Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
Qin-Feng Zhng, Hu-Ming Xio, Jin-To Zhn, Bi-Feng Yun,b,∗, Yu-Qi Feng,b,∗
a Department of Chemistry, Wuhan University, Wuhan 430072, China
b School of Public Health, Wuhan University, Wuhan 430071, China
Keywords:Chemical labeling Liquid chromatography-mass spectrometry Indole metabolites Tryptophan Rat feces
ABSTRACT As the connecting part of diet and host physiology, intestinal microbes can convert the ingested diet into a huge number of physiologically active small molecules.Indole metabolites of tryptophan are precursors or signal molecules for many biologically active substances, which are involved in serotonin and microbial catabolism pathways.To understand the influence of tryptophan metabolism in the intestinal environment on the neurological and immune systems at the molecular level, it is important to establish a high-coverage analytical method to comprehensively analyze the metabolites involved in tryptophan metabolism.However, due to a small molecular weight and poor response during mass spectrometry analysis, as well as weak retention on the reversed-phase chromatography, determination of indole metabolites of tryptophan is challenging.Here, we proposed a method for the simultaneous determination of 20 indole metabolites of tryptophan in a single run on reversed-phase chromatography by chemical labeling coupled to liquid chromatography-tandem mass spectrometry analysis.4-(Dimethylamino)benzaldehyde (DMAB) was used for the labeling of indole metabolites of tryptophan, which could significantly improve the detection sensitivities and retention of these metabolites on reversed-phase chromatography.With the developed method, we realized the sensitive detection and comprehensive analysis of 15 endogenous indole metabolites of tryptophan in rat feces samples with functional dyspepsia intervention by acupuncture.The developed method offers a useful tool for studying tryptophan metabolism-related diseases.
Intestinal microorganisms generate a "diet from the microbiome to the host axis" that turns the consumed meal into a huge number of physiologically active small molecules [1–3].A variety of metabolites produced by intestinal microorganisms play essential roles in metabolism, immunology and neurohomeostasis [4–6].Tryptophan and its indole metabolites are precursors or signal molecules for many biologically active substances [7–10].The indole metabolites in the serotonin pathway play important roles in the "gut-brain axis", which activates specific 5-hydroxytryptamine receptors in the gastrointestinal tract and is engaged in a wide range of physiological functions [11–14].Tryptophan also produces a variety of indole metabolites under the action of intestinal microorganisms [7,15,16].These metabolites act as ligands and are essential for intestinal homeostasis [17–19].
To understand the influence of tryptophan metabolism in the intestinal environment on the neurological system and immune system from the molecular level, it is important to establish a high-coverage analytical method to analyze the indole metabolites of tryptophan.At present, the analysis of tryptophan indole metabolites mainly relies on liquid chromatography-mass spectrometry (LC-MS) [20–23].It has been reported that LC-MS-based methods were used to determine tryptophan metabolites in various biological samples [24–26].For example, Chenet al.reported an analytical method for the simultaneous analysis of 16 tryptophan indole metabolites using LC-MS with selected reaction monitoring mode [27].The fragments of these tryptophan indole metabolites under collision-induced dissociation (CID) are similar because they all have modest molecular weights and share the same indole skeleton structure.In general, the fragmentation behavior of parent ions under CID is employed to select distinctive product ions for multiple reaction monitoring (MRM) transitions [28].As a result, establishing the MRM transitions for indole metabolites is difficult.
Since metabolites with carboxylic groups have a poor response in the positive ion mode of mass spectrometry [29–32], some tryptophan indole metabolites such as indole-3-lactic acid (ILA),indole-3-carboxylic acid (ICA), and 3-indoxyl sulfate, are difficult to be analyzed in the positive ion mode.Therefore, researchers used polarity switching to classify tryptophan indole metabolites, and analyzed the corresponding metabolites in positive and negative ion modes [27].However, switching between positive and negative ion modes consumes acquisition time, and frequently reciprocating ion modes switching would affect the MS response to a certain extent.In addition, tryptophan indole metabolites have nitrogencontaining heterocyclic indole, and the modified groups are usually hydrophilic groups such as amino/carboxyl groups, resulting in limited reversed-phase chromatography retention.
Chemical labeling assisted LC-MS strategies have been shown to improve the detection sensitivities of analytes as well as increase the retention on reversed-phase chromatography [33–41].For example, Guoet al.reported a method that used two labeling reagents,N-dimethyl-/N–diethyl-aminonaphthalene-sulfonyl chloride (Dns/Dens-Cl) to label amino and phenolic hydroxyl groups in indole metabolites, thereby realizing the analysis of 9 tryptophan metabolites [42].However, this method requires the simultaneous usage of two labeling reagents.Wanget al.reported a strategy for converting indole butyric acid (IBA) into a cationic derivativeviathe Ehrlich reaction, which could then be combined with negatively charged gold nanoparticles to form a SERS hot spot and finally realize the dual signal enhancement of IBA [43].
In the current study, we successfully established a chemical labeling strategy for indole using 4-(dimethylamino)benzaldehyde(DMAB) reagent to specifically label indole skeleton in indole metabolites of tryptophan (Fig.1).The reaction is a specific electrophilic substitution reaction of DMAB with position 2 on the indole ring under the catalysis of hydrochloric acid.The reaction mechanism is shown in Fig.S1 in Supporting information.The oxygen atom on the carbonyl group of DMAB is first protonated by the hydrogen ion in the strong acid medium.Due to the electrophilicity of the carbocation intermediate, it can undergo an electrophilic substitution reaction with the nucleophilic electron-rich indole ring.DMAB can selectively react with the indole skeleton, and theN,N-dimethyl on DMAB can enhance the signal response during mass spectrometry analysis [44].In addition, the benzene ring on DMAB can increase the hydrophobicity of the analyte, thereby enhancing the retention of the labeled product on the reversed-phase chromatography [45].The DMAB labeling coupled with LC-MS/MS analysis enabled the sensitive and simultaneous analysis of 20 indole metabolites of tryptophan (Fig.2).
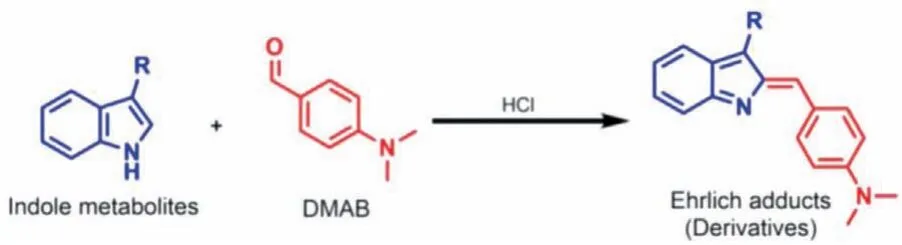
Fig.1 .Chemical labeling of indole metabolites by DMAB.
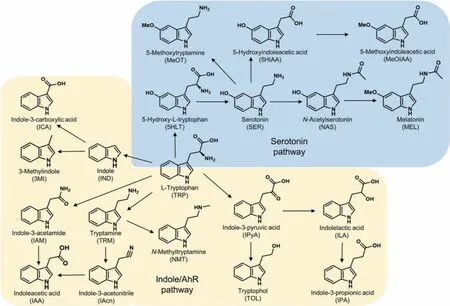
Fig.2 .The chemical structures of indole metabolites of tryptophan involved in serotonin pathway and microbial catabolites.
We first selected indole metabolites with various substituents,including indole (IND), indole-3-acetonitrile (IAcn), indole-3-acetic acid (IAA) and indole-3-acetamide (IAM), as representative metabolites to investigate the fragmentation behavior of DMABlabeled products under CID mode.The results showed that all the four representative indole metabolites could be successfully labeled by DMAB (Fig.3).The DMAB-labeled IND (m/z249.1) producedm/z233.1,m/z219.1, andm/z204.1 product ions (Fig.3a).The DMAB-labeled IAcn produced a product ion ofm/z247.1(Fig.3b), and the DMAB-labeled IAA produced product ion ofm/z261.1 (Fig.3c).The DMAB-labeled IAM produced product ions ofm/z246.1 andm/z218.1 (Fig.3d).All these product ions were produced by the fragmentation of the substituent at the indole ring C3.In summary, the results showed that DMAB could successfully label indole metabolites with different substituents.The optimized MRM parameters of the DMAB-labeled products are shown in Table S1 in Supporting information.With the established MRM mass transitions, we obtained the optimal chromatographic separation of these by optimizing mobile phase compositions, gradients and flow rates (Fig.4).
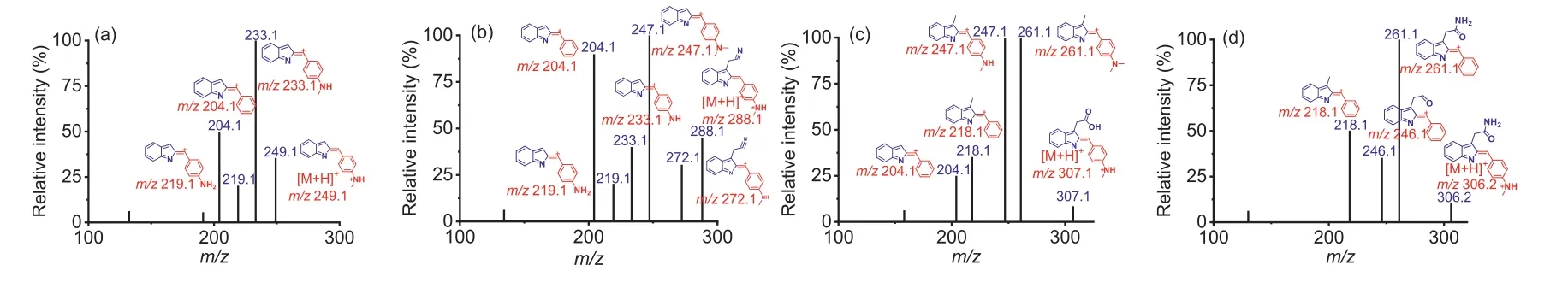
Fig.3 .MS/MS spectrum and fragmentation of DMAB-labeled tryptophan indole metabolite.(a) DMAB-labeled IND.(b) DMAB-labeled IAcn.(c) DMAB-labeled IAA.(d) DMABlabeled IAM.
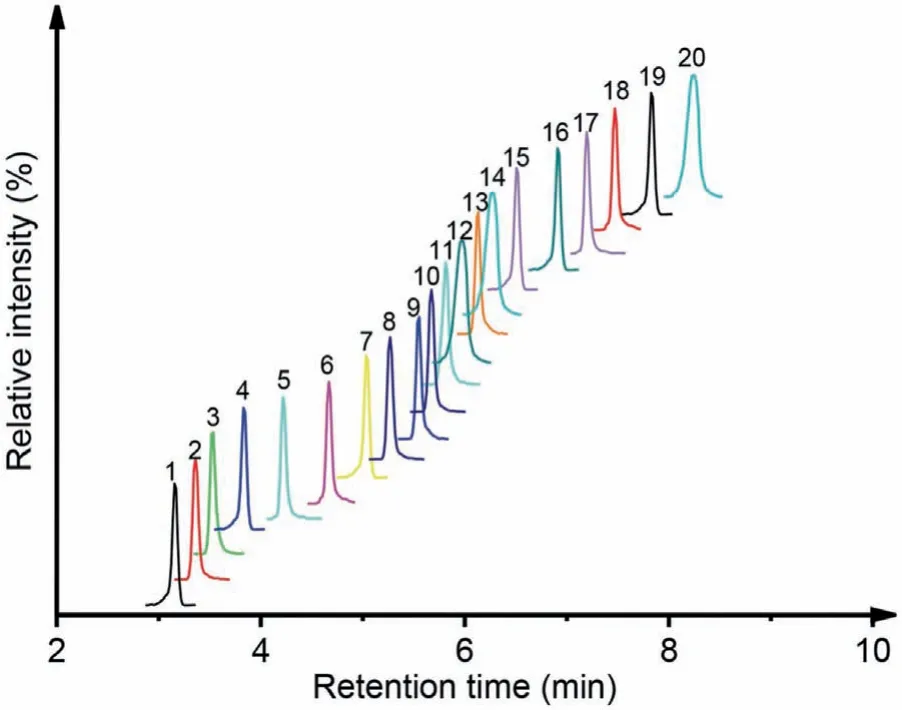
Fig.4 .The extracted-ion chromatograms of 20 indole metabolites standards of tryptophan analyzed by DMAB labeling assisted LC-MS/MS analysis.1.serotonin(SER), 2. N-acetylserotonin (NAS), 3.5-hydroxyindoleacetic acid (5HIAA), 4.5–hydroxy-L-tryptophan (5HLT), 5.melatonin (MLT), 6.indole (IND), 7.3-indolelactic acid (ILA), 8.5-methoxyindoleacetic acid (MeOIAA), 9.tryptamine (TRM), 10.3-indoleacetic acid (IAA), 11.3-methylindole (3MI), 12.indole-3-acetamide (IAM),13.L-tryptophan (TRP), 14. N-methyltryptamine (NMT), 15.indole-3-propionic acid(IPA), 16.5-methoxytryptamine (MeOT), 17.indole-3-carboxylic acid (ICA), 18.3-indoleacetonitrile (IAcn), 19.tryptophanol (TOL), 20.indole-3-pyruvic acid (IPyA).
We next optimized the DMAB labeling reaction conditions, including the types of acid catalyst, the concentrations of acid catalysts, the amount of DMAB, and the reaction time.The results showed that, when HCl was utilized as the acid catalyst in the reaction, the best MS response of the DMAB-labeled product could be obtained (Fig.S2a in Supporting information).We further optimized the concentration of HCl.The result showed that 2 mol/L of HCl offered the best reaction efficiency (Fig.S2b in Supporting information).In addition, the signal of the DMAB-labeled product increased with increasing concentration of the DMAB, and reached the plateau when the concentration of DMAB was 2.5 mmol/L (Fig.S2c in Supporting information).However, due to the ion suppression, further increasing of DMAB will cause a certain degree of signal reduction.Moreover, the signal of the DMAB-labeled product was gradually increased with the increased reaction time, and reached a plateau when the reaction time was 9 h (Fig.S2d in Supporting information).We continued to monitor the signal of the DMAB-labeled tryptophan indole metabolites at 4°C for 24 h to investigate the stability of the DMAB-labeled product.The results showed that the DMAB-labeled product signal within 24 h was within ± 20% (Fig.S3 in Supporting information), which could meet the requirements of subsequent analysis.Collectively, the optimized conditions for DMAB labeling were as follows: acid catalyst, 2 mol/L of HCl; concentration of DMAB, 2.5 mmol/L; reaction time, 9 h.
The calibration curves were prepared to quantify the amounts of tryptophan indole metabolites using the mixtures of 20 tryptophan indole metabolite standards (0.5, 1, 2, 5, 10, 20, 50, 100,200, 500, 1000, 2000, 5000 ng/mL), and the fixed concentrations of internal standards (MEL-d4, TRM-d4, NAS-d3and 5MeOT-d4,10 ng/mL; IAA-d2, 20 ng/mL; Trp-d3, 100 ng/mL).The calibration curves were constructed by plotting the peak area ratio (analytes/internal standards) to the concentrations of tryptophan indole metabolites.The coefficient of determination (R2) ranged from 0.97 to 0.99, indicating the good linearity (Table S2 in Supporting information).The limits of detection (LODs) and limits of quantification (LOQs) are defined as the concentration of analyte when the signal-to-noise ratio (S/N) is 3 and 10, respectively [46].The results showed that the LODs and LOQs of tryptophan indole metabolites were 0.01–0.30 ng/mL and 0.03–1.00 ng/mL, respectively (Table S2 in Supporting information).The reproducibility and accuracy of the method were evaluated by analysis of samples with different spiked concentrations (5, 50 and 100 ng/mL).The intra-day precision was evaluated by repeating 5 times a day, and the inter-day precision was evaluated for 3 consecutive days.The result showed that the method had acceptable intra-day and inter-day precision,and the RSD value was less than 13.7%.In addition, the recovery rate was 78.5%−113.5% (Table S3 in Supporting information).
We then evaluated the matrix effect, the influence of the matrix on the labeling reaction and the extraction efficiency of the proposed method.The matrix effect was defined as the influence of the matrix on the MS response of the analytes and was calculated by the formula (Cstd+endo−Cendo)/Cstd.Cstd+endorepresents the peak area of tryptophan indole metabolites in rat feces samples after spiked with standards, andCendorepresents the peak area of tryptophan indole metabolites in the blank rat feces sample.Cstdrepresents the peak area of tryptophan indole metabolites in the standard solution.The influence of the matrix on the labeling reaction was calculated by the formula(Cpost-spiked−Cendo)/(Cstd+endo−Cendo), whereCpost-spikedrepresents the peak area of tryptophan indole metabolites in rat feces samples spiked with standard solution mixtures after extraction.The matrix effect and the effect of matrix on the labeling reaction were between 80.8%−111.1% and 81.4%−113.2% in rat feces samples, respectively (Table S4 in Supporting information), indicating that rat feces samples have minor effects on the ionization and labeling reaction of analytes.The extraction efficiency was calculated by the formula(Cpre-spiked−Cendo)/(Cpost-spiked−Cendo), whereCpre-spikedrepresents the peak area of tryptophan indole metabolites in rat feces samples spiked with standard solution mixtures before extraction.The extraction efficiency was between 78.5%−92.1% in rat feces samples(Table S4 in Supporting information), indicating that the extraction method can effectively extract the analytes in rat feces samples.
We evaluated the slopes of the quantitative regression curves of the standards solution mixtures of tryptophan indole metabolites and the spiked rat feces samples.The results showed that after sample pretreatment including extraction and DMAB labeling, the regression curves of tryptophan indole metabolites in the standard solution mixtures and spiked rat feces samples had comparable slopes (Table S5 in Supporting information), indicating that the quantitative approach can meet the analytical requirements.We also compared the LOQs of this method with other analytical methods for tryptophan indole metabolites (Table S6 in Supporting information).Previous studies mainly focused on the seven metabolites of the serotonin pathway, including 5HLT, SER, MeOT,5HIAA, MeOIAA, NAS and MEL [26,47–49].In the current study, we could simultaneously detect 20 tryptophan indole metabolites due to the improved detection sensitivity.Therefore, our method could cover the serotonin and microbial catabolism pathways of tryptophan, which is helpful to understand the effect of tryptophan metabolism on the “brain-gut-microbe axis”.
We further applied the established DMAB labeling-assisted LCMS/MS method for simultaneous analysis of a series of metabolites involved in the serotonin metabolic pathway of tryptophan and the microbial catabolism pathway.With the developed method,we successfully detected metabolites in the serotonin pathway (Table S7 in Supporting information), including 5HLT, SER, 5HIAA,MeOIAA, NAS and MEL in rat feces samples.In addition, we detected the existence of a variety of indole metabolites in the microbial degradation pathway, including IND, 3MI, ICA, ILA, TRM, NMT,IAA and IPA (Table S7 in Supporting information).
In summary, we developed a DMAB labeling strategy in combination with LC-MS/MS analysis for the simultaneous and sensitive detection of 20 tryptophan metabolites with indole structure, allowing for a high-coverage analysis of tryptophan metabolites.This method showed good accuracy and precision.Using this method, we successfully detected 15 endogenous indole metabolites of tryptophan in rat feces samples with functional dyspepsia intervention by acupuncture.This method could be applied to research on tryptophan metabolism-related diseases in future study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by the National Key Research and Development Program of China (No.2018YFA0900400), the National Natural Science Foundation of China (Nos.21635006 and 21721005).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.004.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
- Heterostructures of NiFe LDH hierarchically assembled on MoS2 nanosheets as high-efficiency electrocatalysts for overall water splitting
