Two methods of lung biopsy for histological confirmation of acute fibrinous and organizing pneumonia: A case report
Wen-Juan Liu, Shuang Zhou, Yan-Xia Li
Wen-Juan Liu, Yan-Xia Li, Department of Respiratory and Critical Care Medicine, Institute of Respiratory Diseases, The First Affiliated Hospital of Dalian Medical University, Dalian 116011, Liaoning Province, China
Shuang Zhou, Department of Internal Medicine, Dalian Medical University, Dalian 116044,Liaoning Province, China
Abstract BACKGROUND Acute fibrinous and organizing pneumonia (AFOP) is a rare, noninfective lung disease, histologically characterized by a patchy distribution of intra-alveolar fibrin “balls” and organizing pneumonia. The clinical manifestations of AFOP are nonspecific. Diagnosis depends on pathology. Surgical lung biopsy is optimal for tissue sampling to diagnose AFOP. However, many patients have no tolerance to the operation, including mentally and physically. There is still no standard therapy for AFOP and the methods remain controversial. Therefore, further clinical attention and discussion are warranted.CASE SUMMARY A 53-year-old woman presented with fever, cough and dyspnea for 15 d. Antiinfective therapy was ineffective. Chest computed tomography showed bilateral patchy consolidation, especially in the lower lobes. We performed both ultrasound-guided transbronchial lung biopsy and ultrasound-guided percutaneous fine needle puncture at different lung lesion locations. Both samples supported the diagnosis of AFOP. The patient had a good clinical course after treatment with methylprednisolone, and no side effects of steroids.CONCLUSION Percutaneous needle biopsy combined with transbronchial lung biopsies may be a good choice in the absence of surgical biopsy. Methylprednisolone alone is effective in the treatment of idiopathic AFOP.
Key Words: Acute fibrinous and organizing pneumonia; Fibrin balls; Percutaneous needle biopsy; Transbronchial lung biopsies; Methylprednisolone; Case report
INTRODUCTION
Acute fibrinous and organizing pneumonia (AFOP), first described by Beasleyet al[1] in 2002, is a rare histological form of interstitial pneumonia by the American Thoracic Society and the European Respiratory Society[2]. AFOP has been receiving increasing clinical attention in recent years. AFOP is typically characterized histologically by the presence of intra-alveolar fibrin “balls” and organizing pneumonia in a patchy distribution[1]. The most common symptoms are dyspnea, cough and fever, which often lead to misdiagnosis and delayed diagnosis. According to the literature, most cases were initially misdiagnosed as pneumonia and lung tumors[3,4]. Beasleyet al[1] described a mean time from onset of symptoms to diagnosis of 19 d, and Gomeset al[4] reported a mean time of 43.9 d. A definitive diagnosis of AFOP requires histopathological evaluation[1,2]. Chenet al[3] suggested that surgical biopsy is optimal for tissue sampling to make an AFOP diagnosis. However, many patients showed no tolerance to the operation.
We report a case of a 53-year-old female patient with AFOP, who was diagnosed by pathology of percutaneous and transbronchial lung biopsies and successfully treated with steroid monotherapy. The patient was diagnosed 5 d after hospitalization, and discharged after 11 d of hospitalization.
CASE PRESENTATION
Chief complaints
A 53-year-old woman was referred to the First Affiliated Hospital of Dalian Medical University in April 2022 due to fever with cough and dyspnea for 15 d.
History of present illness
The patient had a fever without obvious precipitating causes for 15 d, and her body temperature was 38.5 °C, with cough, sputum and dyspnea. There was no chest pain, hemoptysis, or night sweats. Chest computed tomography (CT) at the local hospital suggested multiple exudative opacities in both lungs (Figure 1A–C). She received penicillin and macrolide antibiotics as treatment for 9 d. However, she had persistent fever, and her symptoms of dyspnea worsened.
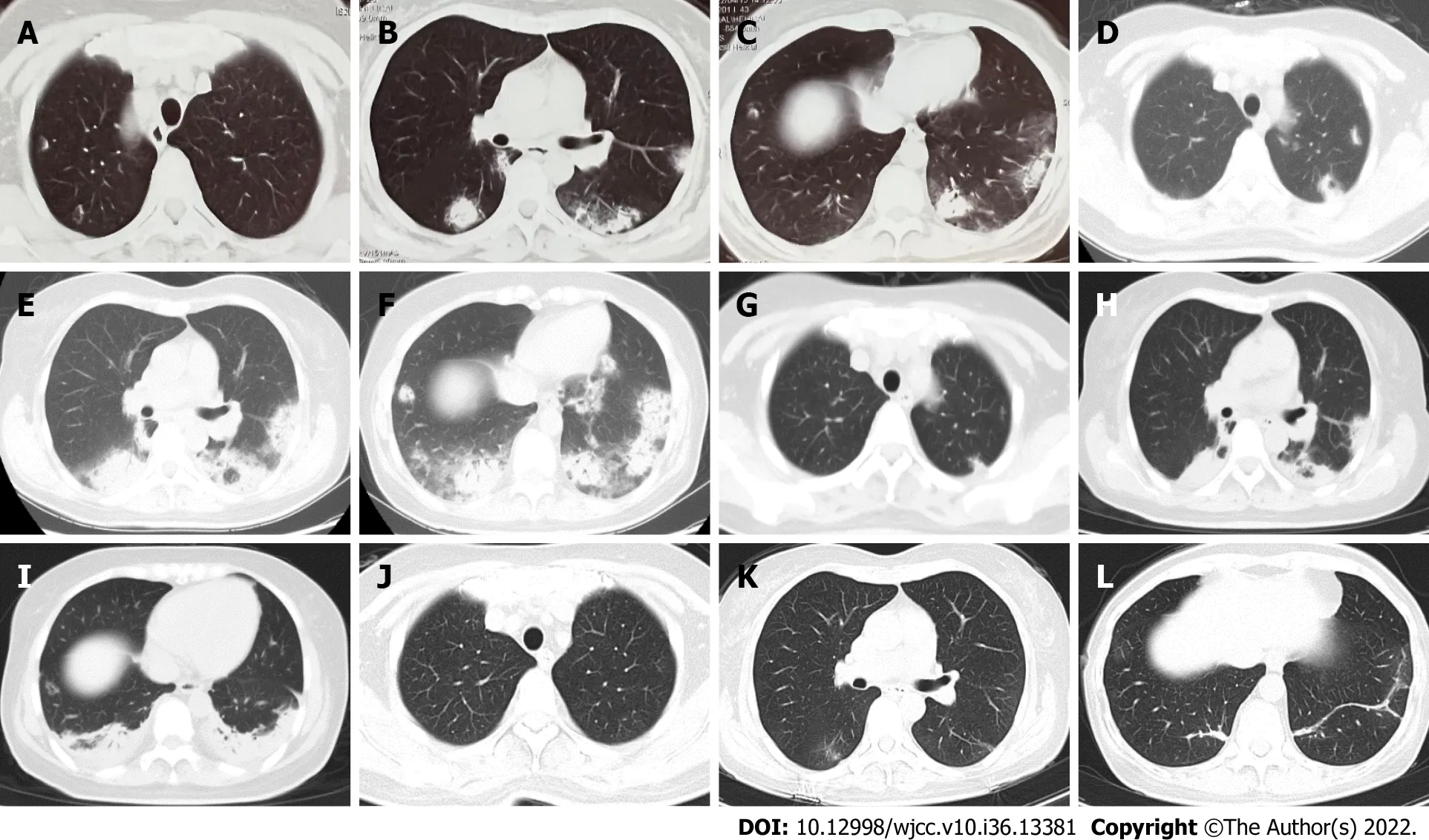
Figure 1 Chest imaging changes. A–C: Chest computed tomography (CT) at the local hospital suggesting multiple exudative opacities in both lungs; D–F:Chest CT on admission revealed patchy, diffuse alveolar opacities and consolidation with bilateral and peripheral distributions after 9 d of anti-infective treatment in the local hospital; G–I: Chest CT shows that the lung opacities have decreased significantly after 3 d of steroid treatment; J–L: Chest CT shows that the lung opacities have almost been entirely absorbed after 1 mo of steroid treatment.
History of past illness
She denied a history of previous illnesses or autoimmune system diseases such as joint swelling and pain, dry mouth and eyes, mouth sores, and rashes.
Personal and family history
She was a nonsmoker. She denied a history of exposure to occupational dust or keeping pets. She had no special drug history. She also denied a family history of lung disease.
Physical examination
On examination the patient was alert, with a temperature of 38.2 ºC, pulse rate 94/min, respiratory rate 18/min, blood pressure 120/70 mmHg, and oxygen saturation with room air 92%. Chest auscultation revealed increased breath sounds with no crackles. The rest of the physical examination was unremarkable.
Laboratory examinations
The patient’s laboratory test results are shown in Table 1.

Table 1 Laboratory examinations
Imaging examinations
High-resolution CT (HRCT) of the thorax on admission revealed patchy, diffuse alveolar opacities and consolidation with bilateral and peripheral distributions, which was significantly more extensive than before (Figure 1D–F).
Further diagnostic work-up
Twodimensional echocardiography and electrocardiography were normal. Blood and alveolar lavage fluid were both examined by next-generation sequencing (NGS), and no bacteria, fungi or viruses were found.
Ultrasound-guided right lung puncture biopsy revealed intra-alveolar fibrin in the form of “fibrin balls” without the formation of hyaline membranes (Figure 2A and B).
Bronchoscopy followed by bronchoalveolar lavage (BAL) was performed. The molecular diagnostic test for tuberculosis was negative; BAL galactomannan was unremarkable. BAL cultures were negative. Transbronchial lung biopsy was performed from the left lower lobe, and the corresponding report revealed AFOP (Figure 2C and D).
FINAL DIAGNOSIS
AFOP.
TREATMENT
Based on the results of the lung biopsy, intravenous methylprednisolone 40 mg twice daily was initiated. The patient’s body temperature returned to normal on the first day of corticosteroid therapy. Her cough and shortness of breath improved significantly. The lung opacities decreased significantly after 3 d of corticosteroid treatment (Figure 1G–I). Then, methylprednisolone was decreased to 40 mg once daily. She was discharged home 3 d later, and continued oral methylprednisolone 40 mg/d for 10 d followed by 20 mg/d for 14 d, 16 mg/d for 14 d, 12 mg/d for 14 d, and 8 mg/d for 1 mo.

Figure 2 Histological findings in the lung. A and B: Ultrasound-guided right lung puncture biopsy reveals intra-alveolar fibrin in the form of “fibrin balls”without the formation of hyaline membranes [Hematoxylin and eosin (HE): 20 ×, HE: 40 ×, respectively]; C and D: Transbronchial lung biopsy of the left lower lobe:fibrin balls are present in the alveolar cavities (HE: 20 ×, HE: 40 ×, respectively).
OUTCOME AND FOLLOW-UP
At follow-up of 1 mo after discharge, chest HRCT showed that most of the lesions had improved in absorption (Figure 1J–L). She is currently being treated with 4 mg methylprednisolone for 2 mo and on regular follow-up. She has no symptoms such as dyspnea, fever or cough, and no side effects of steroids such as obesity, hypertension, hyperglycemia or osteoporosis.
DISCUSSION
In 2002, Beasleyet al[1] described a new histological pattern of lung injury named AFOP. The dominant histological pattern of AFOP is intra-alveolar fibrin deposition and OP without the presence of classical hyaline membranes and eosinophilia, differentiating the disease from diffuse alveolar damage (DAD), OP, and eosinophilic pneumonia[1,2]. Two forms of the disease are described: An acute form with a fulminant course and rapid progression to respiratory failure, and a subacute form with a better outcome[1,3]. AFOP can be idiopathic or associated with a variety of disease conditions, including infections, collagen vascular diseases, adverse drug or chemical reactions, hematological malignancy, altered immune status, inhalation disease, and occupational or environmental exposures[4-10]. Our patient was weakly positive for anti-Smith (Sm) antibody and nuclear ribonucleoprotein/Sm without any symptoms. Possibility of systemic lupus erythematosus should be considered. Therefore, it is necessary to monitor abnormal immune indicators and corresponding symptoms.
The clinical manifestations of AFOP are nonspecific. The most common symptoms are dyspnea, cough and fever. The most common radiological findings are diffuse, patchy opacities with both peripheral and bilateral distributions, and the lesions may be limited to the lung bases[1]. Chenet al[3] suggested that consolidation is manifested more frequently in patients with idiopathic AFOP, while converse ground-glass opacity is seen more frequently in patients with secondary AFOP.
As clinical characteristics associated with this disease are nonspecific, a definitive diagnosis of AFOP requires histopathological evaluation. The methods of lung biopsy often include percutaneous needle biopsy, endobronchial ultrasound-guided transbronchial lung biopsy, and surgical lung biopsy.
Chenet al[3] suggested that surgical biopsy is the best choice for tissue sampling for AFOP diagnosis, as it can minimize the missing areas of the hyaline membrane in DAD. However, our patient showed no tolerance to the operation. To increase the reliability of the biopsy, we performed both ultrasoundguided transbronchial lung biopsy of the posterior basal segment of the left lung and ultrasound-guided percutaneous fine needle puncture of the right lung nodule. Both samples supported the diagnosis of AFOP.
Most patients begin with respiratory symptoms such as fever and cough and often have elevated nonspecific inflammatory indicators such as C-reactive protein and erythrocyte sediment rate, and chest CT suggests a patch in both lungs, hence many AFOP patients are first diagnosed with communityacquired pneumonia (CAP)[4]. This leads to high use of antibiotics. AFOP needs to be differentiated from CAP because the treatment modalities are markedly different. The patient’s blood and BAL fluid were examined with NGS, and no definitive etiological evidence was found, confirming the final diagnosis of AFOP. It is important to exclude infection. Luet al[11] suggested that NGS technology plays an important role in the diagnosis of infectious diseases, and has potential for exclusion of noninfectious diseases.
The most common therapy for AFOP is corticosteroids. Other options include immunosuppressants and antibiotics, depending on the cause of AFOP. Usually 0.5–1 mg/kg/d prednisone (or equivalent) is prescribed initially[12,13]. A maximal dose of methylprednisolone was reported to be up to 1 g/d[8]. Corticosteroids should be reduced after remission, and the total course of treatment should be maintained in small doses for 6–12 mo[12,13]. Relapse is possible during corticosteroid tapering, and symptoms may be reduced when higher corticosteroids doses are resumed[3]. Considering that our patient weighed 75 kg, had no previous hypertension or diabetes, and had a wide range of imaging lesions with rapid progression, we chose 40 mg methylprednisolone twice daily as the initial treatment. After 3 d, due to the improvement of symptoms and imaging, and considering the side effects of steroids, we reduced the dose of methylprednisolone to 40 mg once daily. After 5 mo of gradual reduction, the dose of methylprednisolone was 4 mg/d. The patient has no symptoms such as cough, dyspnea or fever, or side effects of steroids such as obesity, hypertension, hyperglycemia or osteoporosis.
CONCLUSION
Percutaneous needle biopsy combined with transbronchial lung biopsies may be a good choice in the absence of surgical biopsy. Methylprednisolone alone is effective and relatively safe in the treatment of idiopathic AFOP.
FOOTNOTES
Author contributions:Liu WJ contributed to data analysis and wrote the paper; Zhou S contributed to data collection; Li YX contributed to the conception and design of the study; all authors revised the paper and approved the submitted version.
Supported byNatural Science Foundation of Liaoning Province, No. 2021-MS-287.
Informed consent statement:The patient provided informed written consent prior to study enrollment.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Wen-Juan Liu 0000-0002-0846-6580; Shuang Zhou 0000-0002-7048-4405; Yan-Xia Li 0000-0002-1586-5352.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Clinical Cases2022年36期
World Journal of Clinical Cases2022年36期
- World Journal of Clinical Cases的其它文章
- Liver injury in COVID-19: Holds ferritinophagy-mediated ferroptosis accountable
- Amebic liver abscess by Entamoeba histolytica
- Living with liver disease in the era of COVID-19-the impact of the epidemic and the threat to high-risk populations
- Cortical bone trajectory screws in the treatment of lumbar degenerative disc disease in patients with osteoporosis
- Probiotics for preventing gestational diabetes in overweight or obese pregnant women: A review
- Effectiveness of microwave endometrial ablation combined with hysteroscopic transcervical resection in treating submucous uterine myomas
