Selective recognition of methyl viologen by an endo-functionalized naphthobox
Weier Liu, Linghui Kong, Mo Qun, Hun Yo, Liupn Yng,∗, Ho Yu Au-Yeung,∗,Wei Jing,∗
a Guangdong Provincial Key Laboratory of Catalysis and Department of Chemistry, Shenzhen Grubbs Institute, Southern University of Science and Technology, Shenzhen 518055, China
b Department of Chemistry, The University of Hong Kong, Hong Kong, China
Keywords:Molecular recognition Host-guest chemistry Endo-functionalized cavity Hydrogen bond Naphthobox
ABSTRACT Highly selective binding of structurally similar substrates is common for biomolecular recognition, but is often challenging to realize in synthetic hosts.Herein, we report highly selective binding of methyl viologen over other analogues by an endo-functionalized naphthobox.X-ray single crystal structure and Density Functional Theory (DFT) calculations revealed that the endo-functionalized groups in the cavity of the naphthobox is important for the high binding selectivity through the formation of multiple C–H∙∙∙N,C–H∙∙∙π, and π∙∙∙π interactions with methyl viologen.
Biological receptors can selectively recognize specific substrate and distinguish minor differences of substituent groups, which lays a foundation on a variety of biological function.These natural receptors usually feature a deep cavity with inward-pointing functional groups which are essential for the high binding selectivity and/or affinity [1].One well-known example is the unerring precision of substrate specificity of enzymes [2], such as urease which catalyzes only the hydrolysis of urea to NH3and CO2, but cannot catalyze the hydrolysis of methyl urea [3].High selectivity is also a most important goal of the synthetic host [4].However, for synthetic hosts, it is often challenging to achieve highly selective molecular recognition of guest molecules with similar structures[5].In order to distinguish the guests with minor structural differences, for example, with the differences in only one atom or one group, it would require directional noncovalent interactions, for example, hydrogen bonding, to be involved together with a rigid cavity.Nevertheless, it is still claimed to be difficult to construct a rigid cavity with functional groups pointing inwards [6].
Molecular boxes such as “blue box” [7–9], “Texas-sized molecular box” [10,11] and others [12–16] are one class of box-like hosts which possess rigid structures and well-defined cavities.They have relatively good guest-binding properties and have a wide range of applications in supramolecular chemistry and related areas [7,10–15].However, their binding selectivity is often not high for structurally similar guests, which may be due to the lack of functional groups inside the deep cavity.
Over the past years, we reported several naphthol-based macrocyclic hosts [17,18].Among them,endo-functionalized naphthotubes [19–26] were found to be really effective in the selective recognition of organic molecules due to the inward-directed functional groups.Recently, we have reported a naphthobox consisting ofπ-electron-rich naphthalene subunits, which is able to recognize planar aromatic cations [27].However, the binding selectivity is not high.We anticipated that the binding selectivity of naphthobox may be greatly improved when functional groups are appropriately introduced in their internal cavities.This may be realized by the naphthobox 1 (Fig.1a) with inward-pointing nitrogen atoms from naphthyridine pillars which was reported by Katz and co-workers [28].This naphthobox has been used to recognize neutral aromatic compounds, such as toluene, benzonitrile, phenol, benzoic acid, ando-salicylic acid.The association constant too-salicylic acid is only 306 L/mol (dichloromethane), and binding affinities for other guests have not been reported, and the role of theendo-functionalized groups has not been clearly demonstrated.In this research, we report the highly selective binding of thisendo-functionalized naphthobox to methyl viologen over other analogues through involvement of theendo-functionalized groups.
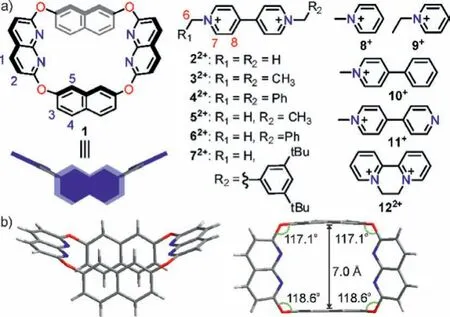
Fig.1 .(a) Chemical structures and cartoons of naphthobox and methyl viologen and several pyridinium derivatives.(b) X-ray single crystal structure of 1.
The crystal structure (Fig.1b) of naphthobox 1 reveals the following properties of this naphthobox: a) it has a relatively rigid and well pre-organized structure consisting of two naphthyl groups and two naphthyridinyl groups bonded by four ether linkages; b)it has anendo-functionalized structure with two naphthalene walls and four inward directing nitrogen atoms, which could formπ-πstacking and C–H∙∙∙N hydrogen bonding with guest molecules; c)the two electron-rich naphthalene walls are co-planar and the distance between them is 7 ˚A, thus the cavity is suitable for planar organic cations such as viologen derivatives.
Initially, the host-guest chemistry of naphthobox 1 and a series of viologen derivatives were studied by1H NMR spectroscopy.The noncoordinating tetrakis[3,5-bis(trifluoromethyl)phenyl]-borate anion (BArF−) is used as the counterion [29].A mixed solvent of CDCl3/CD3CN (1:1, v/v) was chosen to perform1H NMR experiment to ensure both the host and guest are fully dissolved.Indeed, for all the studied guests, obvious complexation-induced shifts of NMR signals on both host and the guests were observed (Fig.2 and Figs.S1–S8 in Supporting information), indicating that all these guests can be encapsulated in the cavity of 1.However, careful analysis indicates that the binding modes are quite different for different guests.Taking the complex with methyl viologen 22+as an example (Fig.2b), the1H NMR spectra of a 1:1 mixture of 1 and 22+show that the proton signals of 6 and 8 on 22+and naphthalene protons (3 and 4) of the host are upfield shifted (Δδ: 0.22 to 0.32 ppm), indicating 22+is encapsulated in the cavity of host 1, and both 22+and 1 experience the mutual anisotropic shielding effect of each other’sπelectron clouds.Moreover, the downfield shifts observed for the proton signals of 7 on 22+and naphthyridine rings (1 and 2) of the host suggests the formation of C–H∙∙∙N hydrogen bonds between the naphthyridinyl groups of 1 and 22+.Surprisingly, a slightly altering of theN-substituent of the guest is found to have a great influence on the binding behavior.For example, the binding of ethyl viologen 32+to 1 has resulted in only very tiny shifts (Δδ: 0.01 to 0.02 ppm) of the protons of 32+(Fig.2d).Similar phenomena were also observed for benzyl viologen 42+.This indicates that 1 has a rather high binding selectivity toward methyl viologen over similar analogues.
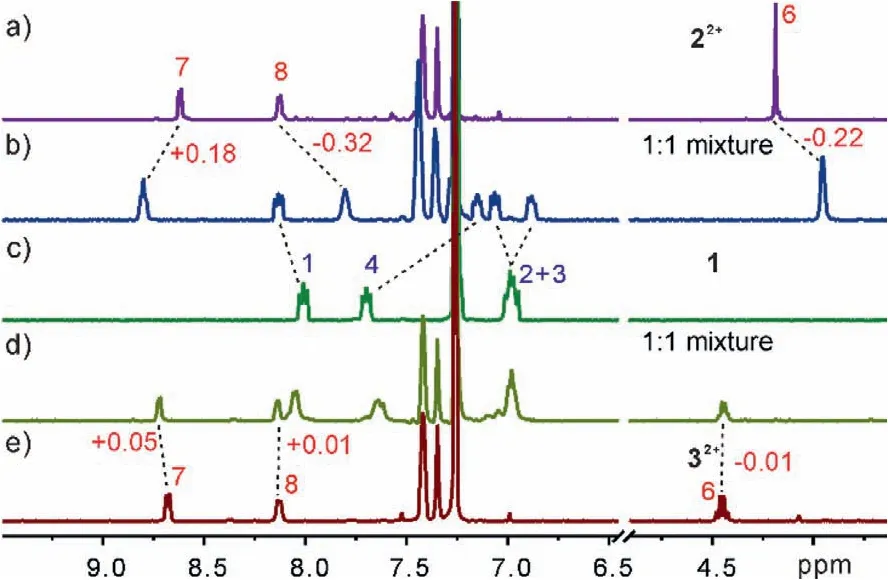
Fig.2 .Partial 1H NMR spectra (0.5 mmol/L, 500 MHz, CDCl3+CD3CN (1:1, v/v), 25°C) of (a) 22+, (c) 1, (e) 32+, and the equimolar mixture of (b) 1 and 22+, and (d) 1 and 32+.
Moreover, the ability of naphthobox 1 to differentiate minor structural differences of viologen derivatives was also demonstrated by viologens with two different substituents (52+−72+,Fig.3, Figs.S2 and S3 in Supporting information).As shown in Fig.3, protons 6–8 of the methyl pyridinium of 52+undergo greater shifts (Δδ:0.28 to 0.66 ppm) than proton 6′, 7′ and 8′of the ethyl pyridinium of 52+(Δδ: 0.03 to 0.04 ppm) in the 1:1 mixture of 1 and 52+.This indicates that methyl pyridinium is preferentially encapsulated in the cavity of 1 over the ethyl pyridinium, thus experiencing a stronger shielding effect from the two naphthalenes and forming C–H∙∙∙N hydrogen bonds with two naphthyrides.Similar site preference on a single guest was also observed for guests 62+and 72+.
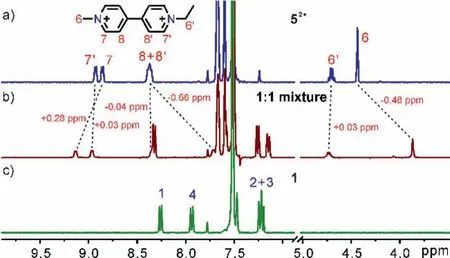
Fig.3 .Partial 1H NMR spectra (0.5 mmol/L, 400 MHz, CDCl3+CD3CN (1:1, v/v), 25°C) of (a) 52+, (c) 1, and (b) their equimolar mixture.
In order to quantify these bindings and understand the selectivity of 1, association constants were determined by NMR titration experiments (Figs.S9–S41 in Supporting Information) and are listed in Table 1.Job plot and molar ratio curves suggest a 2:1 binding stoichiometry for the binding of 1 with 22+(Fig.S9).Since the Job plot method cannot offers the accurate stoichiometry [30,31], our result was further supported by the X-ray crystal structure (Fig.4).A 1:1 binding stoichiometry was found for all other guests (Figs.S10–S19).In the titration experiments, the stock guest solutions were prepared with host solution (0.5 mmol/L), so the concentration of host is kept constant upon the titration.As expected, the largest association constant (1.7×104L/mol) was observed for the formation of the binary complex of methyl viologen 22+with 1.However, the association constant for ethyl viologen 32+is only 2.9×102L/mol, and for benzyl viologen 42+is even lower (0.7×102L/mol).The selectivity (S) for methyl viologen and ethyl/benzyl viologen (S2/3andS2/4) are 59 and 250, respectively, which are much higher than those reported in other studies[32,33].Additionally, the selectivity of 1 to methyl and ethyl pyridinium could be further revealed by its association constants with 8+and 9+, which are 3.4×103and 2.9×102L/mol, respectively.Moreover, naphthobox 1 also shows a clear preference for 22+over 122+, in which the latter is another commonly used pyridiniumbased herbicide.
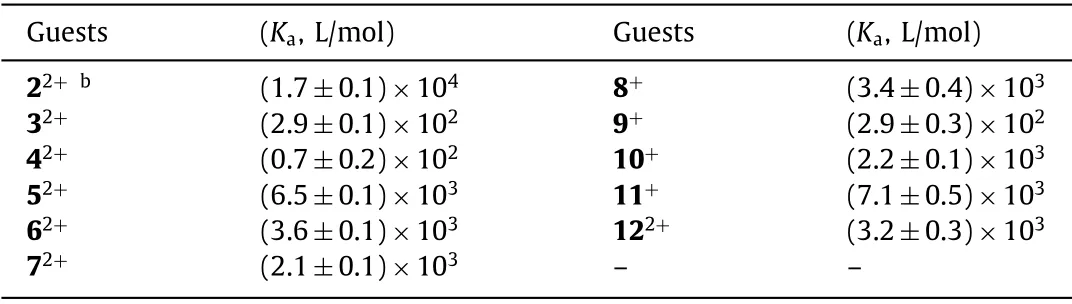
Table 1 Association constants (Ka, L/mol) of naphthobox 1 with pyridinium derivatives in CDCl3+CD3CN (1:1, v/v) at 25°C as determined by NMR titrationsa.
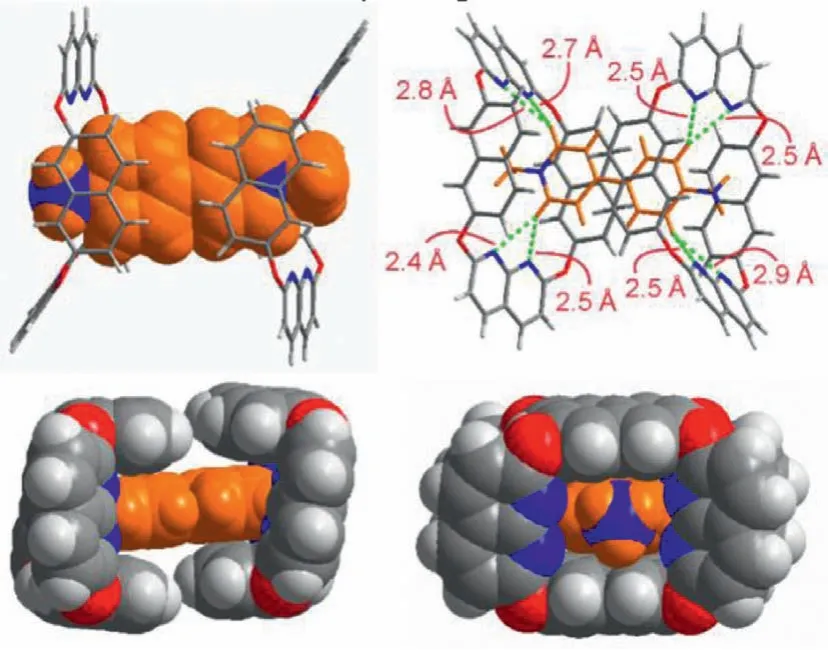
Fig.4 .X-ray single crystal structure of 22+@12.Green dotted lines represent the C–H…N hydrogen bonds.
In addition, the association constant for binding the second macrocycle (K2) is 6.4×102L/mol as determined by NMR titration experiment (Fig.S21).A negative cooperative effect is clearly demonstrated (α=4K2/K1=0.16) [34], which is probably due to the reduced electron deficiency of unbounded pyridinium and steric hindrance between the first and second macrocycles [35–37].
Single crystals of the ternary complex 22+@12were obtained by slow evaporation of a solution of 22+@12in 1:1 mixture of CH2Cl2/CH3CN.The X-ray structure clearly shows that one methyl viologen is wrapped by two box molecules with host-guest-host sandwich structure (Fig.4).The two box molecules are arranged in back to back fashion, with the adjacent naphthalenes being nearly coplanar and sit close with each other, which might account for the aforementioned negative cooperativity.Multiple noncovalent interactions are observed between 22+and 1 in the complex 22+@12.Each of the methyl pyridinium moieties is surrounded by four inner-cavity nitrogen atoms of naphthyridine so that they can form eight intramolecular C–H…N hydrogen bonds ranging from 2.4 ˚A to 2.9 ˚A.In addition,π…πstacking, C–H…πand cation…πinteractions are also observed between the naphthalene panels of the host and guest molecules.These multiple noncovalent forces revealed from the X-ray structure work cooperatively that leading to the formation of the ternary complex.
To understand the high binding selectivity of 1 to methyl viologen and the origin of the host’s ability in differentiating methyl and ethyl groups, molecular modeling of complexes 8+@1 and 9+@1 was performed.As shown in Fig.5a, the calculated structure of 8+@1 is similar to the crystal structure of 22+@12, in which the methyl pyridinium fits snuggly into the cavity of 1, and C–H…N hydrogen bonds,π…πstacking, C–H…πand cation…πinteractions are observed between the host and guest.For the complex with the ethyl viologen 9+, the host cavity of 1 is slightly enlarged and twisted, as shown by the obvious increase of the angle (∼2°) of four ether bonds after binding the guest (Fig.5b).Thus, steric effects of bulky substituent groups have certainly been one reason for the high selectivity.
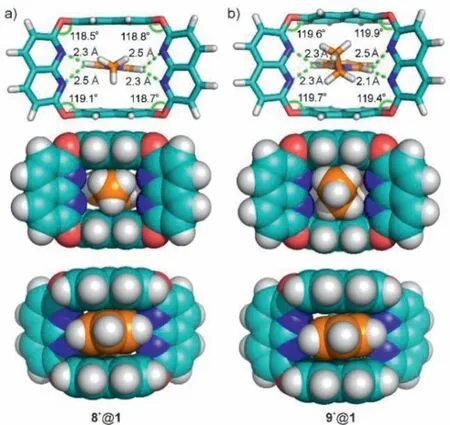
Fig.5 .Energy favourable orientational isomers of 8+@1 and 9+@1 as calculated by DFT (wB97XD/6–31G(d)) with the SMD solution model in CH2Cl2 at 298 K.
In addition to steric effects, electronic effects of substituent group also play an important role in the binding between 1 and pyridinium guests.This could be revealed by the binding affinities of 1 with guests 8+, 10+, 11+.Guest 10+and 11+are derivatives of 8+with an electron donating phenyl group or an electron withdrawing pyridyl group, respectively.As a result, the association constant of 1 to 11+is twice than that of 8+, while the association constant of 10+is only half of that of 8+.Guest 52+,62+and 72+also show a similar trend, in which the bipyridinium guests with one ethyl, phenyl or di–tert-butylphenyl group have generally decreasing association constants (6.5×103, 3.6×103and 2.1×103L/mol).The reason may be that the increase in electron density of the guests could weaken the C–H…N hydrogen bonds andπ…πstacking, thus affect the binding strength.
Furthermore, although the steric and electronic properties of methyl viologen 22+are similar to that of 11+, and even the DFT results show that the binding modes of 1 to the methyl pyridinium side of 22+, 10+, 11+are similar (Figs.S42–S44 in Supporting Information), the association constant of 22+is much higher than that of 11+.This phenomenon could be explained as followed: the strong binding of 1 to 22+could occurred at both sides of the methyl viologen molecule (Fig.6a) and statistically more favourable binding will lead to a stronger binding affinity [38].In contrast, 1 could only have a very weak binding to the pyridyl group of 11+, and even repel the phenyl group of 10+(Fig.6b).As a result, methyl viologen 22+could still be selectively recognized by naphthobox 1 over 10+and 11+.
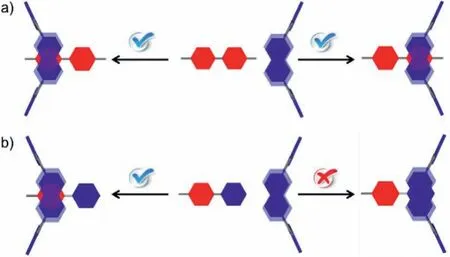
Fig.6 .Cartoon representation of the binding of 1 to (a) both sides of 22+ and (b)the methyl pyridinium side of 10+ and 11+.
In summary, we report anendo-functionalized naphthobox that is capable of binding viologen derivatives.Based on the relatively rigid structure and inner-cavity nitrogen atoms, this naphthobox shows a stronger binding affinity to methyl viologen over other pyridinium derivatives.The selectivity for methyl viologen and ethyl viologen is 59, which is the highest among the hosts reported in the literature.The single-crystal structure and DFT calculations reveal that the multiple C–H…N hydrogen bonds, C–H…π,andπ…πinteractions are essential for the binding affinity and selectivity.The present research first presented the concept ofendofunctionalized cavity to a box-like molecular host and showcases again that inward-directed functional groups can endow the corresponding host with unique recognition abilities [17,39–44].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No.22125105), the Shenzhen Science and Technology Innovation Committee (No.JCYJ20180504165810828), Guangdong Provincial Key Laboratory of Catalysis (No.2020B121201002), Shenzhen “Pengcheng Scholar”,Guangdong High-Level Personnel of Special Support Program (No.2019TX05C157) and the Croucher Foundation.We thank SUSTech CRF and the Center for Computational Science and Engineering for the technical support.We also thank Bonnie Yan, and Dr.Kam-Hung Low at the Department of Chemistry, the University of Hong Kong for their help with NMR, and XRD measurements.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.076.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
