Optically probing molecular shuttling motion of [2]rotaxane by a conformation-adaptive fluorophore
Chengyun Yu, Xiodong Wng, Ci-Xin Zho, Shun Yng, Jin Gn, Zhuo Wng,Zhnqi Co, D-Hui Qu,∗
a Key Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China
bShanghai Gantian Optical Material Co., Ltd., Shanghai 201512, China
c College of Science, Henan Agricultural University, Zhengzhou 450002, China
Keywords:[2]Rotaxane Optically probe Mechanical shuttling Molecular conformations Conformation-adaptive macrocycle
ABSTRACT A bistable [2]rotaxane with a conformation-adaptive macrocycle bearing a 9,14-diphenyl-9,14-dihydrodibenzo[a,c]phenazine (DPAC) unit was synthesized, which could be utilized to optical probe the molecular shuttling motion of the functionalized rotaxane system.The UV–vis, 1H NMR and PL spectroscopic data clearly demonstrated that the DPAC ring was interlocked onto the thread and the fluorescence intensity of the DPAC unit in the macrocycle was effectively regulated by the location change of the macrocycle along the thread under acid/base stimulation, which was attributed to the modulation of the intramolecular photo-induced electron transfer between the DPAC unit and the methyltriazole (MTA)unit.This bistable rotaxane system containing a conformation-adaptive fluorophore unit in the macrocycle moiety opens an alternative way to design functional bistable mechanically interlocked molecules.
Since the first introduction of supramolecular chemistry [1,2],macrocyclic molecules [3] as an important branch have gained particular attention and developed numerous potential applications in various fields.Macrocycles are often structured with a special ring motif, such as crown ether [4], in which these cyclic motifs could be efficiently interacted with guest molecules.The crown ether ring embellished with fluorophores has been introduced into mechanical interlocking molecules (MIMs) [5] to construct smart fluorescence systems, which have been widely adopted in photoelectric switches [6,7], molecular recognition [8], fluorescent probes[9,10], drug delivery [11] and smart materials [12–15].A typical MIMs system consists of a macrocyclic host structure, such as a rotaxane, which a rod-shaped guest axle and two big blocking groups to prevent the separation of the ring components.Among the many macrocycle-based MIMs, one of the most common types is the rotaxane that could produce luminescence upon different stimulus (acid/base [16,17], ion binding [18,19], light [20,21] or oxidation state change [22]).The fluorescent signal was proven to be an efficient approach to monitor the ring’s mechanical movement,taking advantages of the Förster resonance energy transfer (FRET),photo-induced electron transfer (PET) mechanism.Meanwhile, the mechanical shuttling motion of the host molecule can also be effectively monitored and even regulated based on its fluorescence performance [23].Nevertheless, more efforts are necessary for developing new fluorescent macrocyclic systems, in order to widen the range of the potential application for current supramolecular macrocycles.
A single-molecule conformation-adaptive fluorophore based on 9,14-dihydrodibenzo[a,c]phenazine derivatives (DPAC) [24] has been reported, which has uniquely large Stokes shift and multiple fluorescence properties and been used for fluorescent probes [25],bioimaging [26].Recently, Tianet al.realized geometric changes and inversions from bending to plane in the exciting process, using chemical approaches, such as intramolecular cyclization [27] and substituent effects [28], both which can precisely tune the fluorescence properties for the dihydrophenazine molecules.Apart from these, we also reported a [2]catenane consisting of a DPAC-based fluorescent macrocycle [29], in which the conformation-adaptive emission of DPAC ring was precisely controlled in the interlocked environment.Thus, it is possible to apply the single fluorophore in fluorescent switches to regulate the multi-color or intensity emission.Therefore, this controllable fluorescence property of the DPAC-ring would be useful for developing MIMs’applications.
Herein, we report a unique DPAC-crown ether ring-modified[2]rotaxane system, a dedicatedly designed optical probe that could be precisely regulated by the unique dynamic adaptive mechanism of the fluorescence unit.As shown in Fig.1, the target [2]rotaxane 2-H·2PF6contained a DPAC-functional crown ether macrocyclic component and two large blocking groups at both ends to prevent the departure of cyclic components.There were two structured blocking units on the rod-shaped component, that was dibenzylammonium (DBA) andN-methyltriazolium (MTA), as the binding sites of the ring.The experimental results suggested that the DPAC ring could recognize the binding sites based on the different binding affinity of the stations towards the ring upon stimuli-response.The conformation freedom of the DPAC ring varied with the recognition sites (i.e., at different recognition sites, diverse sized guest molecules could bind to the host ring), resulting in the change of the fluorescence (wavelength and intensity).This supramolecular host macrocyclic structure with vibrationintroduced emission characteristics can optically monitor different guest molecules through the conformation freedom of the vibration motion of the DPAC’s aromatic backbone.The phenomena provide a good strategy for developing advanced supramolecular system and designing functional bistable mechanically interlocked molecules.
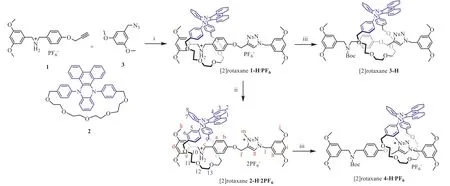
Fig.1 .The synthesis routes of the [2]rotaxanes.Reaction conditions: (i) Cu(CH3CN)4PF6 (1.1 equiv.), anhydrous dichloromethane, 25°C for 4 h; (ii) CH3I (6.0 equiv.), NH4PF6,methanol, 40°C for 36 h; (iii) Et3N (5.0 equiv.), (Boc)2O (5.0 equiv.), dichloromethane, Ar, 25°C for 12 h.
To synthesize the target [2]rotaxane 2-H·2PF6, we started with the one-step synthesis of the [2]rotaxane 1-H·PF6from the known compounds (Fig.1, Supporting information for details).The compound [2]rotaxane 2-H·2PF6was obtained from the methylation of the triazole unit of [2]rotaxane 1-H·PF6.This was confirmed by the characteristic chemical shifts of the methyl hydrogen and Hg(Fig.1) on methyltriazole (MTA) according to the1H NMR.Meantime, a significant displacement of the characteristic protons H9–13on the ring and Ha, Hbon the axle was observed.Two-dimensional1H NOE signals were found between NH2+and hydrogens (H11,H12, H13) on the DPAC ring (Figs.S10-S12 in Supporting information), which confirmed that: 1) the correlation between the ring and axle; 2) the DPAC-ring well fit at the dibenzylammonium (DBA) recognition site.Additionally, the high-resolution ESIMS spectrum of [2]rotaxane 2-H·2PF6showed an intense peak atm/z1317.5280, which was assigned to the [2]rotaxane 2-H·2PF6after losing one of the PF6counterions.
The DBA salt in [2]rotaxane 2-H·2PF6was deprotonated and protected with di–tert–butyl dicarbonate ((Boc)2O), then a reference [2]rotaxane 4-H·PF6was therefore obtained.In COZY and NOESY NMR spectra (Figs.S16-S18 in Supporting information),there were the appearance of new cross peaks (representing the three characteristic protons H6and Hl, Hf).It proved that the DPAC-ring was located on MTA station away from the Bocprotected DBA recognition site, and the signal of characteristic hydrogen Hfupshifted due to the shielding effect of the crown ether.By using a similar synthesis method of [2]rotaxane 4-H·PF6,another reference compound [2]rotaxane 3-H was obtained from[2]rotaxane 1-H·PF6.The structures of these compounds were further confirmed by mass spectrometry (Figs.S28-S31 in Supporting information).
Then the intrinsic optical properties of the [2]rotaxane was particularly characterized (Fig.2).A absorption band with a maximum wavelength (λabs) at 345 nm was observed for [2]rotaxane 2-H·2PF6.Comparing with the free DPAC ring [29], the maximum absorption of [2]rotaxane 2-H·2PF6exhibited a faintly blueshifted, which was similar with that of [2]rotaxane 1-H·PF6in dichloromethane solution (Fig.2a).The slight difference between the free DPAC ring and [2]rotaxane 2-H·2PF6mainly resulted from the deplanarization of the aromatic backbone and the enhancement of DPAC ring conformation tension after binding the DBA site in [2]rotaxane 2-H·2PF6.Similar phenomenon in which the macrocycles with distinctly shifted optical properties before and after the host-guest recognition have been reported before [30].Interestingly, the fluorescence quantum yields of the two [2]rotaxanes were greatly different and the emission intensity of [2]rotaxane 2-H·2PF6was 5% less than that of [2]rotaxane 1-H·PF6.It was believed that the cation dipole interaction between the crown ether ring and the positive charge of MTA resulted in the decrease of the fluorescence quantum yield [31].Meanwhile, it was found that the DPAC ring could be a good optical monitor when the subtle chemical environment was changed.In blank experiments, [2]rotaxane 3-H (λabs=362 nm) showed a larger red-shifted absorption because the conformation of the DPAC ring was free with no recognition sites.And the absorption and emission peak of [2]rotaxane 4-H·PF6was consistent with the [2]rotaxane 2-H·2PF6, the latter was comparatively larger in the degree of blue-shift.Since the binding ability of crown ether and MTA was weaker than that of the crown ether and DBA group, the aromatic skeleton planarization of the DPAC ring was relatively smaller when the ring was constrained by surrounded glycol chains.In addition, the luminescence almost quenched owing to the intramolecular photo-induced electron transfer effect (PET) between the electron-positive DPAC chromophore and electron-deficient MTA molecule, indicating that the DPAC ring can be used as a conformational adaptive fluorophore optically detecting the molecular shuttle motion of [2]rotaxane.
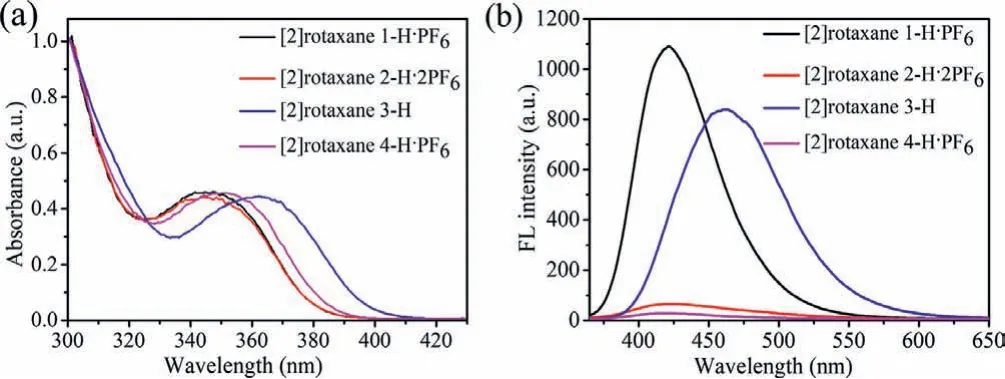
Fig.2 .The photophysical properties of [2]rotaxanes.UV–vis absorption spectra(a) and fluorescence spectra (b) of target [2]rotaxanes in dichloromethane (pathlength=10 mm, c=40 μmol/L, λex=350 nm).
Subsequently, the reversible shuttling movement of target [2]rotaxane 2-H·2PF6and the changes of photophysical properties of the DPAC-ring were investigated by1H NMR, UV–vis and PL spectroscopy under acid-base stimuli.Upon the addition of 1.2 equiv.1,8-diazabicyclo[5.4.0]undec–7-ene (DBU) to neutralize the DBA group of [2]rotaxane 2-H·2PF6in dichloromethane, the characteristic hydrogens for Hg, Hl, Hf, Hmwere shifted upfield significantly(Δδg=0.46, 0.4 ppm; Δδl=0.61, 0.59 ppm; Δδf=0.54, 0.98 ppm;Δδm=0.85 ppm) (Figs.3a and b), implying that the shielding effect of the DPAC ring was forced to slip to the MTA station away from the DBA recognition site.According to the literature upfield shifts within different degrees and the disappearance of the NH2+signal in 2D NMR spectroscopy (Figs.S12 in Supporting informaiton)suggested that the formation of hydrogen bonds between the MTA station and the macrocycle.Furthermore, the deprotonated [2]rotaxane 2·PF6was again mixed with 1.2 equiv.of trifluoroacetate(TFA), the correlation of the [2]rotaxane 2-H·2PF6and with the DPAC-ring at DBA site was reformed again, leading to the chemical peak returned to its initial position (Fig.3c).Therefore, the molecular shuttling motion of [2]rotaxane 2-H·2PF6can be easily realized between the DBA and MTA recognition sites upon acid or base conditions.
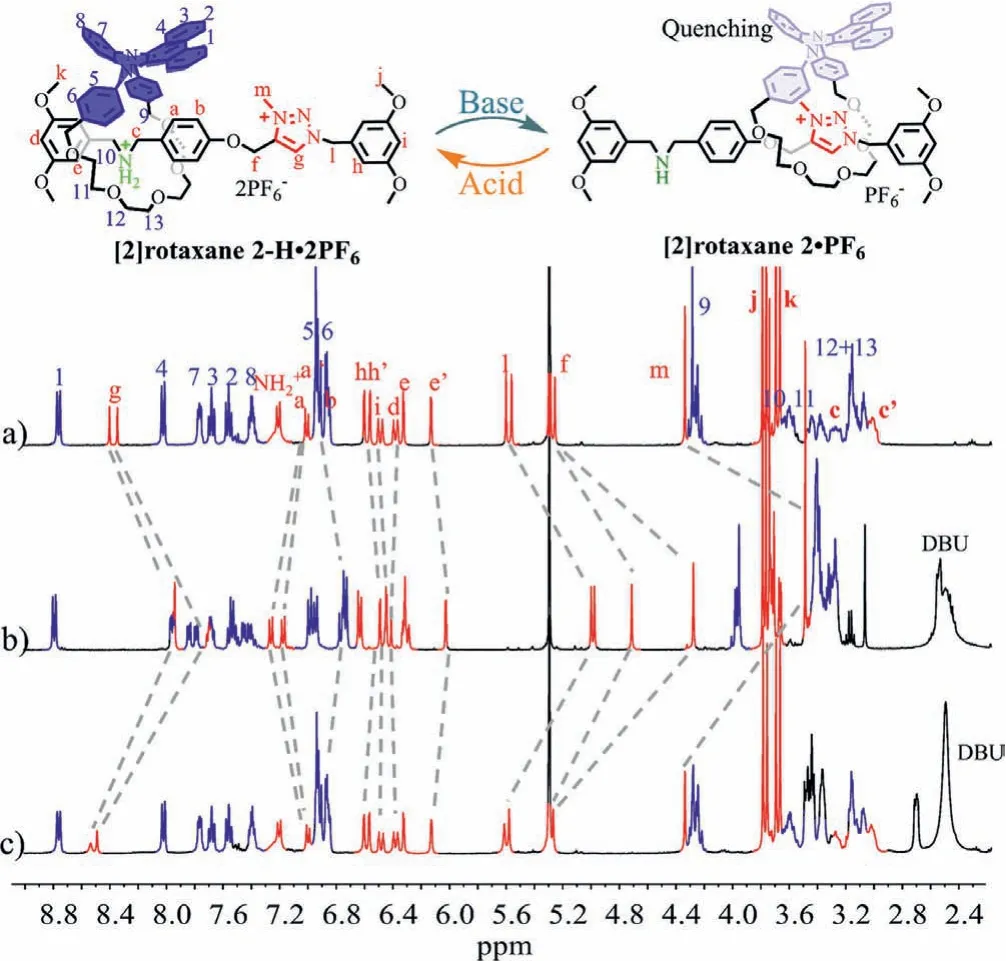
Fig.3 .(a) Partial 1H NMR (400 MHz, CD2Cl2, 298 K) spectra of [2]rotaxane 2-H·2PF6, (b) [2]rotaxane 2-H·2PF6 after adding 1.2 equiv.DBU, (c) [2]rotaxane 2-H·2PF6+1.2 equiv.DBU after adding 1.2 equiv.TFA.
Then, the changes of the optical properties of the shuttling motion [2]rotaxane 2-H·2PF6was further investigated.Under base treatment, the absorption of the deprotonated [2]rotaxane 2·PF6showed a slight red-shift and a maximum peak (λabs=351 nm)(Fig.4).Interestingly, a fluorescence quenching effect accompanied with a weak blue-shift was observed.The weaker hydrogen bonds between crown ether and MTA released the conformation tension of the DPAC, the DPAC ring was prone to planarization on the excited state, resulting in the fluorescence emission blueshift.Since the MTA structure was an electron-deficient acceptor and easily to generate cation-dipole interaction, when the ring located at MTA recognition site, strong intramolecular PET effect occurred, which caused the fluorescence quenching.The similar phenomenon was also observed in the reference compound [2]rotaxane 4-H·PF6(λabs/λem=350/421 nm in dichloromethane), which further confirm us demonstration, that the DPAC ring had slipped at toward the triazole MTA from the DBA recognition site, and the fluorescence quenching was closely related to the triazole MTA group.After addition of TFA into the deprotonated [2]rotaxane 2·PF6, the NH group of DBA site reprotonated and the macrocyclic returned to its original location.In the current state, the DPAC ring adopted a deplanarized conformation due to strong hydrogen bonds and the intramolecular PET phenomenon disappears, resulting in the reversion of the absorption and fluorescence.These obvious fluorescence changes indicated that the DPAC-functionalized ring could be used as an optical probe to monitor the molecule reversibly shuttle among the DBA and MTA recognition sites.
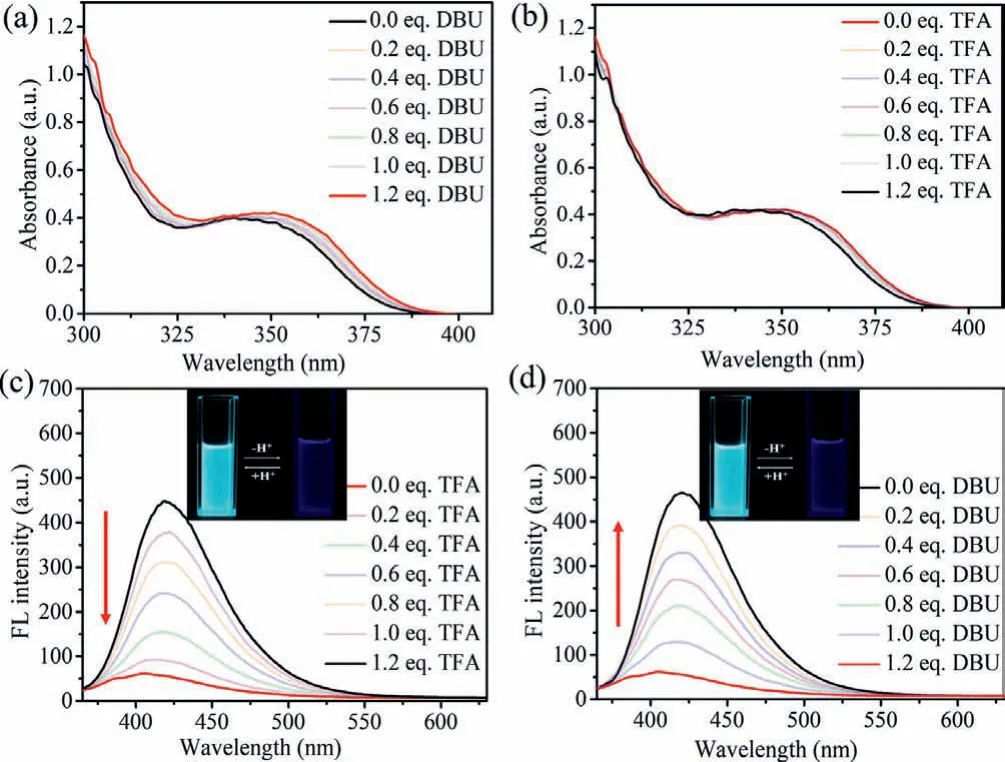
Fig.4 .The photophysical characteristics of [2]rotaxane 2-H·2PF6 driven by acidbase in dichloromethane.UV–vis absorption spectra (a) and fluorescence spectra(c) of [2]rotaxane 2-H·2PF6 titrated with 1.2 equiv.DBU.UV–vis absorption spectra(b) and fluorescence spectra (d) of [2]rotaxane 2-H·2PF6+1.2 equiv.DBU and then adding 1.2 equiv.TFA (pathlength=10 mm, c=80 μmol/L, λex=350 nm).
Furthermore, in order to verify our hypothesis, addition excessive base or acid to the dichloromethane solution of [2]rotaxane 4-H·PF6, it was found that there was no change of the UV–vis absorption, fluorescence emission or1H NMR (Figs.S3 and S6 in Supporting information).Because the DPAC ring has been recognized on the MTA site and the conformation was fixed.And the fluorescence emission of [2]rotaxane 4-H·PF6was always quenched,which was consistent with the phenomenon of [2]rotaxane 2-H·2PF6driven by the base.For [2]rotaxane 3-H, after adding 1.2 equiv.TFA, the absorption of [2]rotaxane 3 exhibited a blue-shift,but its fluorescence intensity was quenched (Fig.S2 in Supporting information).This may be attributed to the intramolecular PET effect between electron-rich donor DPAC chromophore and the formation of electron-deficient triazole groups in an acidic condition.If an equivalent amount of DBU was added in slowly, its absorption and emission spectra (wavelength and intensity) returned to its original state.
Therefore, except for [2]rotaxane 4-H·PF6, [2]rotaxane 2-H·2PF6, [2]rotaxane 1-H·PF6and [2]rotaxane 3-H all achieved reversible fluorescence wavelength or intensity switching upon acid or base stimulation.The key feature of the [2]rotaxane 2-H·2PF6was that the conformation-adaptive macrocycle leading the functionalized rotaxane to undergo optically probe molecular shuttling motions by addition acid or base.
In summary, we have successfully synthesized [2]rotaxane 2-H·2PF6and three reference compounds ([2]rotaxane 1-H·PF6,[2]rotaxane 3-H, [2]rotaxane 4-H·PF6) based on conformationadaptive macrocycle DPAC.The DPAC ring was proved to shuttle between the recognition sites with different binding abilities, leading to the discrepancy of conformational distortion and fluorescence properties of the DPAC molecule.The results were found that the intrinsic luminescence of the DPAC molecule was restricted by the unique dynamic adaptive mechanism and the fluorophore quenching of DPAC was closely related to the intramolecular PET effect.Therefore, the DPAC chromophore can be used as a signal output to monitor the molecular shuttling motion and a new main fluorescence macrocycle on MIMs.In addition, [2]rotaxane 1-H·PF6, [2]rotaxane 2-H·2PF6can be used as fluorescent molecular probes with excellent performance under acid-base conditions,which provides a new inspiration for the simple design of a multimode stimulating fluorescent output system.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.22025503, 21790361 and 21871084),Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX03), the Fundamental Research Funds for the Central Universities, the Program of Introducing Talents of Discipline to Universities (No.B16017), the Shanghai Science and Technology Committee (No.17520750100).This project has received funding from China Postdoctoral Science Foundation funded project (No.J100–5R-20130).We thank the Research Center of Analysis and Test of East China University of Science and Technology for help on the material characterization.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.004.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
