Effectiveness of microwave endometrial ablation combined with hysteroscopic transcervical resection in treating submucous uterine myomas
Toshiyuki Kakinuma, Kaoru Kakinuma, Ayano Shimizu, Ayaka Kaneko, Masataka Kagimoto, Takafumi Okusa,Eri Suizu, Koyomi Saito, Yoshio Matsuda, Kaoru Yanagida, Nobuhiro Takeshima, Michitaka Ohwada
Toshiyuki Kakinuma, Kaoru Kakinuma, Ayano Shimizu, Ayaka Kaneko, Masataka Kagimoto,Takafumi Okusa, Eri Suizu, Koyomi Saito, Yoshio Matsuda, Kaoru Yanagida, Nobuhiro Takeshima,Michitaka Ohwada, Department of Obstetrics and Gynecology, International University of Health and Welfare Hospital, Nasushiobara 329-2763, Japan
Abstract BACKGROUND Hypermenorrhea is characterized by excessive menstrual bleeding that causes severe anemia and interferes with everyday life. This condition can restrict women’s social activities and decrease their quality of life. Microwave endometrial ablation (MEA) using a 2.45-GHz energy source is a minimally invasive alternative to conventional hysterectomy for treating hypermenorrhea that is resistant to conservative treatment, triggered by systemic disease or medications, or caused by uterine myomas and fibrosis. The popularity of MEA has increased worldwide. Although MEA can safely and effectively treat submucous myomas, some patients may still experience recurrent hypermenorrhea postoperatively and may require additional treatment.AIM To investigate the efficacy of MEA combined with transcervical resection (TCR).METHODS Participants underwent cervical and endometrial evaluations. Magnetic resonance imaging and hysteroscopy were performed to evaluate the size and location of the myomas. TCR was performed before MEA using a hystero-resectoscope. MEA was performed using transabdominal ultrasound. The variables included operation time, number of ablation cycles, length of hospital stay, and visual analog scale cores for hypermenorrhea, dysmenorrhea, and treatment satisfaction at 3 and 6 mo postoperatively. The postoperative incidence of amenorrhea, changes in hemoglobin concentrations, and MEA-related complications were evaluated.RESULTS A total of 34 women underwent a combination of MEA and TCR during the study period. Two patients were excluded from the study as their histopathological tests identified uterine malignancies (uterine sarcoma and endometrial cancer). The 32 eligible women (6 nulliparous, 26 multiparous) had a mean age of 45.2 ± 4.3 years (range: 36–52 years). Patients reported very severe hypermenorrhea (10/10 points on the visual analog scale) before the procedure. However, after the procedure, the hypermenorrhea scores decreased to 1.2 ± 1.3 and 0.9 ± 1.3 at 3 and 6 mo, respectively (P < 0.001). The mean follow-up duration was 33.8 ± 16.8 mo. Although 10 women (31.3%) developed amenorrhea during this period, none experienced a recurrence of hypermenorrhea. No surgical complications were observed.CONCLUSION Reducing the size of uterine myomas by combining MEA and TCR can safely and effectively treat hypermenorrhea in patients with submucous myomas.
Key Words: Dysmenorrhea; Endometrial ablation techniques; Menorrhagia; Microwaves; Myoma; Uterus
INTRODUCTION
Hypermenorrhea is characterized by excessive menstrual bleeding that can cause severe anemia and interfere with everyday life. This condition can restrict women’s social activities and decrease their quality of life. Microwave endometrial ablation (MEA) using a 2.45-GHz energy source is a minimally invasive alternative to conventional hysterectomy for treating hypermenorrhea, which is resistant to conservative treatment, triggered by systemic disease or medications, or caused by uterine myomas and fibrosis[1,2]. The popularity of MEA continues to increase worldwide, including that in Japan where the national health insurance program has covered it since April 2012. This procedure has been performed at the International School of Medicine and Welfare Hospital since January 2016, and a previous report has demonstrated that it is reliable and effective in treating hypermenorrhea-induced anemia[3]. Although MEA can safely and effectively treat submucous myomas, some patients may still experience recurrent hypermenorrhea postoperatively and may require additional treatment[4]. Therefore, this study evaluated whether a combination of MEA and transcervical resection (TCR) was effective in treating submucous uterine myomas by reducing the size of uterine myomas in patients with hypermenorrhea, thereby decreasing the possibility of recurrence and additional treatment.
MATERIALS AND METHODS
Study design
The retrospective study protocol was approved by the Ethics Committee of the International University of Health and Welfare Hospital (20-B-399, date: May 7, 2020). All patients provided written informed consent prior to the procedure. Patients were considered eligible if they underwent MEA in addition to TCR for submucous uterine myomas after a primary complaint of hypermenorrhea between January 2016 and June 2020. All patients were followed up for ≥6 mo, and their medical records were reviewed to evaluate their outcomes.
Procedures
All participants underwent cervical and endometrial cytological evaluations as well as histological evaluations, if necessary, to exclude cases of uterine malignancies. In addition, magnetic resonance imaging and hysteroscopy were performed to evaluate the size and location of the myomas, as well as the degree of protrusion into the uterine cavity. The procedure was initiated under general anesthesia in the lithotomy position. The TCR procedure was performed before MEA using a hystero-resectoscope (26-Fr outer sheath; Olympus Corp., Tokyo, Japan), and D-sorbitol (3%) was used as the irrigation solution. Resected tissue specimens undaerwent histopathological examinations in all cases. Therefore, cervical dilatation was performed on the day before surgery using Lamicel (Japan Medtronic Co. Ltd., Tokyo, Japan). MEA was then performed under transabdominal ultrasound guidance using the Microtaze AFM-712 unit (Alfresa Pharma Corp., Osaka, Japan) and a Sounding Applicator (CSA-40CBL-1006200C; Alfresa Pharma Corp., Osaka, Japan) (Figure 1). According to the guidelines, the 2.45 GHz microwave Microtaze device was set to an output power of 70 W for 50-s cycles[5]. Before terminating the MEA, the uterine cavity was evaluated using a hysteroscope to confirm that ablative necrosis at the endometrium did not extend to the internal orifice or endocervix.
Study variables
The variables included operation time, number of ablation cycles, length of hospital stay, and visual analog scale (VAS) scores for hypermenorrhea, dysmenorrhea, and treatment satisfaction at 3 and 6 mo postoperatively. In addition, we evaluated the postoperative incidence of amenorrhea, changes in hemoglobin (Hb) concentrations, and MEA-related complications.
Statistical analysis
Numerical data were reported as mean ± standard deviation (range). All statistical analyses were performed using JMP®software, version 14.2 (SAS Institute Japan Co. Ltd, Tokyo, Japan). Statistical analysis consisted of single-factor ANOVA and multiple parametric comparisons. APvalue < 0.05 was considered statistically significant.
RESULTS
A total of 34 women underwent a combination of MEA and TCR during the study period. Two patients were excluded from the study as their histopathological tests identified uterine malignancies (uterine sarcoma and endometrial cancer). Patients who wish to have children were excluded from this treatment since MEA completely destroys the endometrium’s functional layers. The patients’ characteristics are shown in Table 1. The 32 eligible women (6 nulliparous, 26 multiparous) had a mean age of 45.2 ± 4.3 years (range: 36–52 years). The cases included 10 cases of single myoma (only submucosal fibroids) and 22 cases of multiple myomas (submucosal and intramuscular fibroids) with a mean major axis diameter of 26.3 ± 12.3 mm and a mean protrusion degree of 51.3% ± 11.3%. According to the International Federation of Gynecology and Obstetrics classification of submucosal fibroids, 6 cases presented with Type 1 and 26 cases presented with type 2 fibroids.
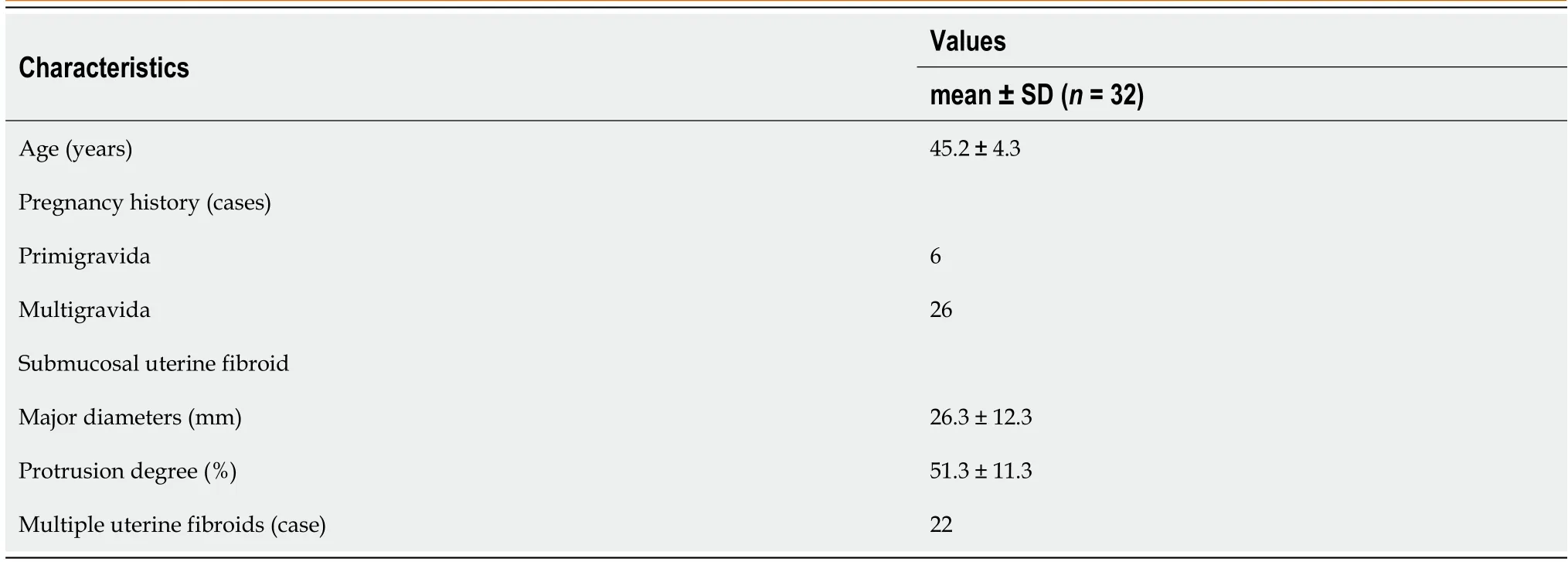
Table 1 Patient characteristics
The mean procedure duration was 45.5 ± 21.0 min (range: 19–110 min) and involved 7.0 ± 1.3 ablation cycles (range: 5–11 cycles). The mean postoperative hospital stay was 2.5 ± 0.5 d (range: 2–3 d). The patients reported having very severe hypermenorrhea and dysmenorrhea at 10 mo before the procedure, based on average VAS scores of 10/10 for both items. However, the hypermenorrhea scores significantly decreased to 1.2 ± 1.3 (range: 0–5) at 3 mo postoperatively and 0.9 ± 1.3 (range: 0–5) at 6 mo postoperatively (bothP< 0.001) (Figure 2A). Similarly, the dysmenorrhea scores significantly decreased to 1.3 ± 1.8 (range: 0–7) after 3 mo and 1.3 ± 1.8 (range 0–5) after 6 mo (bothP< 0.001) (Figure 2B). Figure 3 shows that the circulating Hb concentrations improved significantly from 8.7 ± 1.9 g/dL (range: 5.1–12.5 g/dL) preoperatively to 13.5 ± 1.1 g/dL (range: 11.3–15.2 g/dL) postoperatively (P< 0.001). No surgical complications were observed. The patients reported being highly satisfied with the procedure’s ability to relieve hypermenorrhea, based on a mean VAS score of 9.5 ± 0.8 (range: 7–10) for satisfaction. The mean follow-up duration was 33.8 ± 16.8 mo (range: 6–60 mo). During follow-up, amenorrhea was reported by 10 patients (31.3%), and there were no instances of hypermenorrhea recurrence.

Figure 1 Microtaze AFM-712 generator and sounding applicator (Alfresa Pharma Co, Osaka, Japan).
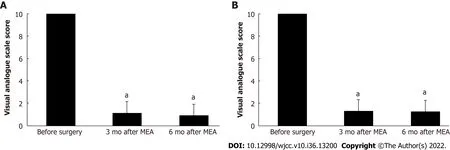
Figure 2 Changes in the visual analog scale score. A: Hypermenorrhea after microwave endometrial ablation. Values are expressed as the mean ±standard deviation (error bars). aP < 0.01 relative to the result before surgery; B: Dysmenorrhea after microwave endometrial ablation. Values are expressed as the mean ± standard deviation (error bars). aP < 0.01 relative to the result before surgery. MEA: Microwave endometrial ablation.
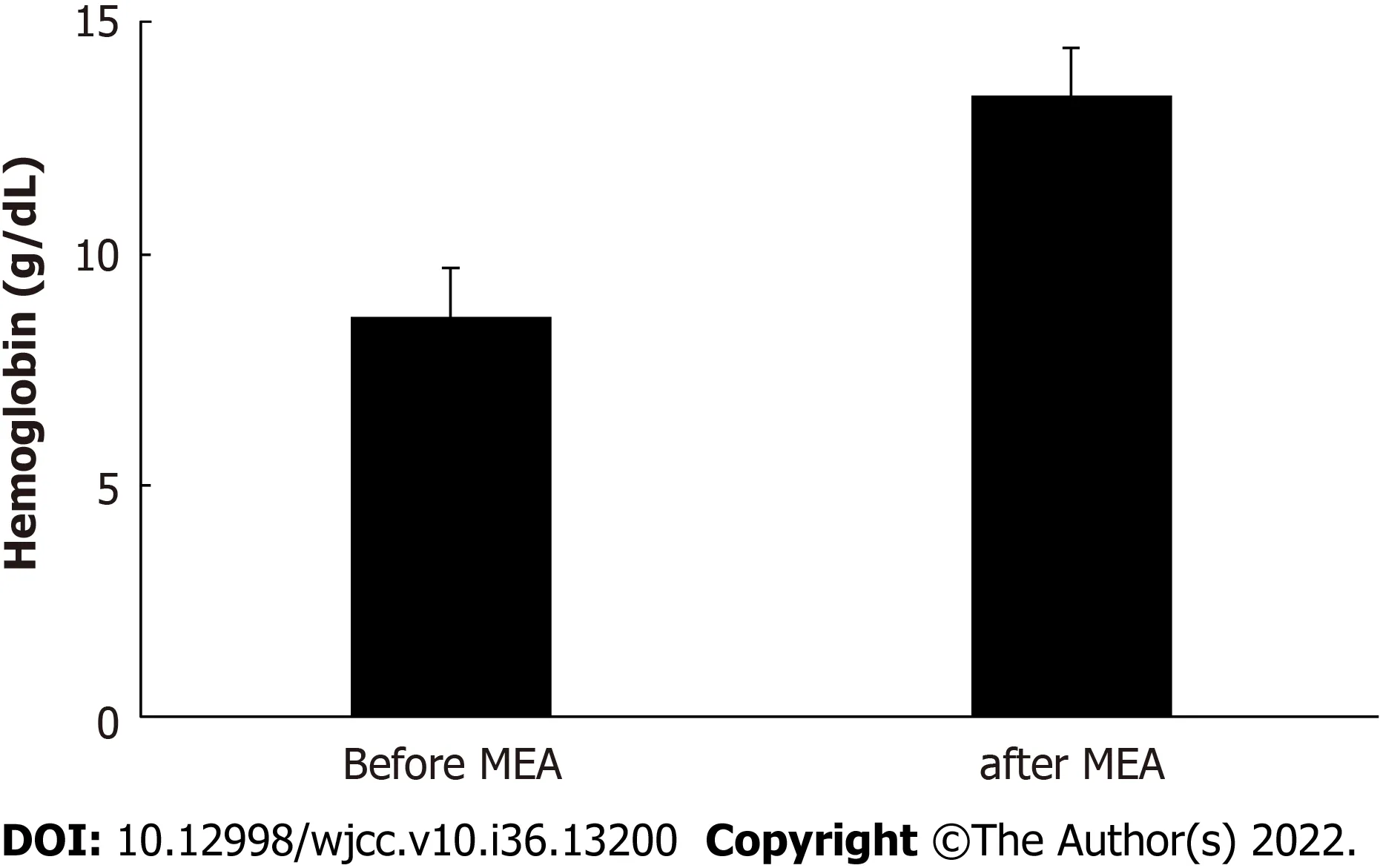
Figure 3 Comparing blood hemoglobin concentrations before and after microwave endometrial ablation. MEA: Microwave endometrial ablation.
DISCUSSION
Previous studies have indicated that MEA is an alternative to hysterectomy for patients requiring treatment for hypermenorrhea caused by submucous myomas[1-3]. This procedure has gained popularity in Japan, where the national health insurance program has covered it since 2012. Although MEA can safely and effectively treat submucous myomas, some patients may experience recurrent hypermenorrhea postoperatively and require additional treatment. This is believed to be caused by endometrial regeneration over time, as the uterine cavity expands because of post-procedural myoma growth. The present study evaluated the efficacy and safety of the combination of MEA and TCR under hysteroscopic guidance for women with submucous myomas. The patients experienced dramatic improvements in their hypermenorrhea symptoms and a significant increase in their circulating Hb concentrations. Furthermore, none of the women experienced a recurrence of hypermenorrhea during the follow-up period. Submucous myomas deform the uterine cavity, complicating the endometrial ablation process, depending on the shape of the microwave applicator. A resectoscope can be used for pre-ablation excision to improve the outcomes of MEA by smoothing the irregular inner surface, although it is important to be aware of potential issues regarding the scope of myoma resection during this step. Problems are unlikely in patients who have a sufficiently thick residual myometrium, even if the submucous myoma is completely removed. However, excessive thinning is possible if complete myoma resection is attempted. Thus, the Japanese clinical practice guidelines indicate that MEA requires a myometrial thickness of ≥ 10 mm to prevent damage to the surrounding organs[5]. Therefore, the scope of the TCR must be limited to partial resection before MEA if there is a risk of excessively thinning the uterine musculature. We reviewed the patients’ TCR procedures and noted that only six patients underwent partial resection, although they experienced no recurrence and reported the same improvements in their clinical symptoms compared with patients who underwent complete resection. The efficacy of the combined procedures can be attributed to the reduction in the fibroid mass and the use of a hysteroscope after MEA to evaluate the uterine cavity and perform cauterization for sites with inadequate ablation.
The levonorgestrel-releasing intrauterine system (LNG-IUS) is another minimally invasive treatment for hypermenorrhea. This system provides promising outcomes, based on a > 50% reduction in menstrual flow (vsthe preoperative volume) experienced by 84.8% of patients and amenorrhea only occurring in approximately 20% of patients[6]. Based on our findings, the MEA procedure appears to be more effective than the LNG-IUS, although a direct comparison is precluded because we only used subjective VAS ratings to evaluate menstrual flow. Nevertheless, from a long-term perspective, the effects of MEA may be more stable, as only 21% of patients who undergo MEA require another treatment for recurrent hypermenorrhea, while re-treatment is required for 42% of patients who receive the LNG-IUS[6,7]. Furthermore, the LNG-IUS could potentially slip out of place in a uterine cavity that has been deformed by the presence of submucous myomas. Therefore, we suggest that the combination of MEA and TCR is a more effective treatment option for hypermenorrhea caused by submucous uterine myomas. The concurrent use of a resectoscope with MEA permits a histopathological characterization of the lesion, which is not possible if MEA is performed alone. Furthermore, we have previously reported that abnormal findings were observed in endometrial specimens collectedviatotal endometrial curettage during MEA, despite these patients having initially negative results for uterine malignancy from cytodiagnostic and histological screening before MEA[8]. The present study also identified two patients with uterine malignancy, which was confirmed based on the histopathological findings from specimens that were excised during the combination of TCR and MEA, despite the preoperative diagnosis being hypermenorrhea caused by submucous uterine myoma. Similarly, histopathological examinations of the TCR specimens can be helpful in identifying uterine malignancies.
It is important to consider the safety of MEA, as complications include heat injury to the pelvic organs, fluid retention in the uterus (because of cervical stenosis or the ablated endometrium), hematometra, pelvic inflammation (e.g., endometritis because of ascending infection), and pyometra[9]. However, we did not detect any of these complications, which may be related to resectoscope usage to evaluate the endometrial lining immediately after MEA. This step allowed for the maximum removal of the necrotic tissue leftover by ablation, and we believe that it can improve the safety of MEA by reducing the rate of complications. Although MEA is not indicated for treating dysmenorrhea and was not originally expected to alleviate its symptoms, some studies demonstrate its effectiveness in treating this condition[10,11]. Our patients reported improvements in dysmenorrhea as soon as their first menses after MEA. We speculate that this was a secondary effect of improvements in hypermenorrhea, as reductions in menstrual volume were accompanied by less severe dysmenorrhea. Thus, MEA might be effective for reducing dysmenorrhea that is associated with hypermenorrhea caused by uterine myomas. Our patients were highly satisfied with MEA, based on an average VAS score of 9.5 ± 0.89 out of 10 points. Furthermore, most patients continued menstruating, although amenorrhea was reported by 10 patients (31.3%). Thus, we suspect that women with hypermenorrhea may desire a reduction, but not necessarily complete elimination, of menstrual flow while preserving their uterine function. The safety and efficacy of MEA in combination with TCR may also contribute to patient satisfaction. Further studies are needed to consider various issues related to whether a combination of MEA and TCR can effectively treat hypermenorrhea secondary to uterine myomas. For example, hypermenorrhea outcomes should be considered in cases where the fibroids do not protrude into the uterine cavity. Furthermore, long-term follow-up is needed to clarify the hypermenorrhea recurrence rate and time to recurrence. Moreover, screening criteria are needed to identify women who are especially susceptible to hypermenorrhea recurrence. Finally, these studies could also consider novel ablation techniques.
CONCLUSION
The combination of MEA and TCR may safely and effectively reduce the size of uterine myoma and, thus, treat hypermenorrhea caused by submucous myomas.
ARTICLE HIGHLIGHTS
Research background
Hypermenorrhea is characterized by excessive menstrual bleeding that causes severe anemia and interferes with everyday life. This condition can restrict women’s social activities and decrease their quality of life. Microwave endometrial ablation (MEA) using a 2.45-GHz energy source is a minimally invasive alternative to conventional hysterectomy for treating hypermenorrhea that is resistant to conservative treatment, triggered by systemic disease or medications, or caused by uterine myomas and fibrosis. The popularity of MEA has increased worldwide. Although MEA can safely and effectively treat submucous myomas, some patients may still experience recurrent hypermenorrhea postoperatively and may require additional treatment.
Research motivation
Although MEA can safely and effectively treat submucous myomas, some patients may still experience recurrent hypermenorrhea postoperatively and may require additional treatment. This study evaluated whether a combination of MEA and transcervical resection (TCR) was effective in treating submucous uterine myomas by reducing the size of uterine myomas in patients with hypermenorrhea, thereby decreasing the possibility of recurrence and additional treatment.
Research objectives
To investigate the efficacy of MEA combined with TCR.
Research methods
Participants underwent cervical and endometrial evaluations. Magnetic resonance imaging and hysteroscopy were performed to evaluate the size and location of the myomas. TCR was performed before MEA using a hystero-resectoscope. MEA was performed using transabdominal ultrasound. The variables included operation time, number of ablation cycles, length of hospital stay, and visual analog scale cores for hypermenorrhea, dysmenorrhea, and treatment satisfaction at 3 and 6 mo postoperatively. The postoperative incidence of amenorrhea, changes in hemoglobin concentrations, and MEArelated complications were evaluated.
Research results
A total of 34 women underwent a combination of MEA and TCR during the study period. Two patients were excluded from the study as their histopathological tests identified uterine malignancies (uterine sarcoma and endometrial cancer). The 32 eligible women (6 nulliparous, 26 multiparous) had a mean age of 45.2 ± 4.3 years (range: 36–52 years). Patients reported very severe hypermenorrhea (10/10 points on the visual analog scale) before the procedure. However, after the procedure, the hypermenorrhea scores decreased to 1.2 ± 1.3 and 0.9 ± 1.3 at 3 and 6 mo, respectively (P< 0.001). The mean follow-up duration was 33.8 ± 16.8 mo. Although 10 women (31.3%) developed amenorrhea during this period, none experienced a recurrence of hypermenorrhea. No surgical complications were observed.
Research conclusions
Reducing the size of uterine myomas by combining MEA and TCR can safely and effectively treat hypermenorrhea in patients with submucous myomas.
Research perspectives
MEA may be ineffective in menorrhagia with uterine myomas. Combining TCR and endometrial ablation can help reduce the tumor volume, and cauterization can ensure the effectiveness of this procedure. This can facilitate the pathological evaluation of the excised specimen. The therapeutic effect of this method is considered to be a reduction in the number of uterine myomas. Furthermore, patient satisfaction with this surgical procedure is high.
FOOTNOTES
Author contributions:Toshiyuki Kakinuma: methodology, software, validation, and formal analysis, writing-original draft preparation, writing-review and editing, visualization, supervision, and project administration; all authors did investigation, resources, data curation, and have read and agreed to the published version.
Institutional review board statement:The study was reviewed and approved by the Ethics Committee of the International University of Health and Welfare Hospital.
Informed consent statement:All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:All the data related to the study have been disclosed in the manuscript. No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Japan
ORCID number:Toshiyuki Kakinuma 0000-0001-7853-4860; Kaoru Kakinuma 0000-0003-4647-9582; Yoshio Matsuda 0000-0002-2890-2802; Kaoru Yanagida 0000-0002-5348-3603; Michitaka Ohwada 0000-0002-0396-6228.
S-Editor:Xing YX
L-Editor:A
P-Editor:Xing YX
 World Journal of Clinical Cases2022年36期
World Journal of Clinical Cases2022年36期
- World Journal of Clinical Cases的其它文章
- Liver injury in COVID-19: Holds ferritinophagy-mediated ferroptosis accountable
- Amebic liver abscess by Entamoeba histolytica
- Living with liver disease in the era of COVID-19-the impact of the epidemic and the threat to high-risk populations
- Cortical bone trajectory screws in the treatment of lumbar degenerative disc disease in patients with osteoporosis
- Probiotics for preventing gestational diabetes in overweight or obese pregnant women: A review
- Antibody and complement levels in patients with hypersplenism associated with cirrhotic portal hypertension and therapeutic principles
