Precautions before starting tofacitinib in persons with rheumatoid arthritis
Raktim Swarnakar, Shiv Lal Yadav
Raktim Swarnakar, Shiv Lal Yadav, Department of Physical Medicine and Rehabilitation, All India Institute of Medical Sciences, New Delhi, New Delhi 11049, Delhi, India
Abstract Tofacitinib is an immunosuppressive and disease-modifying therapy in rheumatoid arthritis. It may result in many infections flaring up. It is important to take precautions of all kinds (cardiovascular, malignancy, infections etc.) before starting tofacitinib. In this article, we have highlighted important steps where we need to take precautions before starting tofacitinib.
Key Words: Tofacitinib; Rheumatoid arthritis; DMARDs; Disease-modifying; Precaution;Side-effects
TO THE EDITOR
?
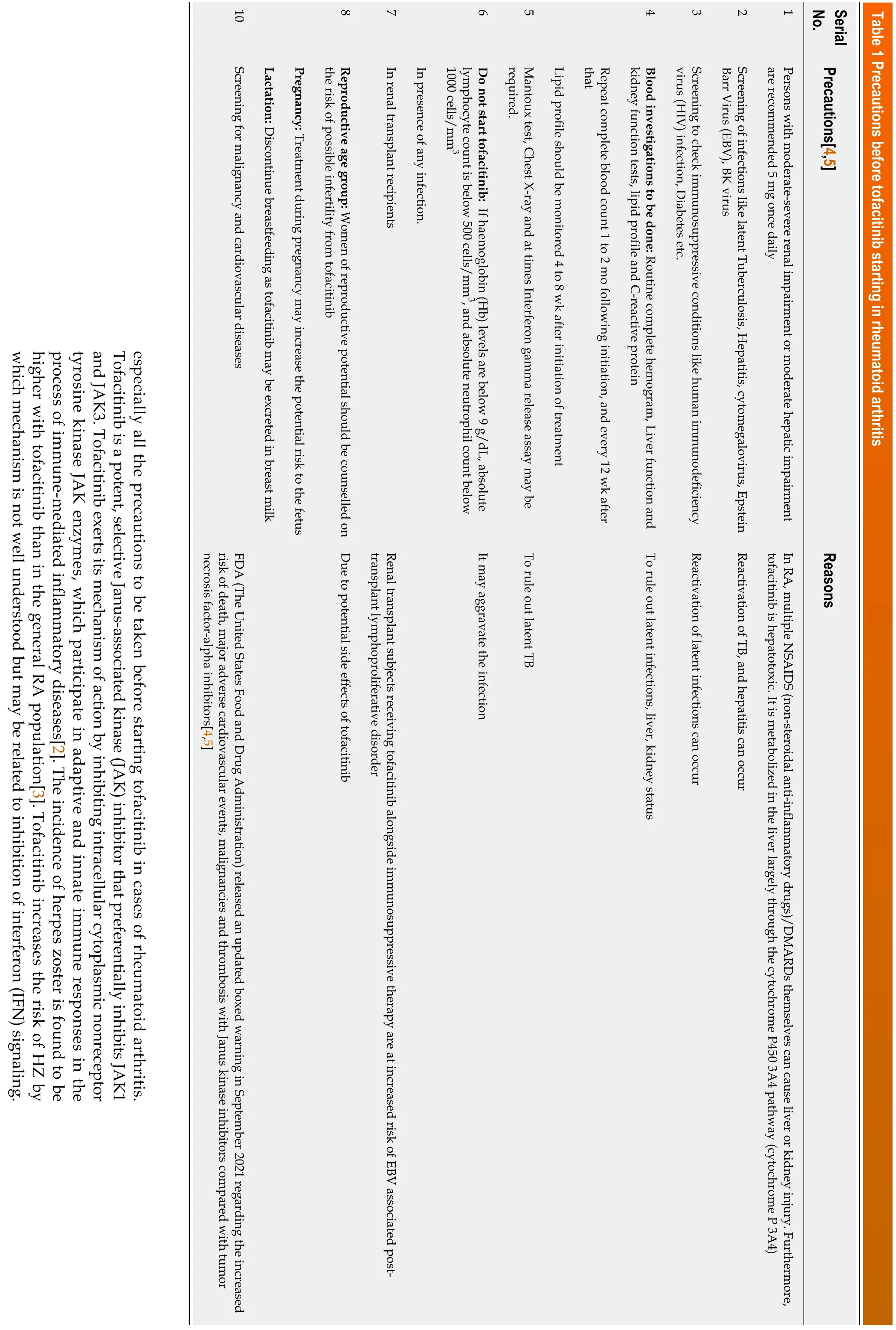
We read with interest the article by Linet al[1] where authors have reported one case report of recurrent herpes zoster (HZ) in rheumatoid arthritis (RA) patients treated with tofacitinib. We would like to highlight important aspects regarding tofacitinib, Antiviral defenses depend on type I and II IFN signalingviathe JAK/STAT pathway and it is inhibited by tofacitinib. Tofacitinib is United States Food and Drug Administration (FDA) approved drug for RA. Oral tofacitinib 5 mg twice daily is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant of, one or more disease-modifying antirheumatic drugs (DMARDs). It can also be used in sequence with first-line therapy methotrexate or conventional DMARDS or can also be used as monotherapy for RA. Detailed precautions are listed in Table 1.
Screening for malignancy and cardiovascular diseases
FDA released an updated boxed warning in September 2021 regarding the increased risk of death, major adverse cardiovascular events, malignancies and thrombosis with JAK inhibitors compared with tumor necrosis factor inhibitors[4,5]. Hence, before starting tofacitinib in a case of rheumatoid arthritis a doctor has to keep in mind those precautionary measures to avoid untoward adverse reactions or incidents.
FOOTNOTES
Author contributions:Swarnakar R and Yadav SL contributed to conception and design; Swarnakar R and Yadav SL contributed to literature search and writing.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:Raktim Swarnakar 0000-0002-7221-2825.
S-Editor:Xing YX
L-Editor:A
P-Editor:Xing YX
 World Journal of Clinical Cases2022年36期
World Journal of Clinical Cases2022年36期
- World Journal of Clinical Cases的其它文章
- Liver injury in COVID-19: Holds ferritinophagy-mediated ferroptosis accountable
- Amebic liver abscess by Entamoeba histolytica
- Living with liver disease in the era of COVID-19-the impact of the epidemic and the threat to high-risk populations
- Cortical bone trajectory screws in the treatment of lumbar degenerative disc disease in patients with osteoporosis
- Probiotics for preventing gestational diabetes in overweight or obese pregnant women: A review
- Effectiveness of microwave endometrial ablation combined with hysteroscopic transcervical resection in treating submucous uterine myomas
