Solitary hyoid plasmacytoma with unicentric Castleman disease: A case report and review of literature
Yan-Hui Zhang, Yi-Feng He, Hao Yue, Yue-Ni Zhang, Lei Shi, Bin Jin, Pin Dong
Yan-Hui Zhang, Bin Jin, Pin Dong, Department of Otolaryngology-Head and Neck Surgery,Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai 200080, China
Yi-Feng He, Department of Radiology, Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai 200080, China
Hao Yue, Department of Pathology, Shanghai Cancer Center, Fudan University, Shanghai 200032, China
Yue-Ni Zhang, Lei Shi, NHC Key Laboratory of Molecular Probes and Targeted Diagnosis and Therapy, The Fourth Affiliated Hospital of Harbin Medical University, Harbin 150028,Heilongjiang Province, China
Abstract BACKGROUND Solitary plasmacytoma and unicentric Castleman disease (UCD) are rare lymphoproliferative disorders characterized by monoclonal plasma cells and a single set of locally enlarged lymph nodes, respectively.CASE SUMMARY A 48-year-old Han Chinese man presented to our department with a neck mass and progressive foreign body sensation in his throat. 18F-FDG positron emission tomography revealed focally increased radioactivity centered around the hyoid, and computed tomography (CT) revealed osteolytic lesions. Histopathology revealed Castleman-like features and CD138/CD38-positive mature plasma cells. Systemic work-up ruled out the possibility of POEMS syndrome, lymphoma, and multiple myeloma, leading to a final diagnosis of solitary hyoid plasmacytoma with UCD. The patient underwent partial hyoid resection and selective neck dissection, followed by intensity-modulated radiotherapy. 99mTc-MDP singlephoton emission computed tomography/CT reevaluation showed neither local recurrence nor distant bone metastasis at the 40-mo follow-up.CONCLUSION The diagnostic process and differential diagnosis of this rare case provided valuable educational information to clinicians.
Key Words: 18F-FDG; Positron emission tomography/computed tomography; Plasmacytoma; Hyoid bone;Castleman disease; Case report
INTRODUCTION
Solitary plasmacytoma (SP) is a rare disease characterized by abnormal proliferation of monoclonal plasma cells, with a cumulative incidence of 0.15/100000[1,2]. Solitary bone plasmacytoma (SBP) is a subtype of SP that occurs primarily in the red-marrow-containing axial skeleton and long bones[3] and accounts for 70% of all SP cases[1]. SBP with minimal (< 10%) bone marrow plasma cells progresses to multiple myeloma (MM) within 3 years in approximately 60% of patients[2,4]. All SP are radiationsensitive; therefore, radiotherapy (RT) is recommended as the first-line treatment strategy for the longterm disease control in these patients[3,5].
Unicentric Castleman disease (UCD) is a rare lymphoproliferative disorder with unknown prevalence and belongs to a heterogeneous group of conditions characterized by similar histopathological features but has distinct pathological etiologies, clinical symptoms, management, and outcomes[6,7]. For patients with resectable UCD, complete surgical excision is the optimal first-line treatment, with a 5-year overall survival of up to 90%; even after a 10-year follow-up, disease relapse is rare[6,8].
Here, we report the case of a 48-year-old man with SBP of the hyoid and UCD along with the clinical diagnosis, management process, and follow-up. Moreover, we reviewed the literature of related SBP cases to provide a comparative experience in guiding the management of this rare disease.
CASE PRESENTATION
Chief complaints
A 48-year-old Han Chinese man presented with a neck mass and progressive foreign body sensation in his throat.
History of present illness
The patient reported no sore throat, dyspnea, or dysphagia during the course of the disease.
History of past illness
The patient had no history of past illness.
Personal and family history
The patient had no personal or family history.
Physical examination
Basic physical examination showed no obvious abnormalities.
Laboratory examinations
Laboratory tests showed that the routine blood and urine results as well as blood biochemical indices, including serum calcium-albumin-creatinine, blood urea nitrogen, lactate dehydrogenase,β2-microglobulin, and 24-hour urine for total protein, moreover, the results of serum immunoglobulin and complement assay (Supplementary Table 1), were all within normal limits.
Imaging examinations
Electronic laryngoscopy and plain computed tomography (CT) showed thickening of the left epiglottis (Figure 1A and C), arytenoid and aryepiglottic folds (Figure 1B and D), osteolytic lesions of the left hyoid (Figure 1E), and thyroid cartilage plate (Figure 1F). The left piriform fossa was occluded, and enlarged cervical and submandibular lymph nodes were observed bilaterally. The patient underwent selective neck dissection and partial hyoid resection to relieve neck compression, and a final diagnosis was made.18F-FDG positron emission tomography (PET)/CT revealed focally increased radioactivity centered around the hyoid body and its left greater horn (SUVmax= 8.8) and the left submandibular and submental soft tissue (SUVmax= 4.8) exhibited on PET (Figure 1G-I), CT (Figure 1J-L), and PET/CT fusion (Figure 1M-O). Osteolytic lesions were identified on the plain CT bone window. PET maximumintensity projection image (Figure 1P) showed no other sites with abnormal18F-FDG uptake, except for the perihyoid region. These findings were suggestive of a malignancy.
Further diagnostic work-up
Histological results revealed angiofollicular lymph node hyperplasia of the hyaline-vascular type arranged in an onion-bulb appearance (Figure 2A and B, 200 × and 400 ×), and the presence of mature plasma cells characterized by an oval shape, abundant basophilic cytoplasm, perinuclear halo, round eccentric nuclei, and indiscernible nucleoli[6,7,9] (Figure 2C and D, 200 × and 400 ×). Histopathological results were positive for plasma cell markers [CD138 (Figure 2E), CD38 (Figure 2F), κ (Figure 2G) and λ (Figure 2H) light chains, CD79α (Figure 2I), IgG, IgM], and focal expression of T-cell markers [CD43 (Figure 2J), CD45], and CD56; however, deficiency of B-cell markers [CD20 (Figure 2K), CD19] was observed. The Ki-67 proliferation index was 3% (Figure 2L). No paraproteins and pathological elevation of κ and λ chains were detected by the serum protein electrophoresis and free light-chain assays[4,10]. Bone marrow biopsy indicated normal trilineage hematopoiesis with only 0.2% plasma cells in all leukocytes[4]. Additionally, polyneuropathy and other minor symptoms were absent.
FINAL DIAGNOSIS
Systemic work-up ruled out the possibility of POEMS syndrome, lymphoma, and MM[4,11,12], thus leading to the diagnosis of SP of the hyoid, with UCD. Inflammatory factors, such as TNFα, IL-6, IL1β, TGFβ1, and VEGFα (Supplementary Figure 1), were increased, confirming the multiple cytokineproducing property of SBP[13,14].
TREATMENT
The patient underwent intensity-modulated RT[5] for the hyoid bone and high-risk subclinical areas, with a total dose of 50.4 Gy, divided into 28 fractions[10].
OUTCOME AND FOLLOW-UP
No acute toxicity was observed during RT. After 3 mo, contrast-enhanced CT revealed a slightly shrunken tumor boundary. The patient underwent CT and18F-FDG PET/CT or 99mTc-MDP singlephoton emission computed tomography (SPECT)/CT examinations at 6 and 12 mo, respectively. Recent 99mTc-MDP SPECT/CT reevaluation showed a slight increase in radioactivity around the hyoid, considering postoperative changes rather than local recurrence on SPECT (Figure 3A), CT (Figure 3B), and SPECT/CT fusion (Figure 3C). Focal bone destruction and secondary hyperosteogeny were also observed in the CT bone window (Figure 3D). Whole-body SPECT revealed no evidence of distant bone metastasis (Figure 3E and F). The patient was symptomatic and progression-free at the 40-mo follow-up visit.
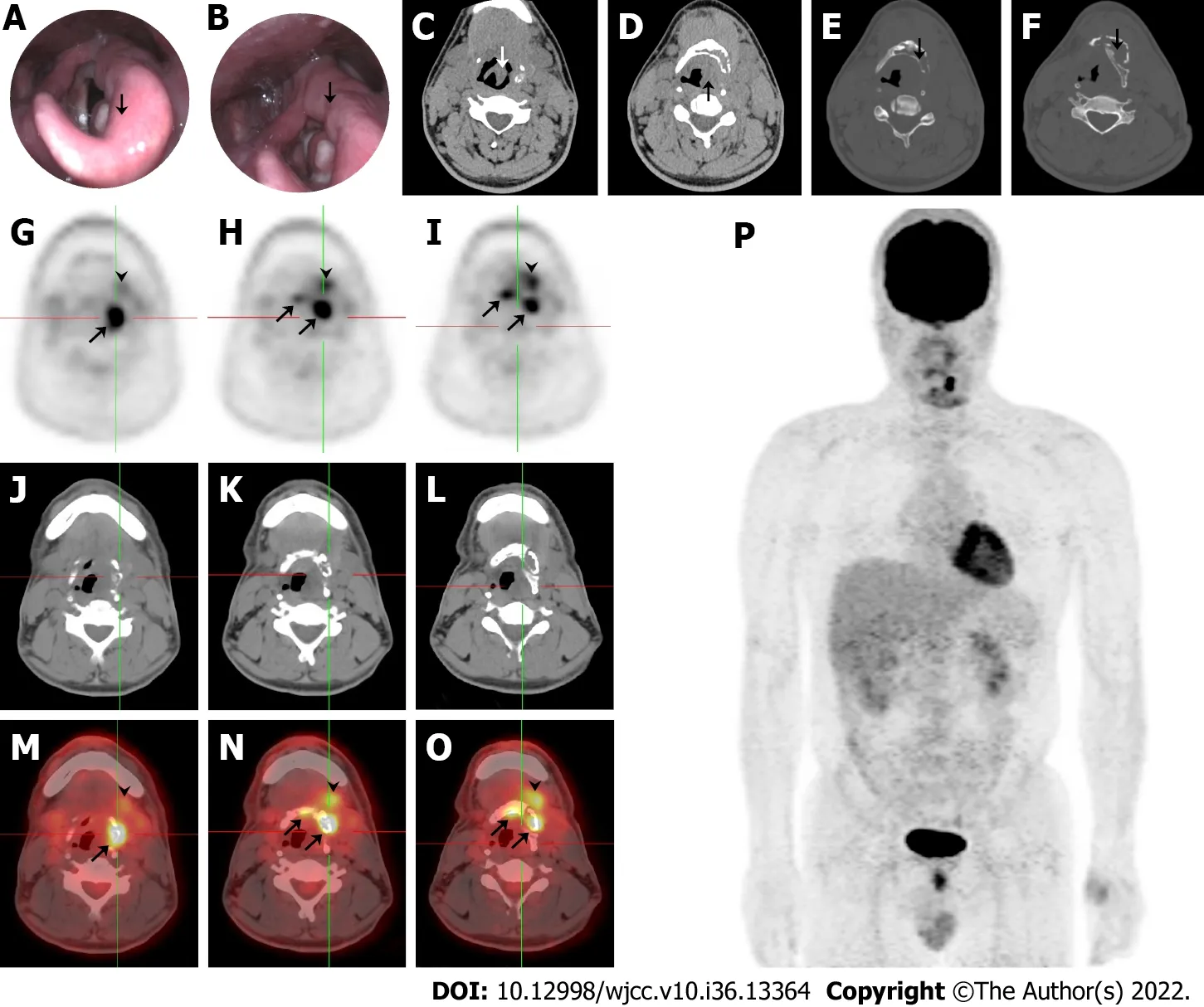
Figure 1 Imaging findings during initial admission. A and C: Thickening of the left epiglottis; B and D: Arytenoid and aryepiglottic fold; E: Osteolytic lesions of the left hyoid; F: Thyroid cartilage plate; G-I: 18F-FDG centered around the hyoid body and its left greater horn (SUVmax = 8.8), and left submandibular and submental soft tissue (SUVmax = 4.8) exhibited on positron emission tomography (PET); J-L: Computed tomography (CT); M-O: PET/CT fusion; and P: PET maximumintensity projection image demonstrated no other sites with abnormal 18F-FDG uptake.
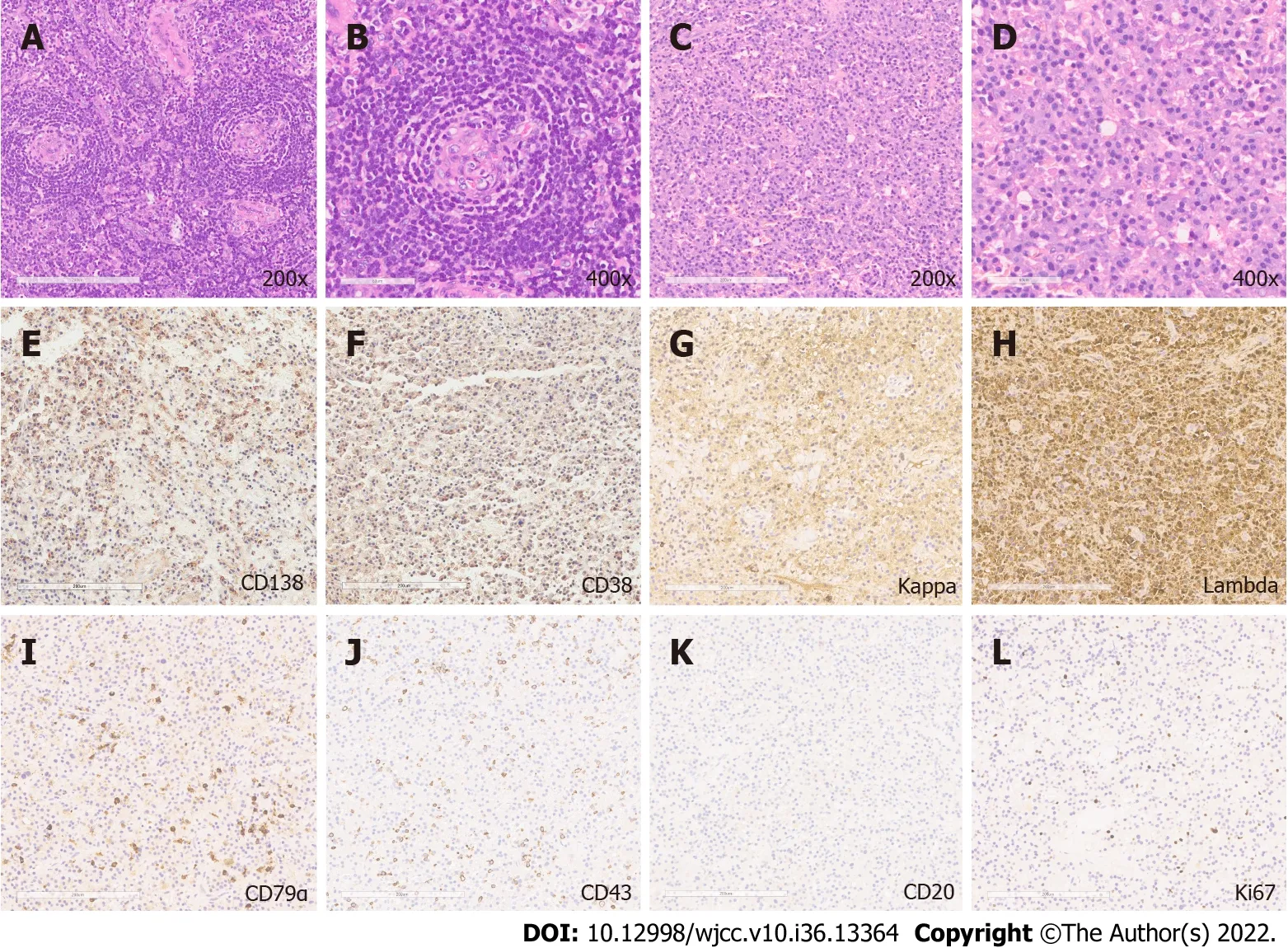
Figure 2 Histopathological and immunohistochemical analysis. A and B: Hematoxylin and eosin staining revealed angiofollicular lymph node hyperplasia(200 × and 400 ×); C and D: The presence of mature plasma cells (200 × and 400 ×); E-J: Positive immunohistochemical staining for plasma cell markers: CD138,CD38, κ light chains, λ light chains, CD79α, T-cell marker CD43; K: Negative staining for B-cell marker CD20; and L: The Ki-67 positive staining.

Figure 3 Molecular imaging in the evaluation of local recurrence and ectopic metastasis during follow-up. A-C: 99mTc-MDP radioactivity around the hyoid exhibited on single-photon emission computed tomography (SPECT), computed tomography (CT), and SPECT/CT fusion; D: Focal bone destruction and secondary hyperosteogeny exhibited on CT bone window; E and F: No evidence of distant bone metastasis on whole-body SPECT.
DISCUSSION
Plasma cell disease comprises a heterogeneous group of disorders characterized by an increase in the number of monoclonal plasma cells[4]. Abnormally proliferative cells can secrete a large quantity of monoclonal immunoglobulin and invade and damage various tissues or organs, such as the bones, bone marrow, kidneys, hearts, lungs, and immune system. Precision clinical intervention is needed for the accurate diagnosis of these diseases. In the present study, the histology of the neck mass showed Castleman’s lesion, such as vitreous degeneration with an "onion skin" appearance, and monoclonal proliferation of plasma cells, resembling the typical features of CD variant of POEMS syndrome. However, the patient did not meet the other major or minor criteria required for a definite diagnosis of POEMS syndrome or MM[4,11,12]; therefore, led to a final diagnosed with SBP of the hyoid and UCD. The National Comprehensive Cancer Network recommends using primary RT of a total dose of 40–50 Gy in 1.8–2.0 Gy per fraction over 4 wk, for patients with SP in whom the surgery is depended on clinical necessity[10]; in addition, for patients with UCD, excisional surgery is the optimal strategy with the benefit of long-term disease control[6,8]. The patient received intensity-modulated RT according to the guidelines after extended dissection of the neoplastic mass adjoining the primary lesion.
The hyoid bone is an atypical location and is extremely rare for the growth of SP; to the best of our knowledge, only three cases have been reported[15-17]. Goelet al[15] presented the first case treated with local RT followed by chemotherapy, and the hyoid swelling was significantly reduced in size during the 18-mo follow-up. Danaciet al[16] described the second case of a 60-year-old man. This patient underwent total excision of the hyoid mass but was not subsequently treated with RT or chemotherapy, and was alive and progression-free during the 24-mo follow-up period[16]. The third patient was diagnosed with non-secretory MM stage IIIA and presented with plasma cell infiltration into the hyoid bone. This patient received chemotherapy and anti-hypercalcemic therapy; however, the prognosis was not referred to in the report[17].
SP can be divided into SBP and solitary extramedullary plasmacytoma based on its origin from the bone marrow or soft tissue organs[4]. Abnormal monoclonal plasma cells and bone marrow stromal cells (BMSCs) in SBP strongly express multiple cytokines and their corresponding receptors that promote the growth and survival of tumor cells or angiogenesis through autocrine and paracrine mechanisms[13,14]. Plasma cell-derived matrix metalloproteinases participate in the degradation of the extracellular matrix and basement membrane, leading to chronic inflammation and bone-destructive lesions[18]. Abnormal plasma cells also induce local angiogenesis and stimulate neovascularization depending on secreted cytokines, such as ANG-1, CXCL12, and VEGFα, which supply nutrition for tumor cell growth[19-21]. Cytokine homeostasis, particularly that secreted by BMSCs, acts as a regulatory mechanism for tumor growth[22]. Some reports indicate that aberrant activation of gp130-dependent and Notch signaling may provide strong advantages for the growth of plasmacytomas[19,23-25]. CD and POEMS syndrome are also associated with the elevation of several classical cytokines in serum, such as IL-6, VEGFα, TNFα, IL1β, and TGFβ1 secreted by proinflammatory cells, as found in SBP. CD and SP can present independently, concurrently, or sequentially with POEMS[26-28]. Patients with CD and/or POEMS syndrome usually have an upregulation of serum VEGFα and IL-6 mediated cytokine storm, which might impair cerebral vessels, resulting in vasculopathy of the central nervous system and an increased risk of cerebral infarction[26,29,30].
Recently, with the development of high-throughput sequencing technology, genetic analyses have been broadly applied to reveal the molecular mechanisms of UCD and idiopathic multicentric Castleman disease (iMCD)[31-35]. Overall, two recurrent hotspot somatic mutations,PDGFRB(NM_002609.4, c.1997A > G, p.N666S) andNCOA4(NM_005437.4, c.781 > T, p.L261F), were identified in 17% and 18% of the tested UCD and iMCD cases, respectively[33,34]. In a patient with iMCD accompanied by severe peripheral neuropathy, somatic alterations were identified in genes related to neuronal development, such asPDLIM5,SEC24B,ZFHX3, andPACRG[36]. Another patient harbored a somaticJAK1missense mutation, but normal serum IL-6 had a complete response rate to the IL-6 antibody siltuximab, implicating the essential roles of the IL-6/IL-6R/JAK1/STAT pathway and its mutations in the pathogenesis of iMCD[37]. Somatic mutations were prone to enrichment in the mitogen-activated protein kinase and interleukin pathways in UCD and iMCD; however, genes affecting chromatin remodeling were solely enriched in iMCD[38]. Moreover, patients with iMCD have a high prevalence of germlineMEFVvariants that modify their clinical phenotypes and treatment responses[39,40]. Transcriptome profiling also revealed that UCD and MCD are involved in unique genes, pathways, and cell types[35].
18F-FDG PET/CT exhibits superior sensitivity and specificity for SBP lesions. It can detect minimal residual disease inside and outside the bone marrow at the same time in one examination, which cannot be identified by χ-ray and magnetic resonance imaging[41,42]. In the early stages of patients with SBP after RT,18F-FDG PET/CT performs poorly in disease staging; however, a high SUVmaxvalue is a regular prognostic indicator for progression[43]. 99mTc-MDP scintigraphy shows a relatively low sensitivity to detect bone lesions of SBP due to the suppressed differentiation of osteoblasts[44] and can be more applicable for evaluating the complications of SBP[45]. 99mTc-MIBI scintigraphy shows the same sensitivity and specificity as18F-FDG PET/CT in the detection of SBP lesions[46] and even better performance in the detection of diffuse bone marrow involvement, but it is inefficient in the detection of focal lesions[47].18F-FDG PET/CT has the ability to simultaneously collect anatomical structure and metabolic information and can be used for the initial staging and response monitoring of CD[48]. It has high specificity and sensitivity for the identification of lymph node lesions in patients with CD[49] and is an important imaging marker for judging the severity and prognosis of the disease. A variety of metabolic parameters in18F-FDG PET/CT, including SUVmax, SUVmean, SUVpeak, metabolic tumor volume, and total lesion glycolysis, can be used for the differential diagnosis of UCD and other diseases[50].
CONCLUSION
To our knowledge, this is the first reported case presented with these two rare clinical entities (SP of the hyoid bone and UCD). In addition to comprehensive histopathological analysis,18F-FDG PET/CT and 99mTc-MDP SPECT/CT are routinely used in the clinical practice of differential diagnosis, disease staging, and follow-up monitoring, highlighting the advantages of nuclear medical and other imaging techniques in the management of SP in the head and neck. In addition, the diagnostic and management processes provided valuable educational information to clinicians.
ACKNOWLEDGEMENTS
We sincerely thank Dr. Ya-Qiong Ren and Yu-Qi Shen at the Chinese Academy of Medical Sciences & Peking Union Medical College for staining of inflammatory factors of SBP and their independent figure combination and optimization.
FOOTNOTES
Author contributions:Shi L, Zhang YH and Zhang YN wrote this report; Jin B and Dong P reviewed and revised the manuscript; Zhang YH and Jin B involved in the clinical treatment and follow-up of the patient; Yue H, He YF, Zhang YN and Shi L interpreted the histopathology and radiology; and all authors had made a substantial contribution to this work and approved it for publication.
Supported byKey Program of the Medical Engineering Cross Research Fund of Shanghai Jiao Tong University, No. YG2019QNA55; and Tou-Yan Innovation Team Program of Heilongjiang Province, No. 2019-15.
Informed consent statement:Informed written consent was obtained from the patient for the publication of this report and any accompanying images.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Lei Shi 0000-0002-1913-8355.
S-Editor:Liu XF
L-Editor:A
P-Editor:Liu XF
 World Journal of Clinical Cases2022年36期
World Journal of Clinical Cases2022年36期
- World Journal of Clinical Cases的其它文章
- Liver injury in COVID-19: Holds ferritinophagy-mediated ferroptosis accountable
- Amebic liver abscess by Entamoeba histolytica
- Living with liver disease in the era of COVID-19-the impact of the epidemic and the threat to high-risk populations
- Cortical bone trajectory screws in the treatment of lumbar degenerative disc disease in patients with osteoporosis
- Probiotics for preventing gestational diabetes in overweight or obese pregnant women: A review
- Effectiveness of microwave endometrial ablation combined with hysteroscopic transcervical resection in treating submucous uterine myomas
