Ruthenium-modified porous NiCo2O4 nanosheets boost overall water splitting in alkaline solution
Rui Yng, Xuezho Shi, Ynyn Wng, Jing Jin, Hnwen Liu, Jie Yin,Yong-Qing Zho, Pinxin Xi,∗
a State Key Laboratory of Applied Organic Chemistry Frontiers Science Center for Rare Isotopes College of Chemistry and Chemical Engineering, Lanzhou University Lanzhou 730000, China
b Lanzhou Jinchuan Advanced Materials Technology Co., Ltd., Jinchang 737100, China
Keywords:Ru modification Porous nanosheets Oxygen vacancy Spinel-based oxides Water splitting
ABSTRACT Exploring efficient oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) electrocatalysts is crucial for developing water splitting devices.The composition and structure of catalysts are of great importance for catalytic performance.In this work, a heterogeneous Ru modified strategy is engineered to improve the catalytic performance of porous NiCo2O4 nanosheets (NSs).Profiting from favorable elements composition and optimized structure property of decreased charge transfer barrier, more accessible active sites and increased oxygen vacancy concentration, the Ru-NiCo2O4 NSs exhibits excellent OER activity with a low overpotential of 230 mV to reach the current density of 10 mA/cm2 and decent durability.Furthermore, Ru-NiCo2O4 NSs show superior HER activity than the pristine NiCo2O4 NSs, as well.When assembling Ru-NiCo2O4 NSs couple as an alkaline water electrolyzer, a cell voltage of 1.60 V can deliver the current density of 10 mA/cm2.This work provides feasible guidance for improving the catalytic performance of spinel-based oxides.
Electrocatalytic water splitting is regarded as a technology with great prospects for production of clean hydrogen energy [1–5].However, there still exist great challenges to fabricate exceedingly efficient catalysts to accelerate the oxygen evolution reaction(OER) and the hydrogen evolution reaction (HER) in alkaline media, due to their sluggish kinetics and unseemly thermodynamics[6–10].Especially for OER process, it contains complicated multistep proton-coupled electron transfer processes, responsible for a large overpotential [11].At present, the commonly recognized OER and HER catalysts with prominent performance are Ir-based and Pt-based materials, respectively [12,13].But high price and limited reserve of these noble metals restrain their widespread application.Among all kinds of alternative catalysts for expensive materials, spinel-based oxides have gained enormous attention owing to rich element composition, variable valence state and good stability during electrocatalytic process [14–17].Nevertheless, the electrocatalytic activity of spinel-based oxides without further modulating is still far from people’s satisfaction.
Obviously, the catalytic activity of catalysts is highly associated with their elemental composition and structure traits [18,19] Introducing heteroatoms into substrate materials has been certified a useful strategy to optimize materials’surface property and improve their catalytic performance [20,21].A critical point lies in the choice of heteroelement.It has been recorded that Ru has moderate H∗and OH∗intermediates absorption energy, due to the similar Ru-H bond energy (∼65 kcal/mol) with Pt-H bond energy(∼70 kcal/mol) and reasonable oxophilic character [22–25].And the price of Ru is the cheapest among Pt-group metals [26].Therefore, Ru has great potential to be used in electrocatalytic water splitting realm.For instance, Huang and co-workers reported the synthesis of Ru-doped CoCr LDHs as efficient a OER catalyst due to synergetic charge transfer [27].Besides, Chen and co-workers designed RuCo nanoalloys catalyst, displaying impressive HER performance, with the overpotential of 28 mV being need to reach the current density of 10 mA/cm2[26].More importantly, Qi and co-workers engineered Co MOF by introducing Ru, forming hollow (Ru-Co)Oxcompound which can promote overall water splitting performance [28].In addition, the morphology of catalysts also plays a pivotal role in catalytic process.The two-dimension (2D)nanomaterial with largely exposed surface atoms and edge sites is deemed as an appealing electrocatalyst for water splitting, which is not only in favor of the absorption of reactant species due to its large parts of superficial coordination-unsaturated atoms but also helpful to abridged diffusion path of reactants and products[21,29,30].It has been reported that nanosheets array structure of various catalysts show excellent performance such as NiFe-LDH[21], CoOOH [31] and Co-NiS2[32].Inspired by these relevant reports, it may be working to construct 2D spinel-based oxides catalysts integrated with Ru to achieve efficient overall water splitting.
Herein, we synthesized porous NiCo2O4nanosheets (NSs) arrays coupled with Ru aligned on carbon fiber cloth (CFC) (denoted as Ru-NiCo2O4NSs) through a facile impregnating method to introduce Ru.The obtained porous Ru-NiCo2O4NSs features desirable elements composition and favorable structure virtues which showcase higher oxygen vacancy concentration, decreased charge transfer impedance and more accessible active sites.Moreover,the introduction of Ru greatly reduces the activation energy of OER process.Accordingly, Ru-NiCo2O4NSs manifest superior OER performance with only an overpotential of 230 mV at the current density of 10 mA/cm2and long-term stability in alkaline media.Furthermore, Ru-NiCo2O4NSs show superior HER activity than NiCo2O4NSs counterpart.The integrated alkaline electrolyzer using Ru-NiCo2O4NSs as bifunctional electrodes materials needs 1.60 V to reach the current density of 10 mA/cm2.And the activity and durability of Ru-NiCo2O4NSs overall water splitting system are both superior to Pt/C||RuO2system.This work presents a viable guide for developing efficient spinel-based oxides electrocatalysts.
The synthesis of NiCo2O4NSs refers to the previous report[20].Firstly, the precursor NiCo-LDH was synthesized by a facile electro-deposition method.0.1 mol/L Ni(NO3)2·6H2O (20 mL) and 0.1 mol/L Co(NO3)2·6H2O (40 mL) were mixed as electrodeposition electrolyte.A piece of carbon fabric cloth (CFC) was used as electro-deposition substrate.The process was performed at −1 V(vs.Ag/AgCl) for 15 min.After finishing, the final product was rinsed with DI water several times, and then dried in 50 °C electrooven.The NiCo2O4NSs were obtained by calcining NiCo-LDH at 300 °C for 2 h with the heating rate (2 °C/min) in air atmosphere.Then, the Ru-NiCo2O4was synthesized by impregnating NiCo2O4NSs in RuCl3solution (10 mg/mL) for 20 min, then dried in 50 °C electro-oven and further annealed at 300 °C for 2 h in air atmosphere.
X-ray diffraction (XRD) measurement was conducted on Rigaku MiniFlex 600 diffractometer from 10° to 90° under a constant voltage of 40 kV and constant current of 15 mA.The morphology characterization was obtained by ThermoFisher Apreo S filedemission scanning electron microscopy (FESEM) with a voltage of 30 kV.Transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HR-TEM) and EDS mapping pictures of samples was collected on a Tecnai G2 F30 filed emission transmission electron microscopy.Raman spectra were measured on a Horiba LabRAM HR Evolution spectrophotometer with a laser line of 532 nm.Electron spin resonance (ESR) experiments were made on JES-FA300 electron spin resonance spectrometer.Xray photoelectron spectroscopy (XPS) was performed by a VG ESCALAB 220I-XL device.The data were corrected using C 1s line as standard.Ultraviolet photoelectron spectrometer (UPS) was conducted on Kratos Axis Supra device.The contact angle test was carried out on a Kruss DSA100 optical contact angle/surface tension meter.
Electrochemical measurements were performed on CHI 760E electrochemical workstation (Shanghai Chenhua Instrument Corp.,China) with a standard three-electrode configuration, with Pt plate and Hg/HgO as counter electrode and reference electrode, respectively, for OER test.While for HER test, the counter electrode was changed to a carbon rod.The catalysts grown on CFC were used as working electrodes directly.1.0 mol/L KOH solution was used as electrolyte and Linear scan voltammogram (LSV) curves were carried out at a scan rate of 2 mV/s.All the electrode potential was converted to the reversible hydrogen electrode (RHE), using the equationERHE=EHg/HgO+ 0.0591 × pH + 0.098 V.
Scheme 1 illustrates the synthesis procedure of catalysts.At first, the phases of samples obtained were analyzed by the Xray diffraction (XRD) technique.Fig.1a shows that the diffraction peaks of the pristine NiCo2O4NSs can well match with cubic NiCo2O4(JCPDS No.20–781, space group F∗3).And the Ru-NiCo2O4NSs possess similar diffraction patterns with the pristine NiCo2O4NSs without related Ru oxides phase being detected,implying Ru was doped successfully in NiCo2O4NSs preliminarily.As shown in Fig.1b and Fig.S1a (Supporting information),the original NiCo2O4NSs presents interlaced nanosheets morphology grown on CFC characterized by scanning electron microscope(SEM).From the transmission electron microscope (TEM) image(Fig.1c), it can be detected that the nanosheets present porous property.It can be seen from Figs.1d and e and Fig.S1b (Supporting information), the Ru-NiCo2O4NSs exhibit similar morphology feature with the pristine NiCo2O4NSs, declaring the introducing of Ru does not ruin porous nanosheets structure of NiCo2O4NSs.It has been documented that porous structure is in favor of accelerating mass transfer process and enhancing the accessibility of active sites [33–35].Furthermore, high-resolution transmission electron microscopy (HRTEM) image of Ru-NiCo2O4NSs exhibits lattice spacing of 0.24 nm, which can be assigned to (311) plane of NiCo2O4(Fig.1f).The high-angle annular dark-field scanning TEM image (Fig.1g) and TEM energy-dispersive spectra (EDS) mapping images (Fig.1h) show the uniform distribution of Ru, Ni, Co and O in the checked region.Furthermore, Fig.1i presents the crystal structure of NiCo2O4.

Scheme 1 .Synthetic scheme of the Ru-NiCo2O4 NSs electrocatalyst.
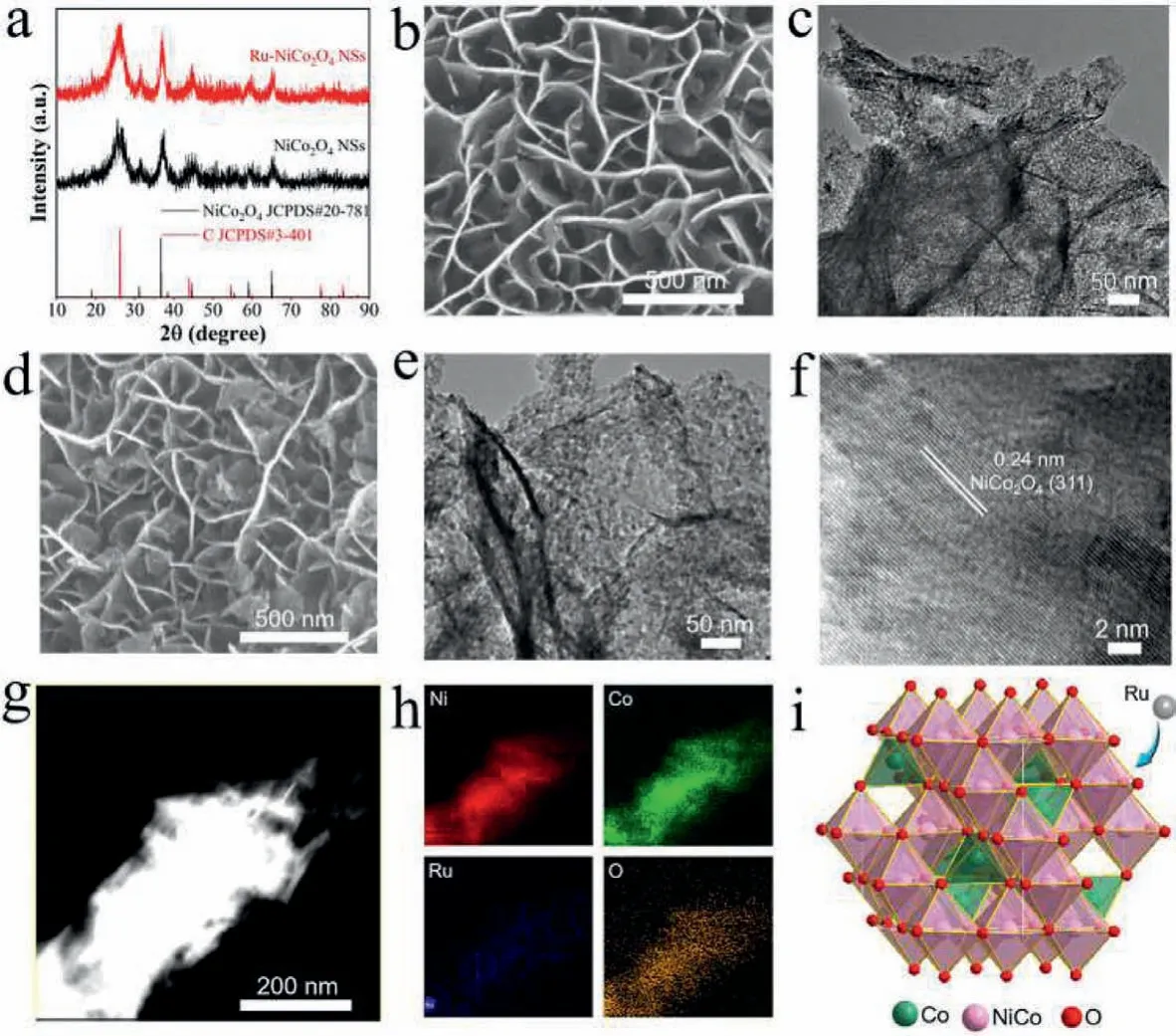
Fig.1 .(a) XRD pattern of NiCo2O4 and Ru-NiCo2O4 NSs.(b, c) SEM, TEM images of NiCo2O4 NSs.(d) SEM, (e) TEM and (f) HRTEM of Ru-NiCo2O4 NSs.(g, h) HAADF-STEM image and corresponding elemental mapping images of Ru-NiCo2O4 NSs.(i) Crystal structure of NiCo2O4.
Moreover, the X-ray photoelectron spectroscopy (XPS), Ultraviolet photoelectron spectrometer (UPS), Raman spectra and electron spin-resonance spectroscopy (ESR) were carried out to further probe the structural information of catalysts obtained.As shown in XPS survey spectrums of Ru-NiCo2O4NSs and NiCo2O4NSs (Fig.S2 in Supporting information), the Ru 3p signal lays in Ru-NiCo2O4NSs, while it does not appear in NiCo2O4NSs, confirming Ru was introduced into NiCo2O4NSs matrix successfully, which is consistent with the TEM-EDS result.The high-resolution O1s XPS spectra (Fig.2a) can be deconvoluted into three parts: lattice oxygen,oxygen vacancy, or hydroxyl group and absorbed oxygen from low binding energy to high binding energy in sequence [36,37] Compared with NiCo2O4NSs, the binding energy of O 1s XPS spectra for Ru-NiCo2O4NSs positively shifts ∼0.2 eV, exhibiting a slight electron-deficiency O state.Furthermore, the binding energy of Ru 3p3/2in Ru-NiCo2O4NSs is 463.7 eV (Fig.2b), which is located between the reference samples RuCl3(Ru3+) and RuO2(Ru4+), implying that the average valence state of Ru is between +3 and +4 in Ru-NiCo2O4NSs [27,38].Besides, there is no distinct difference between NiCo2O4NSs and Ru-NiCo2O4NSs concerning Co and Ni 2p XPS spectra, affirming the preservation of NiCo2O4phase after Ru modification (Fig.S3 in Supporting information).In addition,the UPS was conducted to probe dynamics of electrons on the surface of the as-prepared catalysts [39].As shown in Fig.2c, the Ru-NiCo2O4NSs and NiCo2O4NSs exhibit different secondary electron cutoff energy with 16.34 eV for Ru-NiCo2O4NSs and 16.22 eV for NiCo2O4NSs and thus the corresponding work function are 4.88 eV and 5.00 eV for Ru-NiCo2O4NSs and NiCo2O4NSs, respectively.The smaller work function indicates that Ru modulation facilitates the electronic properties of Ru-NiCo2O4NSs, which is contributed to enhancing catalytic activity [40].Besides, Raman as a surfacesensitive spectrum technology was further applied to gain insights into NiCo2O4NSs before and after Ru modification.As shown in Fig.2d, the Ru-NiCo2O4NSs showcases similar spectra patterns with the pristine NiCo2O4NSs except for a slight blue shift, which is due to the doping of Ru.Furthermore, the Raman spectra of Ru-NiCo2O4NSs exhibit different spectra feature compared with RuO2(Fig.S4 in Supporting information), which implies the Ru may exist as a highly dispersed state in NiCo2O4NSs substrate and further indicating that Ru are doped into NiCo2O4NSs successfully.Moreover, ESR spectra were conducted to investigate the change of oxygen vacancy.From Fig.2e, it can be seen that Ru-NiCo2O4NSs owns a stronger ESR signal originating from the electron trapped at oxygen vacancies than that of NiCo2O4counterpart [41,42].Therefore, we can deduce that the Ru-NiCo2O4NSs possess more oxygen vacancies than NiCo2O4NSs, which is helpful to facilitate water dissociation [43].Furthermore, the excellent hydrophilicity feature of Ru-NiCo2O4NSs supported on CFC enables the easy accessibility of reactants and fast release of product molecules, thus accelerating catalytic process (Fig.2f, the inset shows the contact angle of bare CFC) [44].
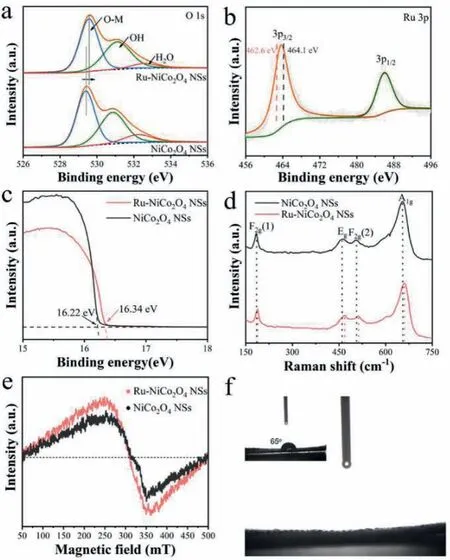
Fig.2 .(a) O 1s spectra of Ru-NiCo2O4 NSs and NiCo2O4 NSs.(b) Ru 3p spectra of Ru-NiCo2O4 NSs.(c) UPS spectra (d) Raman spectra and (e) ESR spectra of Ru-NiCo2O4 NSs and NiCo2O4 NSs.(f) Contact angle of water droplets on the surface of Ru-NiCo2O4 NSs and bare CFC (inset).
The electrocatalytic performance of catalysts prepared was evaluated by a standard three-electrode configuration using 1.0 mol/L KOH as electrolyte.Linear scan voltammogram (LSV) curves of OER(Fig.3a) show that the required overpotentials for Ru-NiCo2O4NSs, NiCo2O4NSs and RuO2to reach the current density of 10 mA/cm2are 230 mV, 340 mV and 329 mV, respectively, manifesting excellent OER activity of Ru-NiCo2O4NSs.Furthermore, the Fig.3b illustrates Ru-NiCo2O4NSs possesses a small Tafel slope(79 mV/dec) lower than that of NiCo2O4NSs (95 mV/dec) and RuO2(82 mV/dec) counterparts, indicating the accelerated OER kinetic for Ru-NiCo2O4NSs.Besides, the stability is also a vital aspect for assessing the performance of catalysts.From Fig.3g (green line), it can be seen that Ru-NiCo2O4NSs show nearly no activity decay in the period of 42 h OER test.To gain the insights about the origin of activity, the electrochemical impedance spectroscopy(EIS) of Ru-NiCo2O4NSs and NiCo2O4NSs were collected to investigate charge transfer ability.As shown in Fig.3c, Ru-NiCo2O4NSs exhibits a smaller radius than that of NiCo2O4NSs counterpart,implying a lower charge transfer (Rct) impedance of Ru-NiCo2O4NSs for OER process, which is conducive to improving OER performance [45].In addition, electrochemical double-layer capacitance(Cdl) was tested to access the electrochemical surface area (ECSA)of the as-prepared catalysts (Fig.S5 in Supporting information)[46].The value of Cdlfor Ru-NiCo2O4NSs and NiCo2O4NSs are 278 mF/cm2and 200 mF/cm2(Fig.3h), respectively, suggesting the larger ECSA of Ru-NiCo2O4NSs.The larger ECSA manifests more accessible active sites on Ru-NiCo2O4NSs surface in catalytic process, thus boosting OER performance.Moreover, LSV curves at various temperature for Ru-NiCo2O4NSs and NiCo2O4NSs were analyzed to evaluate apparent kinetic barriers in OER process.Extended the obtained Arrhenius plots to the OER thermodynamic equilibrium potential, the value of apparent activation energy (Ea)can be obtained [47,48].As illustrated in Fig.S6 (Supporting information), the value ofEaof Ru-NiCo2O4NSs is 26.6 kJ/mol while theEaof NiCo2O4NSs is 45.9 kJ/mol, implying Ru modification availably decreaseEafor OER course.
Due to the similar Ru-H bond energy to Pt-H bond energy [22],the HER performance of NiCo2O4NSs after Ru modification may also be improved.As shown in LSV curves of HER (Fig.3d), an overpotential of 313 mV is needed for NiCo2O4NSs to reach current density of 10 mA/cm2, while the value decreases to 117 mV for Ru-NiCo2O4NSs.The Tafel slopes are analyzed to be 88 mV/dec and 97 mV/dec for Ru-NiCo2O4NSs and NiCo2O4NSs, respectively(Fig.3e), declaring a more favorable HER kinetics for Ru-NiCo2O4NSs.Regarding long-time stability, the chronopotentiometry test shows no obvious activity degradation of Ru-NiCo2O4NSs after 42 h (Fig.3g, blue line).Furthermore, EIS plots were collected to assess charge transfer feature of materials between electrode and electrolyte interface.As illustrated in Fig.3f, the Ru-NiCo2O4NSs possess a smallerRctthan that of NiCo2O4NSs, manifesting that Ru modification accelerates charge transfer in HER process, thus promoting HER activity.Fig.3i displays a largerCdlfor Ru-NiCo2O4NSs (21.3 mF/cm2) than NiCo2O4NSs (2.2 mF/cm2) based on CVs test (Fig.S7 in Supporting information), thereby a larger ECSA of Ru-NiCo2O4NSs for HER catalysis, which is also favorable to promote HER performance.
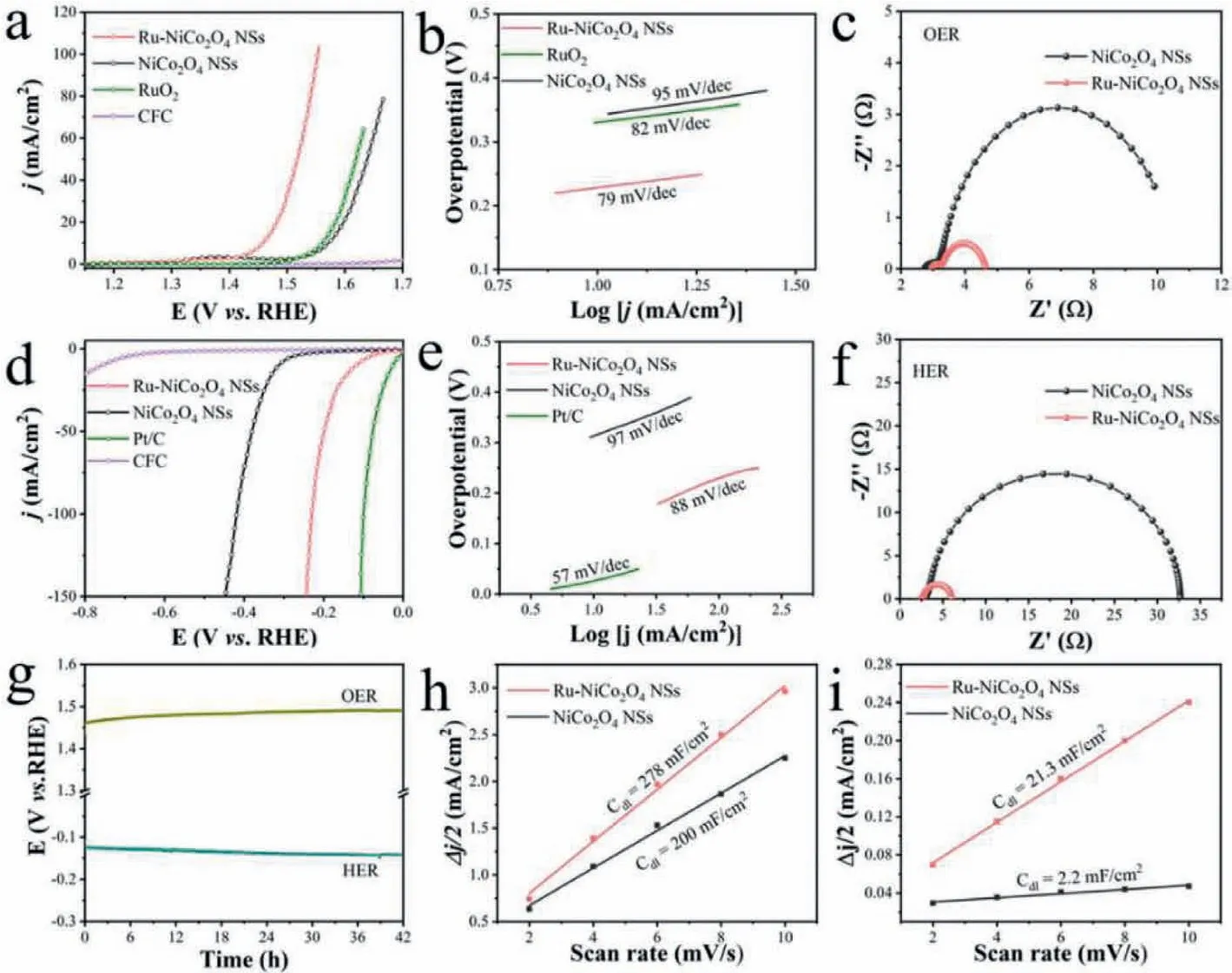
Fig.3 .Electrochemical performance for OER and HER.(a) LSV curves and (b) the corresponding Tafel plots of Ru-NiCo2O4 NSs, NiCo2O4 NSs and RuO2 for OER., (c) EIS of Ru-NiCo2O4 NSs and NiCo2O4 NSs for OER.(d) LSV curves and (e) the corresponding Tafel plots of Ru-NiCo2O4 NSs, NiCo2O4 NSs and Pt/C for HER.(f) EIS of Ru-NiCo2O4 NSs and NiCo2O4 NSs for HER.(g) Chronopotentiometry test of Ru-NiCo2O4 NSs for OER and HER at 10 mA/cm2.(h, i) Capacitive currents of Ru-NiCo2O4 NSs and NiCo2O4 NSs with different scan rates for OER and HER, respectively.
In situRaman spectra characterization analysis was performed to reveal changes on Ru-NiCo2O4NSs electrodes during the OER and HER processes.The peak located at ∼470 cm−1and ∼670 cm−1are attributed toEgphonon modes andA1gphonon modes of Ru-NiCo2O4NSs, respectively (Fig.4).Potentials from open circuit voltage (OCV) to 1.6 Vvs.RHE were applied for OER in suit Raman measurements.The corresponding spectra result are shown in Fig.4a, characteristic peaks associated withEgandA1gphonon modes exhibits no differentiable variation, implying good stability of Ru-NiCo2O4NSs in OER process.For HER, we applied potential from OCV to −0.25 Vvs.RHE.The corresponding spectra (Fig.4b)obtained also show no obvious change, manifesting good HER stability of Ru-NiCo2O4NSs.Besides, SEM characterization was conducted to probe the change of morphology of Ru-NiCo2O4NSs after OER and HER.As shown in Fig.S8 (Supporting information),the morphology of Ru-NiCo2O4NSs after OER and HER remains nanosheets structure.All evidences illustrate the good stability of Ru-NiCo2O4NSs.
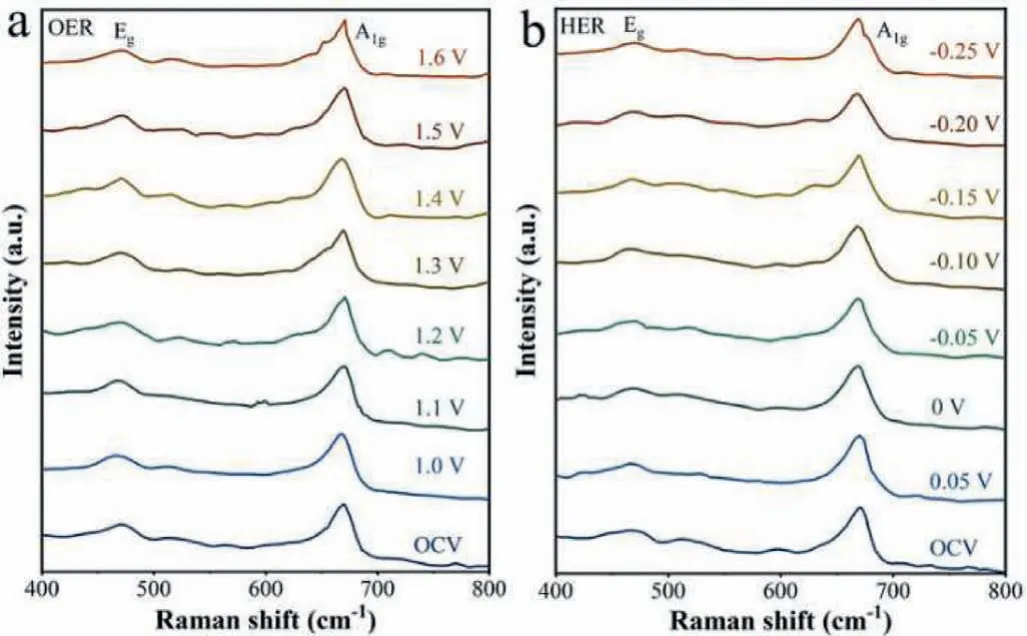
Fig.4 . In situ Raman spectra of Ru-NiCo2O4 NSs during (a) OER and (b) HER.
Based on the enhanced OER and HER performance of Ru-NiCo2O4NSs, the overall water-splitting performance was assessed by utilizing Ru-NiCo2O4NSs as both anode and cathode catalysts in 1.0 mol/L KOH solution.Fig.5a illustrates the configuration of overall water splitting device.Fig.5b showcases the LSV curves of Ru-NiCo2O4NSs for the overall water splitting at various temperatures(25, 45 and 65 °C).A cell voltage of 1.60 V was needed to reach the current density of 10 mA/cm2at 25 °C for Ru-NiCo2O4NSs couple, which is lower than that of Pt/C||RuO2set (1.64 V) (Fig.5c).The overall water splitting performance of Ru-NiCo2O4NSs system can be further improve by increasing temperature.As exhibited in Fig.5d, cell voltages needed to obtain the current density of 10 mA/cm2at 45 °C and 65 °C are 1.54 V and 1.49 V, respectively.In addition, Ru-NiCo2O4NSs set displays good stability on a longtime testing at different current densities 10, 20, 40, 80 mA/cm2(Fig.5e), while for Pt/C||RuO2system, the cell voltages enlarge constantly over time (Fig.5f).All the results verify Ru-NiCo2O4NSs can be used as efficient overall water splitting catalysts.
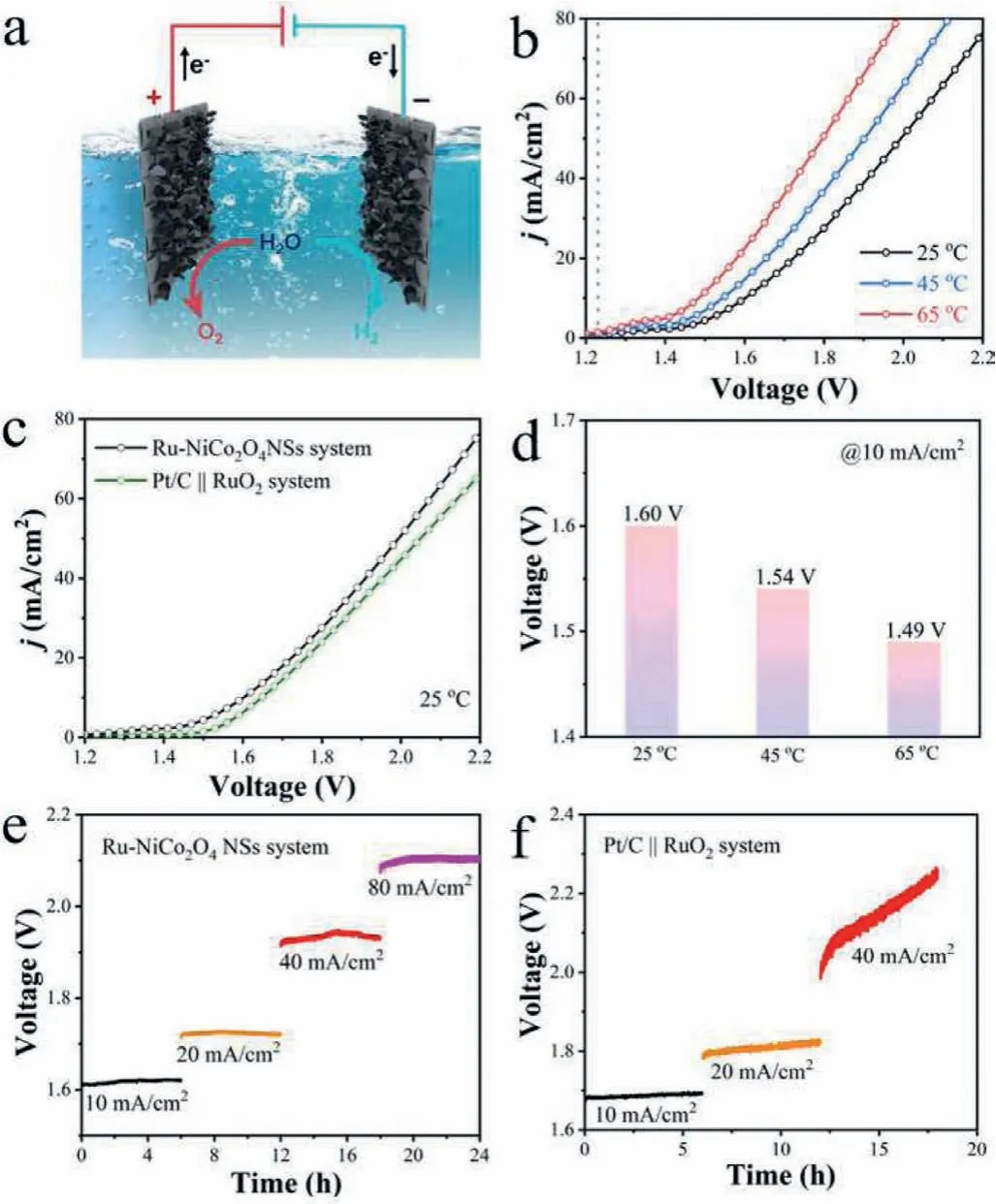
Fig.5 .(a) Schematic illustration of the configuration of alkaline overall water splitting device.(b) LSV curves for overall water splitting applying Ru-NiCo2O4 NSs as both cathode and anode at different temperatures.(c) LSV polarization curves of Ru-NiCo2O4 NSs system and Pt/C||RuO2 system for overall water splitting at 25 °C.(d) Comparison of the cell voltages to reach the current density of 10 mA/cm2 of Ru-NiCo2O4 NSs system at various temperatures., (e, f) Long-term stability test of overall water splitting at various current density of 10, 20, 40 and 80 mA/cm2 for Ru-NiCo2O4 NSs system and Pt/C||RuO2 system, respectively.
In summary, the porous NiCo2O4NSs integrated with Ru through a facile impregnation method is devised.The obtained Ru-NiCo2O4NSs catalysts exhibit superior OER activity with a low overpotential of 230 mV at 10 mA/cm2, the enhanced HER activity and robust stability.The promotion of catalytic performance of Ru-NiCo2O4NSs can be attributed to the low charge transfer impedance, more accessible active sites and higher oxygen vacancy concentration.Experimental results demonstrate that the introduction of Ru efficiently regulates the surface structure property of NiCo2O4NSs.The optimized components and structural advantages facilitate synergistically OER and HER process.This work presents a practicable strategy for improving the catalytic performance of spine-based oxides for both OER and HER.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge support from the National Natural Science Foundation of China (Nos.21922105 and 21931001), the National Key R&D Program of China (2021YFA1501101) as well as the Special Fund Project of Guiding Scientific and Technological Innovation Development of Gansu Province (No.2019ZX-04) and the 111 Project(No.B20027).We also acknowledge support by the Fundamental Research Funds for the Central Universities (Nos.lzujbky-2021-pd04, lzujbky-2021-sp41, lzujbky-2021-it12 and lzujbky-2021-37).J.Yin acknowledges the support of the China Postdoctoral Science Foundation (No.2021M691375) and the China National Postdoctoral Program for Innovative Talents (No.BX20200157).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.058.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
