Distinct structural characteristics define a new subfamily of Mycoplasma ferritin
Wenming Wng, Xioji Liu, Yjie Wng, Yun Wng, Dn Fu, Hongfng Xi,Yi Zho, Hongfei Wng,∗
a Institute of Molecular Science, Shanxi University, Taiyuan 030006, China
b State Key Laboratory of Medicinal Chemical Biology and College of Pharmacy, Nankai University, Tianjin 300350, China
c Shanxi Key Laboratory of Pharmaceutical Biotechnology, Taiyuan 030006, China
Keywords:Ferritin Ferritin subfamily Ureaplasma Mycoplasma Crystal structure
ABSTRACT Ferritins can generally be divided into four subfamilies based on their structural characteristics, namely,the classic ferritins (Ftns), bacterioferritins (Bfrs), DNA-binding proteins from starved cells (Dps’), and encapsulated ferritins (EncFtns).However, the ferritin from Mycoplasma penetrans (Mpef) possesses a particular ferroxidase center with an extreme low activity and exhibits unusual characteristics, indicating that it could be a member of a quite different subfamily of ferritins.Hereby, the crystal structure of the ferritin from Ureaplasma urealyticum (Uurf) is presented, Mpef and Uurf have very similar properties, though they display very low sequence similarity.Thus, ferritins from Mycoplasma with these unique properties do not belong to any known subfamily, but they should rather be placed in a novel ferritin subfamily,which we term Mycoplasma Ferritin (Mfr).
Ferritins are common to almost all kingdoms of life, belonging to the “Ferritin-like superfamily” and possessing a common four-helical bundle domain [1,2].They play a key role in iron metabolism and storage [3,4].Generally, ferritins are highly symmetrical and made up of identical or highly similar subunits forming a roughly spherical in shape [5].Each subunit of ferritin is composed of two antiparallel helix pairs (A, B and C, D) and another short E helix, while an external loop, termed the BC loop,traverses the full length of the helical bundle and links helices B and C [6,7].Up to now, these ferritins have been divided into four subfamilies based primarily on their structural properties, termed Ftns, Bfrs, Dps’, and EncFtns, respectively [1,8–10].
The Ftns are the prototype of ferritins and they can be found in archaea, bacteria, and eukaryotes [1].In this subfamily, 24 similar Ftn subunits assemble into a hollow cage-like structure [11–16].For some Ftns from bacteria, a highly disordered C-terminal extension region following the short helix E has also been observed [17].The residues employed in the ferroxidase center of Ftns are strictly conserved, and harbor the motif: E-6-Y in helix A, ExxH in helix B,E in helix C, and Q/TxxE in helix D [18].These hydrophilic 3-fold or 4-fold channels are thought to be the primary route for Fe2+uptake, although a few Ftns have been reported to uptake iron atomsvia2-fold channels or B-channels [19–21].
The Bfr proteins are restricted to the archaea and bacteria, but possess almost the same three-dimensional structure as Ftns.A ferroxidase center is located within the four helical bundle, and the motif Bfr harbored is slightly different from that of Ftns, namely,it is E-6-Y in helix A, ExxH in helix B, E in helix C, and ExxH in helix D [18].However, the most critical principle for distinguishing Ftns from Bfrs is that up to 12 hemes bind at the 2-fold interface,coaxially ligated by a pair of Met-residues (one from each 2-fold related subunits) [22–24].Moreover, the channels in Bfr, including 3-fold, 4-fold and B-channels, are hydrophilic and can serve as the route for iron uptake alone or simultaneously [3,18,25,26].
The major functionality of Dps is to protect bacteria from oxidative damage, which is obviously different from that of Ftn or Bfr[27].Twelve identical Dps subunits assemble into a compact cagelike structure (95 ˚A outer diameter) with 3–3–2 fold symmetry.In addition, the ferroxidase center in Dps proteins is quite unique and locates at the 2-fold interface, rather than within the four helix bundle as is the case in Ftns or Bfrs, and the residues forming this center are well conserved in the Dps subfamily and are distinct from the Ftn and Bfr subfamilies [28].Furthermore, both types of the 3-fold channels in Dps proteins are acidic and hydrophobic,and they have been suggested to be the main entry route for iron ions [29,30].
Encapsulated ferritins (EncFtns) are a new member of the ferritin family [9], each subunit has two mainα-helices and 10 subunits assembled in an open decameric structure with ferroxidase centers locating at the dimer interfaces.EncFtns, however, are unable to store iron atoms [10].
Mycoplasmas,belonging to the cladMolticutes, contain various pathogens, causing various diseases in human and animals [31].TheMycoplasmashave minimal genomes, and the proteins they encoded are essential to their self-replication.Unexpectedly, someMycoplasmasspecies (Mycoplasmas penetrans,Mycoplasma iowae,andUreaplasma urealyticum(Uurf), for instance) encode ferritin proteins, indicating their importance for their life cycle, although the details of the function and structure of these proteins are completely unknown.Hence, there is a pressing need to investigate their properties, which could help researchers predict which ferritin subfamily they belong to.
To date, the first and the only determined crystal structure of ferritin fromMycoplasmasis Mpef [20], and it possesses some major characteristics: 24 subunits without hemes assemble into a similar protein shell as Ftns, with a ferroxidase center located on the inner face, the constitution of this center is particular, the activity it has is very low, the B-channels are thought to be the routes for iron uptake.Hence, we speculated that ferritins fromMycoplasmawith these characteristics may belong to a new subfamily.However, one key issue was whether the characteristics Mpef exhibited are correlate with the characteristics of otherMycoplasmaferritins.To explore this question, we present another crystal structure of aMycoplasmaferritin here,namely, from Uurf,which has been shown to be associated with adverse pregnancy outcomes [32].As expected, the properties Uurf and Mpef exhibited were almost identical; although they display very low sequence similarity (about 29%), these results demonstrate thatMycoplasmaferritins should belong to a new subfamily of ferritins.
The full-length Uurf protein was expressed and purified (Figs.S1A and B in Supporting information).Dynamic light scattering(DLS) analyses show that the hydrodynamic diameter of Uurf was 11.72±3.81 nm (Fig.S1C in Supporting information).Furthermore,transmission electron microscopy (TEM) results also indicate that Uurf subunits could assemble into a hollow protein shell, which is very similar to the assembly of other Ftns and Bfrs (Fig.S1D in Supporting information).In order to solve the Uurf structure by molecular replacement, the program AlphaFold2 was used to prepare an initial model.Finally, the model of Uurf was refined to 2 ˚A resolution, with a finalRworkvalue of 16.02% (Rfree, 19.08%) and the coordinate has been deposited in the PDB under the following accession code: 7VP8 (Table S1 in Supporting information).Uurf crystallized in the cubic space groupF432 with only one subunit in the asymmetric unit.Each subunit contains a full-length polypeptide, and it is composed of a four-helix bundle (A, B, C, and D), a fifth short helix (E), and an external loop (BCloop) which connects the helix B and helix C (Fig.S2A in Supporting information).As in other Ftns, 24 subunits self-assemble into a hollow cage withF432 symmetry, resulting in 12 2-fold channels, 8 3-fold channels,6 4-fold channels, and 24 B-channels (Figs.S2B–D in Supporting information).Furthermore, some iron atoms were also observed in this Uurf structure.The diameter of the Uurf protein cage and cavity are 12.1 nm and 7.8 nm, respectively (Fig.S2E in Supporting information), which is good agreement with the measured diameter mentioned above.Additionally, the initial model produced by Alphafold2 and the final model we solved were also aligned (Fig.S3 in Supporting information), and the root mean square deviation(RMSD) for Cαand all atoms are as low as 0.474 and 0.516, respectively.
Despite having many overall structural similarities with other ferritins, Uurf subunit still has some characteristics of its own.Compared with Mpef, a longer short helix E in Uurf substitutes the short helix E and disorder region in Mpef (Fig.1A).With respect to other Ftns, Bfrs, and Dps’, Uurf does not possess significant sequence identity and similarity with these ferritins, and the angles between the short helix E and four helix bundle are significantly different, while the helix E is absent in Dps’(Figs.1B–D).The helix E in Uurf is the longest one among all the structures of known ferritins.Moreover, the RMSD values between Uurf andMycoplasmaferritin or Ftns are approximately equal to 1, but much higher for Bfrs or Dps’(Fig.1E).Ferritins from the EncFtn subfamily do not contain the typical structure motif of four helix bundles; hence, the RMSD value is not given here.In all, the ferritins fromMycoplasmaare more closely related to the ferritins from the Ftn subfamily in the overall structural view.
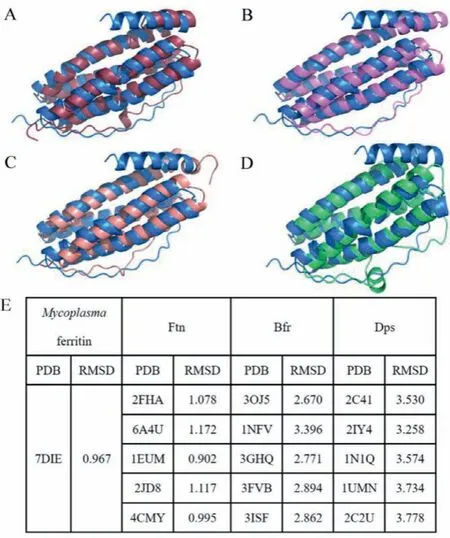
Fig.1 .Subunit structure comparison of Uurf and (A) Mpef (PDB: 7DIE), (B) EcFtnA(PDB: 1EUM), (C) Mycobacterium tuberculosis Bfr (PDB: 3OJ5), and (D) Listeria monocytogenes Dps (PDB: 2IY4).Uurf, Mpef, EcFtnA, Mycobacterium tuberculosis Bfr, and Listeria monocytogenes Dps are colored in blue, dark red, pink, light red, and green,respectively.(E) C-alpha RMSD values between Uurf and other ferritins.
In Uurf, the 3-fold channel is hydrophobic being lined with L112, F120, and K124, and an iron atom is also coordinated at the bottom of this pore with K124viathree water molecules (Figs.2A).In contrast to the 3-fold channels, the hydrophilic residues N149,Q152, Y155, K159, and K162 from the outside in form the 4-fold channel in Uurf, but the aromatic ring of Y155 blocks this channel and no one iron atom could bind in this channel (Fig.2B).In addition, the B-channel is another kind of important channel.This channel is usually hydrophilic, and the residues forming this channel and the diameter of this pore in different ferritins are multitudinous.In Uurf, the B-channel is formed by twelve residues from three adjacent subunits (D33, D34, S37; E62, R65, K66, E68;D135, R138, T139, and D142) (Fig.2C).All of these residues are hydrophilic, and the vast majority of them are charged.Moreover, the entrance area of this channel is up to 46 ˚A2, which is much bigger than that of Mpef, Bfrs, and other Ftns from bacteria.An iron atom is also binding in this channel, it is possibly Fe(II) and is coordinated with D33, E62, D135 and four water molecules, similar to the case of Mpef.
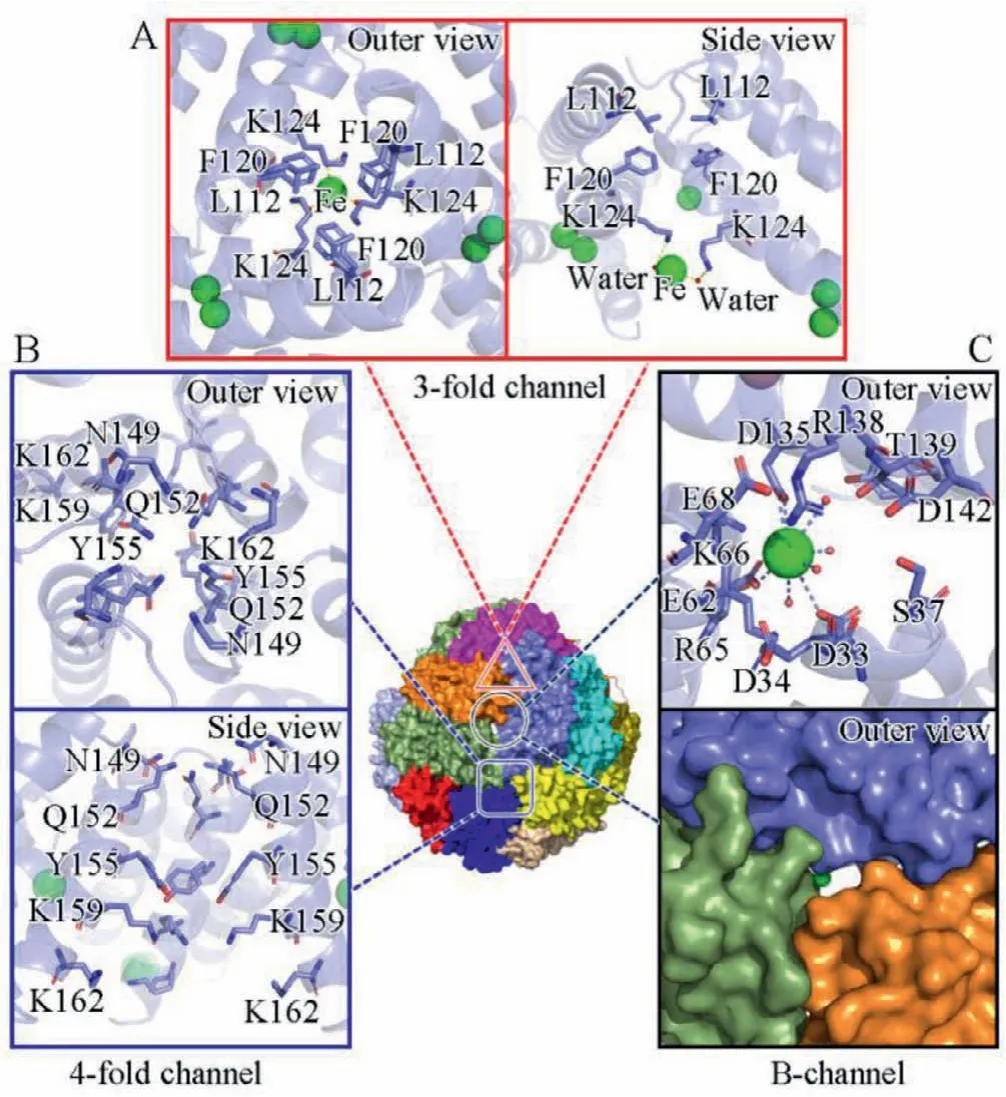
Fig.2 .Channel structures of Uurf.(A) 3-fold channel.(B) 4-fold channel.(C) Bchannel.All of the structures are represented in cartoon or surface presentations and related key residues in stick presentation.Green spheres and small red spheres represent iron atoms and water molecules.The 3-fold channel is labeled with a red triangle, the 4-fold channel is labeled with a blue square and the B-channel is labeled with a black circle.
In ferritins fromMycoplasma, the key residues L112 and F120 forming the 3-fold channels are strictly conserved, while the residues forming the first three layers of 4-fold channels are not conserved (Fig.S4 in Supporting information).Y155 in Uurf, I152 in Mhof and Udif, and S149 and T152 in Miof almost block this channel.Compared to the 3-fold and the 4-fold channel, the formulation of the B-channel is relatively complex and hard to speculate just based on the sequence alignments, owing to the special location between three subunits and the more variable AB-loop in different ferritins fromMycoplasma.The regions and residues participating in the formulation of this channel are also labeled within the red dotted box (Fig.S4 in Supporting information), comprising many charged residues, which could make this channel hydrophilic and bind some iron atoms.
The channels are the major entry routes for iron atoms into the cavity of ferritins, but the properties of these channels are different between ferritins from different subfamilies.In our Uurf model,the 3-fold channel is hydrophobic and the 4-fold channel is hydrophilic but blocked by the aromatic nucleus of Y155, while the B-channel is fully negatively charged and the pore of this channel is big enough for iron atoms to pass through; hence, the only potential route for iron uptake is the B-channel (Figs.2A–C).In contrast to this, according to the surface potential energy, the ferroxidase center and the B-channel are fully negatively charged, forming a huge fully negatively charged region, and iron atoms absorbed by the B-channel could transfer to the ferroxidase center easily (Fig.S5 in Supporting information).Moreover, sequence alignments also indicate that the key residues forming the B-channel, 3-fold and 4-fold channel in Mfr are totally different with those in other ferritin subfamilies (Fig.S6 in Supporting information).Therefore, we conclude that all the ferritins fromMycoplasmause the B-channel as the major route for iron uptake, and this is a pivotal feature for ferritins from this subfamily.
Commonly, ferritins (except for the light subunit) from the Ftn and Bfr subfamilies have a ferroxidase center, which is located within the four helical bundle.The centers from Ftns or Bfrs are similar to each other, while are distinct from that of Dps’.However,the structure of Mpef indicates that the ferroxidase center in Mpef is significantly different in terms of its location and constitution[20].In Uurf, this ferroxidase center is on the inner face of the subunit, and is composed of 6 charged residues (D51, H56, D129, K132,D133, and D136), and all of these 6 residues just come from helix B and helix D (Fig.3A).Similar to other known ferritins, the center in Uurf also contains two iron binding sites, and these two binding sites are occupied by two iron atoms with a distance of 3.0 ˚A.Iron atom binding in site A is coordinated with H56, D51, D129,D133, and a water molecule, while another iron atom binding in site B is D133 and two water molecules (Figs.3A and B).In comparison, the residues forming the ferroxidase center and their conformations are identical in Mpef and Uurf, with the exception of K132 in Uurf being replaced with Glu-in Mpef (Fig.S7A in Supporting information).Besides, these key residues are almost conserved in ferritins fromMycoplasma, indicating that they should employ same ferroxidase centers (Fig.S4 in Supporting information).Furthermore, the structural alignment shows that the ferroxidase center from Uurf and human ferritin heavy chain (PDB code: 4OYN)reveals that these two ferroxidase centers are entirely different in their compositions, conformations of residues and the coordination environment and location of iron atoms (Fig.S7B in Supporting information).In addition, sequence alignments also indicate that the key residues forming the ferroxidase center in Mfr are totally different with those in other ferritin subfamilies (Fig.S6 in Supporting information).In all, the characteristics represented by the ferroxidase center of ferritins fromMycoplasmaare the largest difference from ferritins from other subfamilies.
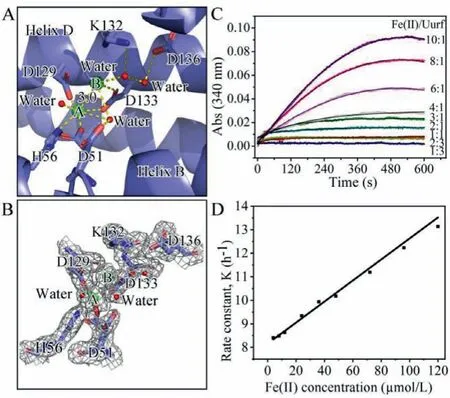
Fig.3 .(A) Detailed structure of the ferroxidase center of Uurf.The interactions of Fe (green), water (red), and ligand residues as well as hydrogen bonds are indicated by dotted lines.(B) A Fo-Fc omit map contoured at 1 σ around the ferroxidase center of Uurf is shown as a gray mesh.Residue D51 in this center has dual conformations.(C) Iron oxidation activity of Uurf.(D) Plot of observed (pseudo-first order)rate constants obtained from fitting the data as a function of Fe(II) concentration.
We also investigated the activity of Uurf and Mpef by following progress curves for iron oxidation (Fig.3C, Fig.S8A in Supporting information).Both ferritins displayed similar activity intensities, but some differences in the shape of the progress curves, and the reaction rate for Uurf was slightly lower than that for Mpef.We analyzed thepseudo-first order rate constant (k0)for each reaction, and gave the second-order rate constants (k)(4.12 ± 0.09)×104L mol−1h−1and (4.23±0.14)×104L mol−1h−1for Uurf and Mpef, respectively (Figs.3D, S8B in Supporting information).Moreover, the oxidized iron atoms could also be stored in Uurf nanocage.Iron mineralization result showed that every ferritin nanocage could store about 495 Fe(III) atoms (20.63 Fe(III)atoms per subunit) (Fig.S9 in Supporting information).Apparently,the lower activities exhibited by ferritins fromMycoplasmaare directly related to the unique ferroxidase centers they respectively possessed.Hence, another important characteristic of ferritins fromMycoplasmais their low rate of Fe(II) oxidation.
Moreover, we selected some typical representatives of ferritins from different subfamilies to construct a phylogenetic tree (Fig.4).As expected, Ftns from vertebrate, invertebrates, plants, and some bacteria were closely related to each other.In addition, ferritins from the EncFtn, Dps, and Bfr subfamilies could be distinguished from each other.At the same time, the ferritins fromMycoplasmacould cluster with each other, but they did not cluster with any other of the major maxi-ferritin branches, indicating that these ferritins should belong to a new subfamily, which we have termed Mfrs.Moreover, it is reported that the Ftn, Bfr and Dps may have arisen through gene duplication of an ancestor of EncFtn [10].The phylogenetic analysis indicated that Mfr should also evolve from EncFtn, and has a closer evolutionary relationship between Ftn.
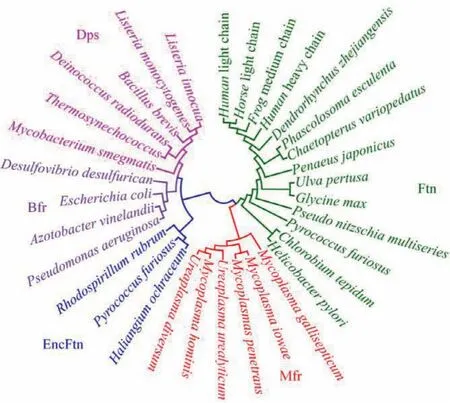
Fig.4 .Ferritin phylogenetic tree shows the relationships between Ftns, EncFtns,Bfrs, Dps’, and Mfrs.
In this work, we report the first crystal structure of ferritin fromU.urealyticum, an important genus ofMycoplasma.The common characteristics ofMycoplasmaferritins from a structural point of view support ferritins fromMycoplasmashould be classified into a new subfamily.Moreover, we demonstrate here that Mfrs have common characteristics: unique ferroxidase centers with novel motif, a much lower iron oxidation activity, and hydrophilic Bchannels for ion passthrough, which allowed them to be distinguished from ferritins from other subfamilies, and highlighting that they belong to the Mfr subfamily.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported partially by the National Natural Science Foundation of China (Nos.62075118, 21601112), the Natural Science Foundation of Shanxi Province (No.20210302123433), Key R&D program of Shanxi Province (International Cooperation, No.201903D421070, 2021XM21), Shanxi Key Laboratory of Pharmaceutical Biotechnology (No.KF202003), and Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi(No.2019L0020).We thank the staffs from BL19U1 beamline of National centre for Protein Sciences Shanghai (NCPSS) at Shanghai Synchrotron Radiation Facility for assistance during data collection.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.119.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
