocompatible polymers such as polyglycolic acid,collagen,or polycaprolactone (PCL) (Meek and Coert,2008;Reid et al.,2013),these hollow tube conduits offered a passive mechanical bridge between the distal and proximal stump whose regenerative function depended on the locally secreted growth factors from the severed nerve ends(Williams et al.,1983;Danielsen and Varon,1995).As such,they are only effective over a short nerve gap (maximum of 3 cm) and their use has been largely limited to the reconstruction of non-critical sensory nerves (i.e.,nerves that do not supply the thumb or index finger,namely the radial sensory nerve).
Fresh nerve allografts taken from deceased donors have been investigated as an alternative to autografts (Mackinnon et al.,1984).They contain Schwann cells (SCs) that provide trophic support to regenerating axons,but the need for immunosuppression has severely limited their use.Hollow tube synthetic conduits were thus introduced as they are inert and do not elicit an immune rejection response.Their success was however limited as they did not contain any of the topographical cues of the native nerve.Those cues are critical for the guidance and support of the growing axons.Processed acellular nerve allografts (ANA) have emerged more recently as a viable option in some clinical scenarios.ANA do provide the native regenerative matrix present in nerve autografts including endoneurial tubes with pro-regenerative laminin in the basement membrane,thereby offering a critical advantage over the hollow tube synthetic conduits that preceded them.A number of studies have demonstrated greater regeneration through ANA in comparison to hollow tube conduits and ANAs have largely replaced hollow tube conduits in clinical use (Buncke et al.,2014;Ao,2016;Means et al.,2016).While they also do not elicit an immune response,they are however devoid of SCs and rely on repopulation by SCs migrating from the proximal and distal nerve stumps of the injured nerve.This process of repopulation was studied by Poppler et al.(2016) They noted that the longer the ANA is the higher the percentage of repopulating senescent SCs and stromal cells is,which explains poor axonal regeneration.That is why autografts are still considered to offer regenerative benefits in comparison to ANA.Those benefits arise from the neurotrophic support provided by SCs within them and tend to be preferred when critical mixed motor nerve defects are present.
Recent advances in tissue engineering and materials science offer the potential to improve upon the currently available options to address the nerve gap.This will require addressing the critical factors affecting nerve regeneration that are discussed below.
Bioengineering Strategies to Improve Outcomes Following Repair of Nerve Gap Injuries
A greater understanding of the critical factors leading to poor functional outcomes after nerve repair provides opportunities to intervene therapeutically.We will present those factors and discuss the specific bioengineering strategies aiming at addressing them.
Engineering biomaterials with topographical cues to guide axonal regeneration
A number of research groups focused on engineering constructs with an architecture that mimics that of the native nerve in an effort to better orient the growing axons (Figure 1A).A crucial component of the native nerve architecture are the elongated tubular fascicles that facilitate axonal guidance towards the target organ.Those are called Bands of Büngner.They are established by proliferating (as opposed to senescent) SCs,forming an architecture characterized by hundreds of microchannels structured along the major axis of the nerve Although the mechanism that results in their formation remains largely elusive,those aligned SCs and their extracellular matrix (laminin,fibronectin) provide indispensable pathways and growth factors for guided axonal regrowth (Ribeiro-Resende et al.,2009).Gonzalez-Perez et al.(2015) loaded chitosan conduits with laminin or fibronectin embedded in a collagen type I-based matrix,both incorporated either as fully hydrated hydrogel fillers or stabilized and organized into longitudinally oriented scaffolds.They used a 15 mm sciatic nerve gap model in rats.They noted that the addition of fibronectin to the collagen matrix enhanced nerve regeneration and that stabilization and organization of the hydrogels into longitudinally oriented structures further increased the cases in which regeneration occurred over the 15 mm long gap.Stabilization and organization of the matrix facilitated SC migration and axonal growth.Their hypothesis is that the matrix served to distribute the collagen fibers in a three-dimensional space,influencing cell adhesion and migration,and vascularization (Gnavi et al.,2013),and this configuration provided an environment promoting axonal regeneration and a surface guiding axonal growth,mimicking the Bands of Büngner of peripheral nerve tissue.The cell signaling triggered by the extracellular matrix molecules functions to potentiate the role of the matrix in repairing long peripheral nerve defects (Gnavi et al.,2013).The role of integrins is worth mentioning here.An elegant review by Nieuwenhuis et al.(2018) highlighted the critical role of integrins as the link between extracellular matrix molecules and internal cell signaling.They participate in axonal regeneration by binding to key ligands that effectuate their signaling,a process known as “outside-in” signaling (Nieuwenhuis et al.,2018).
In another study,Meyer et al.(2016) used fine-tuned chitosan nerve guides enhanced by a longitudinal chitosan film to reconstruct 15 mm sciatic nerve defects in rats.They showed that the chitosan films supported the fibrin matrix that formed following injury and this guided axonal growth.The fibrin-based cable connected the two nerve stumps and the SC migrated along it to proliferate and form the bands of Büngner.This resulted in significantly improved functional and morphological results of nerve regeneration,as measured by electrophysiological tests (20–30%) and nerve histomorphometry (number of myelinated fibers,axon and fiber diameters,and myelin thickness) in comparison to simple hollow chitosan nerve guides.
Both methods,although promising,are however not as successful as an autograft.Meyer et al.(2016) have shown that a native nerve remains superior in terms of latency,CAMP,muscle weight ratio,in addition to the number and myelination of regenerating axons.There is thus room for further improvement.
Engineering biomaterials with biochemical cues to stimulate regeneration
In addition to the topographical cues (discussed above),conduits can be engineered to incorporate growth factors and adhesion cues,such as neurotrophic factors or extracellular matrix proteins (e.g.,laminin) (Figure1B).Cao et al.(2011) incorporated the growth factor ciliary neurotrophic factor (CNTF) to a nerve guidance material (made of linear ordered collagen scaffold,LOCS).LOCS is a collagen membrane made from bovine aponeurosis that undergoes a multi-step treatment to remove the cellular elements and produce linear fibers that help with neurites attachment and guide axonal growth (Lin et al.,2006).LOCS fibers were cross-linked with laminin and a laminin-binding domain was fused to the N-terminal of CNTF.This functional scaffold was placed in a silicon conduit and tested in a rat sciatic nerve transection model (5 mm gap).The system showed it could guide axonal growth,retain CNTF,and enhance nerve regeneration and functional recovery(Cao et al.,2011).In another study,controlled delivery of a combination of growth factors (brain-derived neurotrophic factor (BDNF) and CNTF) also showed improved nerve regeneration and functional recovery in a 4 mm gap rat facial nerve model (Cao et al.,2013).
Other groups used nerve conduits as vehicles for the delivery of growth factors in larger gap models.Nerve growth factor,BDNF,and insulin-like growth factor (IGF) encapsulation into gelatin-based nerve guidance conduits showed promising potential for neurite guidance and extension,as well as functional recovery in the 1 cm sciatic nerve gap model in rats (Chen et al.,2006).Supplementation of multi-luminal conduits with glial-derived neurotrophic factor (GDNF) and pleiotrophin also had a beneficial,but modest effect (with only 37% more axons than the positive control,and partial reinnervation of the anterior tibialis muscle) on inducing axon outgrowth and target reinnervation in a 4 cm critical nerve gap injury model in rabbits (Alsmadi et al.,2018).
Engineering biomaterials with conductive properties to both stimulate and guide regeneration
As mentioned above,many tissue engineering strategies involved reproducing the niche,or microenvironment of the native nerve (architecture,protein composition,and growth factors).Another attribute of the native nerve is the inherent electrical excitability of neurons.There are manyin vitroandin vivostudies showing electrical stimulation increases neurite extension and axonal regeneration (Gordon et al.,2008;Vivó et al.,2008;Wood and Willits,2009).Conductive polymers are already being used in fuel cells,computer displays,and microsurgical tools,and are now finding applications in the field of regenerative medicine.These versatile polymers can be synthesized alone,as hydrogels,combined into composites,or electrospun into microfibers.They can be designed to be biocompatible and biodegradable.Their conductive nature allows cells or tissue cultured on them to be stimulated through the application of an electrical signal.Hence,some research groups focused on engineering electrically conductive biomaterial scaffolds to stimulate nerve regeneration (Figure 1C).Polypyrrole (PPy) is a conductive polymer that satisfies many of the desired criteria for a nerve guidance channel material(biocompatibility,biodegradability,electrical activity,porosity,controllable release of growth factors,and ease of fabrication) (Lee et al.,2009a,b,2012;Schneeberger et al.,2009).In vitroexperiments by Song et al.(2006) and Gomez and Schmidt (2007) utilizing either PPy combined with nerve growth factor and poly-L-lysine/laminin or PPy-PLGA scaffolds conjugated with nerve growth factor demonstrated increased neurite extension and an increased number of neurite-expressing cells (by three times) with electrical stimulation.More interestingly,poly(caprolactone fumarate) (PCLF)-PPy scaffolds were capable of promoting aligned neurite extension in the direction of the applied electrical current (Moroder et al.,2011).In vivoexperiments by Huang et al.(2012) using biodegradable PPy-chitosan scaffolds in a 15-mm sciatic nerve defect showed significantly increased compound muscle action potential(CMAP) amplitude (14.2 ± 0.83 mVvs.12.4 ± 0.79 mV at 8 weeks;P< 0.05),nerve conduction velocity (18.3 ± 0.58 m/svs.15.2 ± 0.65 m/s at 8 weeks;P<0.001),markers of regeneration (S100,P0,P3,and BDNF-2.02 fold,2.31 fold,1.73 fold,and 1.56 fold,respectively),and the number of the regenerated motor and sensory axons (1.16 ± 0.08 mm2vs.1.00 ± 0.07 mm2at 8 weeks;P< 0.05) with electrical stimulation (Huang et al.,2012).
It is worth mentioning a study by Pooshidani et al.(2021) showing that,besides conductivity,interconnected porosity (achieved using a triple-nozzle electrospinning device and sacrificial fiber method) is another required feature for a scaffold to be effective in nerve regeneration.
Bioengineering Strategies to Improve Outcomes Following Direct Epineural Nerve Repair
Minimizing scarring at the repair site
It is well known that axonal growth is limited by the amount of scar tissue that forms at the nerve repair site (Pan et al.,2003;Siemionow and Brzezicki,2009).Scar tissue acts as a mechanical barrier that not only prevents axons from reaching their target but that also impedes their growth rate (Sunderland,1987;Pham et al.,2006;Ozay et al.,2007;Kaplan et al.,2014).Advanced microsurgical techniques aiming at decreasing scarring at the repair site (e.g.,grouped fascicular repair) were in vain.The intraneural dissection performed during these techniques resulted in more tissue trauma and more scarring.Functional outcomes were not superior when compared to simple epineurial repair (Lundborg,2000).
In this regard,modalities that decrease scarring at the repair site are needed,as they carry the potential to increase the number of axons crossing the repair site and hence improve muscle reinnervation.Such modalities will have to address the complex “scarring” phenomenon,which involves an interplay of numerous cell types.One way to regulate this interplay is by providing specific biochemical cues to the key cells directing the inflammatory cascade.The purpose of such cues is to stimulate the inflammatory cells to secrete factors that create a pro-regenerative environment (instead of secreting factors that promote scarring).Many cell types are involved in the nerve injury healing cascade.Macrophages play a central role in tissue repair and remodeling.They secrete cytokines that directly affect the tissue response to injury (Fujiwara and Kobayashi,2005).Some biomaterials can be designed to provide specific micro-environmental signals that polarize the invading macrophages into a regenerative phenotype (Adutler-Lieber et al.,2014).In a previous study,we took advantage of this feature and applied it to nerve regeneration.We created a nanofiber nerve wrap made of nonwoven electrospun PCL fibers with specific design principles (Fiber diameter 1.1 ± 0.5 μm and pore size 6 ± 2 μm,both post-heat treatment) to modify the phenotype of macrophages.We showed that when applied to the coaptation site in a sciatic nerve cut and repair model in rats,the wrap modulated the immune response by polarizing the invading macrophages into a pro-regenerative anti-inflammatory M2 phenotype (Figure 2A).Those M2 macrophages secreted anti-inflammatory factors that altered the cytokine milieu,resulting in decreased scarring at the nerve repair site (Figure 2B-a–d),enhanced axonal regeneration and myelination (Figure 2B-eandf),and decreased target muscle atrophy (Figure 2B-gandh) (Sarhane et al.,2019a).Similarly,chitosan,a widely used biomaterial for peripheral nerve regeneration,was shown by other groups to support axonal growth (Haastert-Talini et al.,2013;Stenberg et al.,2016) and reduce scar tissue formation(Marcol et al.,2011;Neubrech et al.,2018).Chitosan is biodegradable and its degradation product (chito-oligosaccharides) also has a positive effect on nerve regeneration (Gong et al.,2009;Zhao et al.,2017).Zhao et al.(2017)tested nerve regeneration inside a freeze-cast,double-layered chitosan tube,and observed that the conduit porosity allowed good angiogenesis and prevented scar formation.
Therefore,biomaterials designed to provide specific structural cues to modulate the nerve healing cascade towards a favorable immune response may restore the physiological function of regenerating tissues.A recent study suggested that the potential of those biomaterials can be further substantiated when combined with immunomodulatory cell-based therapies,more specifically with adipose-derived stem cells.This combination could further decrease scar tissue formation (by almost 50%,as measured by the percent of collagen-positive surface area with a reference standard of 22%),enhance axonal regeneration,and stimulate remyelination (Di Summa et al.,2018).Combining cell-based therapy with biomaterials is a therapeutic strategy that is yet to be explored.This strategy can be further refined with the use of computer-based analytics that maps the scar tissue evolution over time to aid in the design of immunomodulatory biomaterials (Sergi et al.,2020).
Improving the regenerative environment in the distal stump
After proximal nerve injuries (i.e.when axons have to grow over long distances) or when surgical repair is delayed,full functional recovery is seldom achieved (Brushart,2011).This is attributed to states of chronic axotomy in which axons lose their ability to regenerate in the absence of target innervation,and more importantly to states of chronic denervation,in which SC in the distal nerve senesce and can no longer sustain regeneration (Fu and Gordon,1995a,b).There are multiple etiologies for this deficit in axonal regeneration.These include a decrease in growth-promoting molecules such as GDNF and BDNF (Höke,2005).This lack in endogenous growth factors is further compounded by an increase in growth-inhibiting molecules such as chondroitin sulfate proteoglycans in the distal stump where axons have to re-grow.chondroitin sulfate proteoglycans constitute a group of inhibitory proteins that are upregulated after nerve injury and that suppress axonal growth (Muir,2010).
In this regard,supplementation of the chronically denervated distal stump with exogenous growth factors is a versatile therapeutic strategy to restore its growth-supportive potential and facilitate axonal growth.Sulaiman’s group used a chronic denervation and axotomy rat model and demonstrated that transforming growth factor-β1 alone and with forskolin reversed the deleterious effect of chronic SC denervation and reactivated them to support axonal regeneration (Sulaiman and Gordon,2002;Sulaiman and Dreesen,2014;Sulaiman et al.,2018).However,the simple addition of growth factors to the distal stump,although somehow effective in animal models,might not translate to human applications due to the short half-life and rapid degradation of growth factors in bodily fluids (growth factors are small peptides that get denatured and digested when in contact with human enzymes).To overcome these shortcomings,our group incorporated growth factors into a biodegradable fibrin-based inert biomaterial for a sustained local delivery (Figure 3A,andB).This system was first optimizedin vitroand then testedin vivoin a chronic denervation animal model.We showed that it provided the SCs in the distal stump with a constant amount of GDNF (over 4 days) resulting in their re-activation (Figure 3C-ia).It also supplemented the distal stump with chondroitinase resulting in the degradation of the inhibitory chondroitin sulfate proteoglycans (Figure 3C-ib).Both the number of regenerating sensory and motor neurons (Figure 3C-ii,iii),and the degree of axonal myelination were improved (Figure 3C-iv),by 5.8 and 3.7 times respectively.Our sacrifice endpoint was however not long enough for the regenerating axons to reach their target muscle to assess functional outcomes(Sarhane et al.,2019b).A modality that releases growth factors for a more prolonged period would be more clinically attractive.
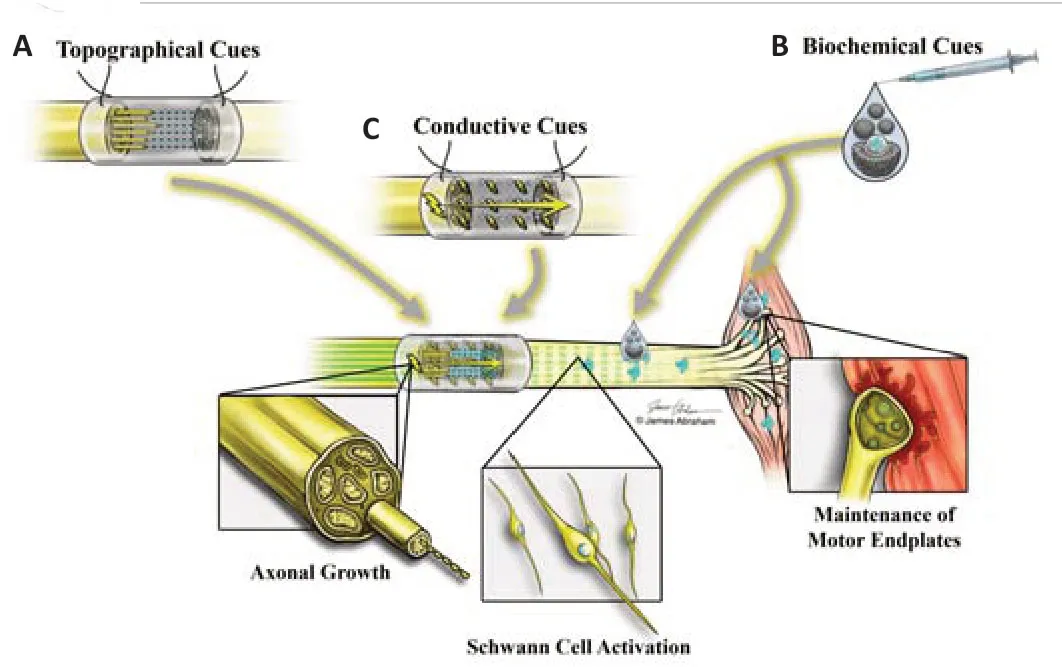
Figure 1|Engineering biomaterials with specific regenerative cues.
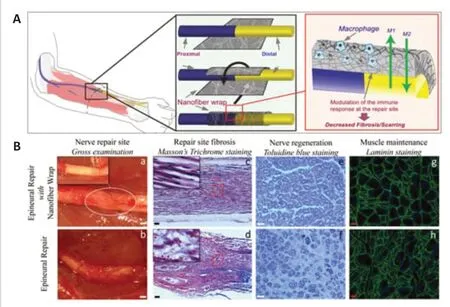
Figure 2|Minimizing scarring at the repair site.
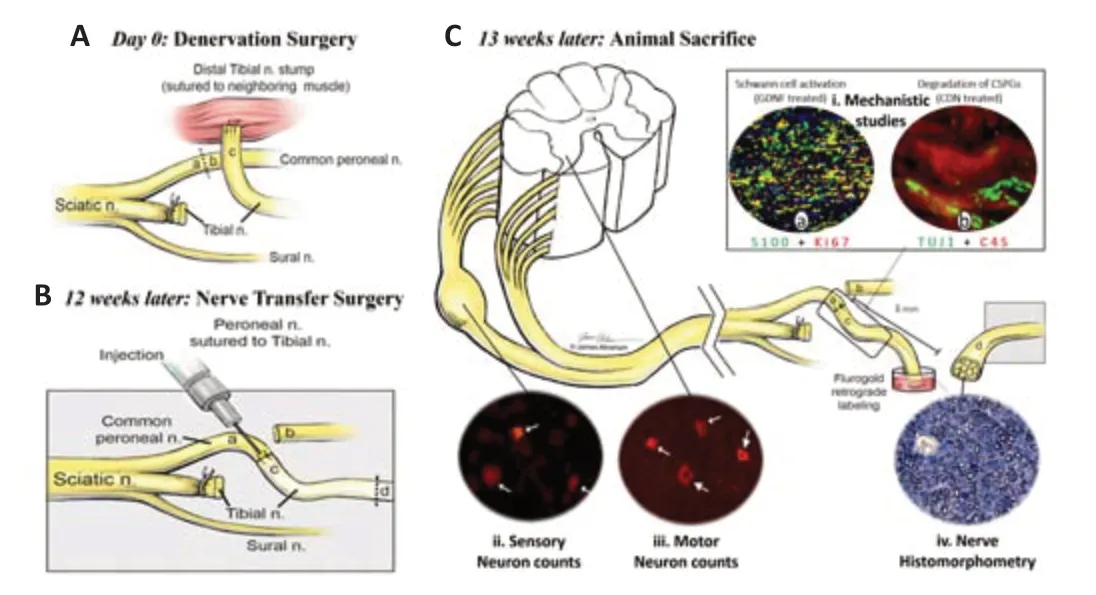
Figure 3|Improving the regenerative environment in the distal stump.
Novel Bioengineering Concepts to Promote Functional Recovery Using a Multimodal Growth Factors-Based Strategy
Most of the previous research efforts aiming at improving functional outcomes following nerve repair have focused on re-activating and enhancing the survival of SCs,and on promoting axonal regrowth and remyelination.The utilization of exogenous growth factors has been the mainstay application to achieve this goal (as the endogenous factors that are upregulated following nerve injury do not satisfy the demand for axonal myelination and nerve outgrowth (Höke et al.,2006)).Engineering biomaterials that incorporate multiple growth factors proved to be the most promising strategy for a controlled delivery of those factors and for maintaining their bioactivity at a safe dose.This strategy (although promising) has not yielded any clinical application in humans.It can be further refined with novel concepts: First,by addressing target muscle denervation atrophy (as the absence of innervation during the lengthy period of axonal growth causes irreversible muscle atrophy that limits its potential for motor recovery);and second,by optimizing an advanced drug delivery system that provides a prolonged and sustained release of growth factors (to both muscle and axons) throughout the entire period of nerve regeneration.These two concepts can be combined to enhance regenerative outcomes.
In 2016,the senior author began investigating the role of systemic growth hormone (GH) therapy on axonal regeneration,SC and muscle maintenance,and end-organ reinnervation in rats.A sciatic nerve transection and repair model was first used.GH therapy not only accelerated axonal regeneration(13,876 ± 2036vs.8645 ± 3279;P=0.0018),but also reduced muscle atrophy(as measured by myofibril cross-sectional area,731.8 ± 157 μmvs.545.2± 144.3 μm;P=0.027),and promoted muscle reinnervation (75.8 ± 8.7vs.38.2 ± 22.6;P=0.0008) (Tuffaha et al.,2016a).Based on those promising histological results on both nerve and muscle,additional investigations using the more stringent chronic denervation model were performed in 2019 (Lopez et al.,2019).Enhanced nerve regeneration (7627 ± 1389vs.3348 ± 283.6;P=0.0046) and motor endplates re-innervation (38.0 ± 3.210%vs.27.9 ±3.230%;P=0.0281) were noted,and a greater recovery of motor function (as measured by grip strength testing,1.81 ± 0.29 Nvs.0.97 ± 0.14,P=0.021)was observed.It was thus decided to further pursue this novel double-target therapeutic approach focusing on both denervation-induced SC senescence(to promote axonal growth and remyelination) and denervation-induced muscle atrophy (to facilitate muscle re-innervation).The necessity to also address target muscle atrophy was substantiated by a study published in 2021,where it was shown that the deleterious effects of muscle denervation are more consequential than the effects of SC denervation on functional recovery (Sarhane et al.,2021).Augmenting the growth hormone axis is a solid double-target therapeutic approach to improve outcomes following nerve injury (Tuffaha et al.,2016b).
Despite those promising experimental results,systemic GH therapy has undesirable systemic side effects (due to its systemic absorption) that limit its clinical translation.These include fluid retention,arthralgias,myalgias,insulin resistance,and the theoretical risk of cancer formation (Souza and Collett-Solberg,2011;Reed et al.,2013).Since the effects of the GH axis are predominantly mediated via systemically and locally produced IGF-1,local delivery of IGF-1 can circumvent the side effects of systemic GH therapy while offering the same benefits (Slavin et al.,2021).Several groups have shown that local infusion of IGF-1 enhances axonal regeneration (1.8x,P< 0.05),neuromuscular junction reinnervation (as qualitatively assessed using laser scanning confocal microscopy),and return of a motor function (assessed by gastrocnemius muscle weight ratio,0.46vs.0.31,P< 0.05) following nerve transection-and-repair (Tiangco et al.,2001;Fansa et al.,2002;Apel et al.,2010).These models however relied on subcutaneously implanted pumps to achieve sustained local delivery of IGF-1.Such delivery methods are unlikely to be adopted for human use.Research efforts have thus been directed at engineering a drug delivery system that is not only clinically translatable but one that also provides sustained local delivery of IGF-1 to denervated nerve and muscle for the entire duration of regeneration until reinnervation occurs.
A novel nanoparticle (NP) delivery system that provides a controlled release of bioactive IGF-1 to denervated muscle and nerve tissue for up to 20 daysin vitrowas recently optimized.Biodegradable PEG-b-PCL NPs with encapsulated IGF-1/dextran sulfate polyelectrolyte complexes were formulated using a flash nanoprecipitation method to preserve IGF-1 bioactivity and maximize encapsulation efficiencies.The encapsulation process protected IGF-1 from conformational changes.The IGF-1 NPs were then loaded into a fibrin gel to facilitate their administration.The efficacy of this system in improving functional recovery was tested in a chronic median nerve denervation model in rats.It was deposited at the nerve coaptation site and also injected into the target muscle at the time of nerve repair.This therapeutic modality resulted in significantly greater recovery of forepaw grip strength (2.1 Nvs.1.3 N,P< 0.05),decreased denervation-induced muscle atrophy (1.9x,P<0.05),decreased SC senescence (86.5 ± 2.8%vs.50.0 ± 3.7%,P< 0.05),and improved neuromuscular reinnervation (40.9 ± 5%vs.26.9 ± 5%,P< 0.05)(Hanwright et al.,2022).
Remaining Challenges
It is important to consider the dosing frequency when designing clinical solutions.A dosing schedule requiring bi-monthly injection (cite the paper)is less favorable.A carrier for the IGF-1 NPs that localizes them next to the nerve and into the muscle for the entire period of IGF-1 release is preferred.The ideal characteristics of such a carrier include tissue compatibility,limited immunogenicity,adjustable viscosity (for muscle and nerve use),and tunable drug release kinetics.In addition,it needs to be engineered with specific topographical cues that actively modulate the tissue response to injury,and not just behave as a passive drug carrier (Sarhane et al.,2020).
Another major challenge is the regeneration across gaps greater than 30 mm.This is of particular clinical importance for patients suffering proximal nerve injuries.In those cases,in addition to the structural,biochemical,and electrical cues,the design of biomaterial systems will need to address the spatial and temporal release of growth factors in response to the speed of regeneration (to avoid axonal trapping).Engineering biomaterials that allow spatiotemporal modulation of biochemical cues will be essential when addressing long-range regeneration.In those challenging cases,the amount of growth factors that is initially delivered to the repair site will have to vary as regeneration progresses and the growth cone advances.
Conclusion
Growth factors play a central role in achieving those goals.The challenge remains in the design of the ideal clinically translatable drug delivery system.Biomaterials that mimic the three-dimensional structure of the target tissue(nerve and muscle) have shown great improvement over unstructured,commercially available drug carriers.That is because such architecture confers biocompatibility and provides a microenvironment similar to the native one(incorporating surface topography cues,biochemical signals,and electrically active properties) and that is favorable for regeneration.However,to achieve clinical translation,an engineered drug should also provide a controlled release of the drug during the entire nerve regeneration process,until axons reach their target muscle and functional reinnervation occurs.
Acknowledgments:The authors would like to thank Mr.James Abraham from Art as Applied to Medicine,Johns Hopkins University,MA,USA for designing the illustrations in Figures 1 and 3.
Author contributions:Writing of the initial draft: KAS;articles search and analysis: KAS,CQ,TGWH,PJH,HQM,SHT;editing and approval of the final draft: KAS,CQ,TGWH,PJH,HQM,and SHT;guidance and supervision: SHT.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neuro faces of beneficial T cells: essential in brain,impaired in aging and neurological diseases,and activated functionally by neurotransmitters and neuropeptides
- Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases:a systematic review
- Cdk5 and aberrant cell cycle activation at the core of neurodegeneration
- Recent advancements in noninvasive brain modulation for individuals with autism spectrum disorder
- Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration
- Cell-based therapeutic strategies for treatment of spinocerebellar ataxias: an update