MUC1 vaccines using β-cyclodextrin grafted chitosan (CS-g-CD) as carrier via host-guest interaction elicit robust immune responses
Hngyn Yu, Hn Lin, Yuntin Xie, Mengyun Qu, Min Jing, Jie Shi, Hofei Hong,Hongrui Xu, Ling Li, Guoho Lio, Zhimeng Wu, Zhifng Zhou,∗
a The Key Laboratory of Carbohydrate Chemistry & Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, China
b Suzhou Municipal Center for Disease Control and Prevention, Suzhou 215004, China
c Joint Laboratory for Translational Cancer Research of Chinese Medicine of the Ministry of Education of the People’s Republic of China, International Institute for Translational Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510006, China
Keywords:β-Cyclodextrin grafted chitosan MUC1 antigen Cancer vaccine Host-guest interaction Vaccine carrier Tn antigen
ABSTRACT We construct MUC1 vaccines using β-cyclodextrin grafted chitosan (CS-g-CD) as carrier via host-guest interaction.These vaccines based on non-covalent assembling can provoke robust immune responses,including high level of specific antibodies and cytokines.The induced antibodies can specifically recognize tumor cells and mediate cytotoxicity against tumor cells.These results indicate that CS-g-CD with strong immunostimulatory activities can be a straightforward platform for peptide-based vaccine construction.
Tumor-associated antigens (TAAs) abundantly expressed on tumor cells are important targets for the development of anti-cancer vaccine [1].Among the TAAs, mucin 1 (MUC1) of tumor cells, a glycosylated protein, has been ranked as the second ideal target for the development of therapeutically vaccines [2,3].The extracellular moiety of tumor-associated MUC1 has variable number tandem repeat region (VNTR) of a core sequence of 20 amino acids(HGVTSAPDTRPAPGSTAPPA) with truncated glycosylation, such as T,Tn and sTn antigens [4,5].Due to its poor immunogenicity and T cell-independent properties, synthetic MUC1 peptides are usually conjugated to carrier protein to improve the immunogenicity.Recently, some novel biomaterials and nanoparticles were developed for constructing MUC1-based vaccines and showed promising results [4,6-14].However, it is still a challenge to develop an effective MUC1-based vaccine.
Chitosan (CS), a cationic polysaccharide comprisingβ(1–4)-linked glucosamine andN-acetyl-glucosamine, is derived from chitin that is one of the most abundant polymers in nature.Due to its biocompatible and biodegradable, chitosan has been widely used in medicine, food, chemical industry, cosmetics and so on[15,16].Chitosan is also considered as a potential vaccine adjuvant because of its abilities to enhance humoral and cellular immunity[17].Depending on the deacetylation degree, chitosan has various amount of –NH2exposure which leads to rich positive charge.This cationic polysaccharide has the superiority to easily attach the cell surface that is negative charged by electrostatic interaction, which may play an important role during the antigen uptake and presentation [18].The positive charge of chitosan can also improve the adjuvanticity including antigen protection and depot formation when the chitosan mixed with antigens.Besides, the protonation of the –NH2group at C-2 position of the D-glucosamine repeat unit also improves the solubility of chitosan which is critical for an adjuvant [19].In addition to these advantages as a delivery cargo, chitosan has specific immunostimulatory activities.It has been proved that chitosan induces type I interferon (IFNs) secretion following with dendritic cells (DCs) activation and Th1 cellular immunity through the cGAS-STING pathway and NLRP3 inflammasome [17].Moreover, after the chitosan capsulated into the lysosome, the protonation of amino groups causes proton sponge effect that may induce endosomal escape and promote the crosspresentation of antigens.It is especially important for provoking CD8+ killer T cells after interacting with major histocompatibility complex (MHC) class I molecules [20].Given the potential adjuvant property of chitosan, it is an excellent candidate for constructing MUC1-based vaccine to improve the immunogenicity of MUC1 peptide.
However, chitosan is only soluble under acidic condition but weakly soluble in neutral pH or physiological conditions which hampers its biomedical applications.To overcome the problem,chitosan derivatives or chitosan-based powder, gel or nanoparticles are developed and used for carrying antigen.Assembling the antigen to chitosan in a more stable and orderly manner is still needed for vaccine construct.Here, we developed a novel strategy to construct vaccine by assembling MUC1 peptide to chitosan derivatives via the host-guest interaction [21].Aβ-cyclodextrin grafted chitosan (CS-g-CD) was prepared, in which the hydrophobic central cavities ofβ-CD were used for assembling the MUC1 peptide with adamantane (ada) modification (Fig.1) [22].This host-guest interaction between CS-g-CD and peptide antigens has the potential to form a complex with high stability for betterin vivodelivery and antigen-depot.Based on the adequate -NH2groups of CS, the inclusion supramolecule can be further prepared as nanoparticles to increase the cellular uptake.
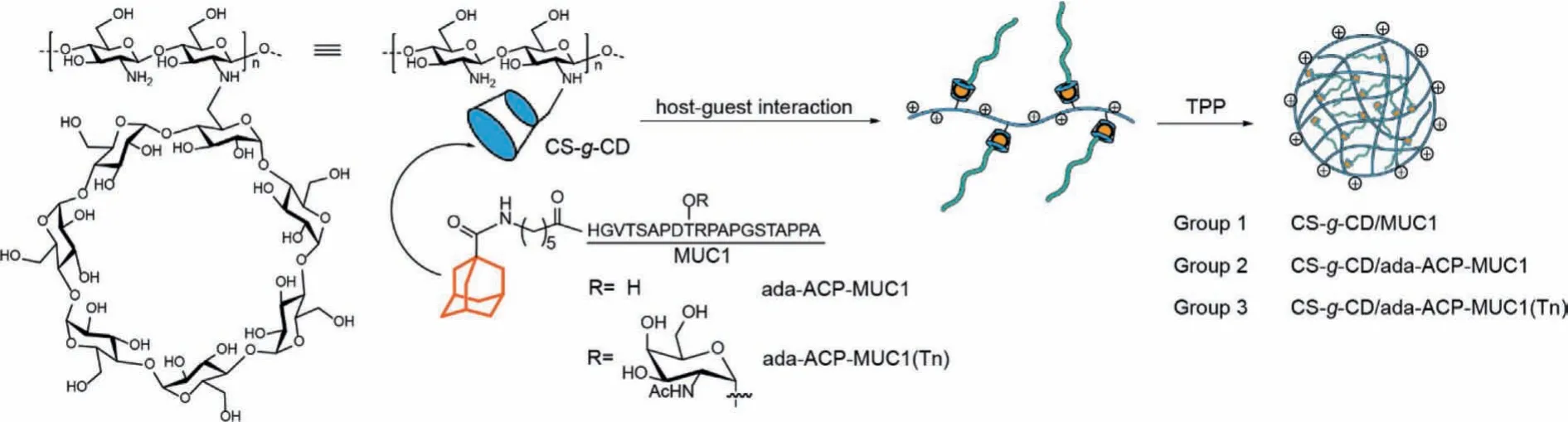
Fig.1 .The construct of MUC1 vaccine using CS-g-CD as carrier by host-guest interaction.
To prove our concept, we synthesized CS-g-CD at first.Chitosan with a deacetylation degree of higher than 90% and a MW of about 100 kDa was employed in our study.Compound 6-OTs-β-CD derived fromβ-CD was grafted to the amino group of CS under acidic conditions (Scheme S2 in Supporting information).To improve the degree of substitution (DS) ofβ-CD, 6-OTs-β-CD was added twice to a raw CS-g-CD product to afford CS-g-CD with a high DS of 15.6% (Fig.S11 in Supporting information) [23].The solubility of CS-g-CD was significantly improved (Fig.S3 in Supporting information) [9].
Subsequently, three groups of MUC1-based vaccines were designed and prepared.The MUC1 peptide without adamantane modification was mixed with CS-g-CD directly as group 1 (Table 1).Group 2 was CS-g-CD including MUC1 antigen with adamantane modification at the N terminal through the aminocaproic acid(ACP) linker (ada-ACP-MUC1), and group 3 was the one including ada-ACP-MUC1(Tn) which had a Tn modification on the residue of threonine to mimic a glycosylated MUC1 antigen (Fig.1).MUC1 peptides were prepared by Fmoc-based solid-phase peptide synthesis (SPPS) strategy.After being deprotected and cleaved from the resin by using a solution of TFA/TIS/H2O (95:2.5:2.5, v/v/v)and purified by HPLC, MUC1 antigens were obtained and identified by mass spectrometry (Fig.S13).The MUC1antigens were then used for vaccine construct.Generally, the CS-g-CD was dissolved in 1% acetic acid solution and then adjusted to pH 4.5.Then the solution of peptide (1.5:1 equiv.toβ-CD) was added to the CSg-CD solution and stirred overnight to form complex.The sodium tripolyphosphate (TPP) was added dropwise to the complex with stirring at 800 rpm for 30 min.The formed nanoparticles were dialyzed with deionized water for 3 days to remove free peptides.The loading of peptide was analyzed according to the amount of free MUC1 antigens in the solution by HPLC determination (Table 1).The host-guest interaction was evaluated by isothermal titration calorimeter (ITC) and theKDvalue was about 26.6×10−6(Fig.S4 in Supporting information).
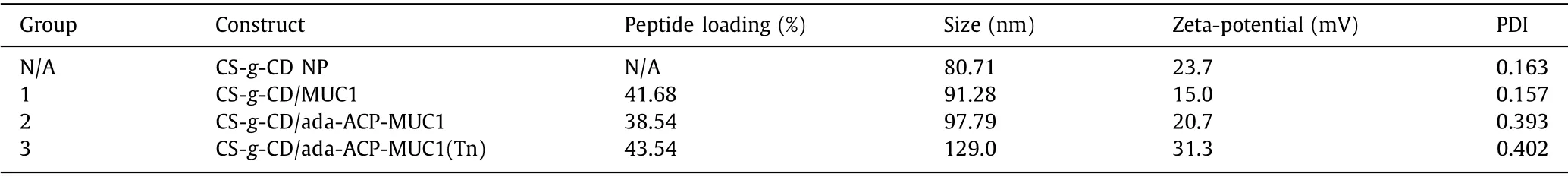
Table 1 The vaccine construct and characterization.
The nanoparticles were characterized by transmission electron microscope (TEM) and dynamic light scattering (DLS).According to the result of DLS, the sizes of the nanoparticles for groups 1, 2 and 3, were 91.28 nm, 97.79 nm and 129.0 nm, respectively (Fig.S2 in Supporting information).Compared with the size of CS-g-CD nanoparticle without MUC1 that was 80.71 nm, these sizes were obviously increased.The zeta-potential of CS-g-CD nanoparticle was 23.7 mV which was mainly caused by the protonation of amino group of CS, while the zeta-potentials of groups 1-3 were all positive as well.This result indicated that the nanoparticles could be stably distributed in solution.The morphology of nanoparticle was further confirmed by TEM which suggested that the size of nanoparticle was about 100 nm and the morphology was irregular sphere (Fig.S1 in Supporting information).These nanoparticles were suitable for the uptake by antigen presenting cells (APCs) according to the report [24] that nanoparticles with the size ranges between 20 to 200 nm were easily swallowed by resident dendritic cells (DC) and could directly target lymph nodes.
Subsequently, the uptake of CS-g-CD nanoparticles by APCs was characterized by using FITC-modified MUC1 peptide.The bone marrow-derived dendritic cells (BMDCs) were incubated with MUC1-FITC peptide, a solution form of CS-g-CD mixed with MUC1-FITC peptide, and CS-g-CD/MUC1-FITC nanoparticle, respectively.The fluorescence of BMDC was then detected by fluorescence microscope and flow cytometry.As shown in Fig.2, only the nanoparticle form of CS-g-CD/MUC1-FITC could be taken in by BMDC effi-ciently and quickly which was represented by the strong fluorescence around the cell nucleus.The result of flow cytometry (Fig.S5 in Supporting information) also indicated that CS-g-CD/MUC1-FITC nanoparticle had the strongest fluorescent intensity and the phagocytic effect was enhanced with increasing incubation time.Therefore, the nanoparticle form was important for a cellular uptake of vaccine candidate.Thein vitrocytotoxicity of nanoparticles was evaluated in BMDCs as well (Fig.S6 in Supporting information).Up to a concentration of 80 μg/mL, no significant cytotoxicity was observed which indicated that the nanoparticle was biocompatible and safe forin vivoapplication.
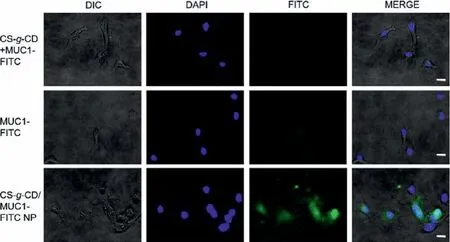
Fig.2 .Laser Scanning confocal microscope (LSCM) images of BMDCs after 2 h of incubation with free MUC1-FITC, CS-g-CD+MUC1-FITC solution and CS-g-CD/MUC1-FITC nanoparticles.Scale bar: 15 μm.
To explore the immunogenicity of vaccine candidates with CSg-CDin vivo, three groups of C57BL/6J mice were employed for the study of humoral and cellular immunity.The CS-g-CD nanoparticles with MUC1 antigens prepared above were further emulsified with Freund’s complete adjuvant as vaccine formulation for mice immunization.Each mouse was inoculated with 100 μL emulsion of 15 nmol of MUC1 antigen on day 1, 7, 14, 21 and 28 through subcutaneous (s.c.) injection.The antisera were prepared from the blood of mice collected through the thigh vein on day 0, 7, 14,21, 28 and 35 for the analysis of antibodies and cytokines.The MUC1-specific antibody titers were determined by enzyme-linked immunosorbent assays (ELISA) using the corresponding peptide as capture reagents.Generally, the antigen coated ELISA plates were treated with blocking buffer and then incubated with half-log serially diluted mouse antisera from 1:300 to 1:218700 in PBS.HRPlinked second antibodies were used to react with TMB substrate solution and then the plates were detected by colorimetric readout at 450 nm wavelength.Antibody titers were calculated from the curves of optical density (OD) against the serum dilution numbers and were defined as the dilution value when OD value reached 0.2[25].
The antibody titers of antisera collected from mice of group 1-3 on different days were exhibited in Fig.3.As shown in Fig.3A, the antibody titers increased when the immunization boosts increased and reached the highest level after the fifth boost.The antibody titers of group 2 were higher than that of group 1 which indicated that the host-guest interaction could improve the immunogenicity of MUC1 peptide.The antibody titer of group 3 was slightly lower than the other two groups which may be caused by the Tn glycosylation on MUC1 [26].The value of OD 450 nm reached 3.0 when the antisera of groups 1 and 2 were 1:300 diluted (Fig.3B).Interestingly, the subclasses of antibodies in groups 1-3 were different (Fig.3C).IgG1 was the main type in group 1, IgG1 and IgG2c were both abundant in group 2.However, in group 3, IgG2c was the highest subclass.This result suggested that the host-guest interaction based on CS-g-CD might regulate the type of immune responses.The T cell immunity may be provoked by the CS-g-CD carrier which had the potential to elicit STING immune activity and induce sufficient cross presentation when the antigens were presented by APCs.To further confirm the specificity of antibodies, the competitive ELISA was performed using the corresponding MUC1 peptide as inhibitor against the antigen coated on plates.The result in Fig.3D suggested that the antibodies in groups 1-3 could successfully recognize MUC1 antigens and could be inhibited by peptides.
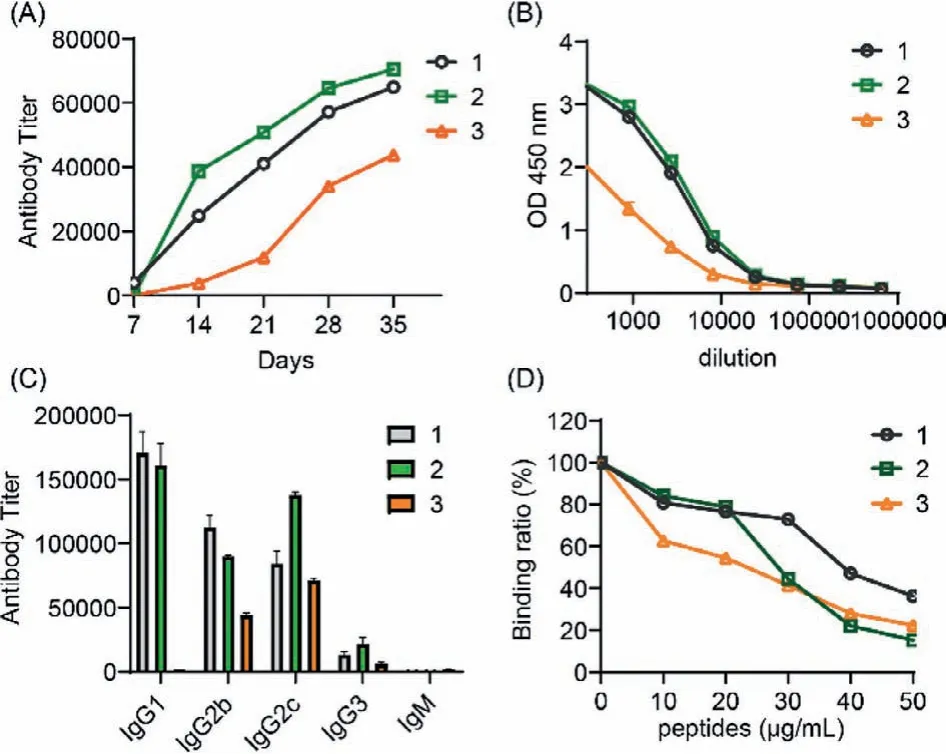
Fig.3 .The ELISA results of antisera collected from mice of groups 1-3.(A) The antibody titers on different days; (B) the OD 450 nm against the dilution number;(C) the subclasses of antibody; (D) the competitive ELISA results.
To further investigate the T cell-mediated immune responses,the expression levels of IFN-γand IL-6 in the serum were analyzed by cytokine ELISA assay.Usually, IFN-γindicates a Th1 type cytokine with critical immunoregulatory properties, which can proliferate and differentiate lymphocyte populations, activate NK cells and enhance antigen presentation.While IL-6 is a Th2-type cytokine that can improve the innate and adaptive immunity, mediate various aspects of B cell and T cell responses, and promote antibody production.As shown in Fig.4A, group 3 produced the highest level of IFN-γcompared to groups 1 and 2 which indicated that the vaccine construct of group 3 has the strongest Th1 type immune responses.This may be caused by the specific inclusion construct between antigen and CS-g-CD in group 3 and the Tn glycosylation on MUC1 antigen.Hence, CS-g-CD/ada-ACPMUC1(Tn) nanoparticle has the potential to elicit strong T cell immune response.In Fig.4B, the level of IL-6 in groups 1 and 3 was similar but higher than that of group 2.This may be generally impacted by several factors including the CFA adjuvant, antigens and CS-g-CD carrier.
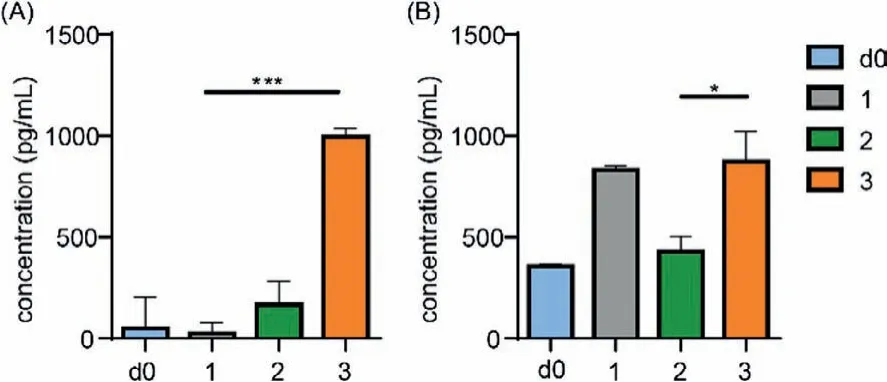
Fig.4 .The levels of IFN-γ (A) and IL-6 (B) in day 0 serum (d0) and antisera of groups 1-3 on day 35.∗∗∗P < 0.001, ∗P < 0.05.
The properties of antibodies in groups 1-3 to recognize and bind to cancer cells were evaluated by using human breast cancer cells, MCF-7 and MDA-MB-468, that can express MUC1 antigen.HEK-293, the human embryonic kidney cells with low MUC1 expression was used as negative control.After treated with antisera, the cells were labeled with FITC-conjugate goat anti-mouse IgG antibody and finally analyzed by flow cytometry.As shown in Fig.S8 (Supporting information), compared with the nonimmunized mouse serum of day 0, the fluorescence intensities of antisera of groups 1-3 incubated with MCF-7 and MDA-MB-468 were significantly increased which revealed that the antibodies produced in mice of groups 1-3 could successfully recognize the MUC1 antigen expressed by tumor cells.As expected,the enhancement of fluorescence intensity had positive correlation with the level of MUC1 expression.Additionally, the antisera of group 3 has the highest fluorescence intensity in both MCF-7 and MDA-MB-468 cells which indicated that the Tn glycosylation may be important for the vaccine construct to achieve higher specificity.
The complement-dependent cytotoxicity (CDC) assay was recruited to investigate the capability of antibodies to mediate cytotoxicity against tumor cells.MCF-7 cells were incubated with antisera following with rabbit complement.The cell lysis of tumor cells was determined by commercial CCK8 kit.As presented in Fig.5,the antisera of each group can effectively activate the complements and induce the death of cancer cells.This result revealed that the vaccines used in groups 1-3 had the potential to elicit robust immune responses against MUC1-expressed tumor cells and the CS-g-CD was a promising carrier for the vaccine construct through hostguest interaction.
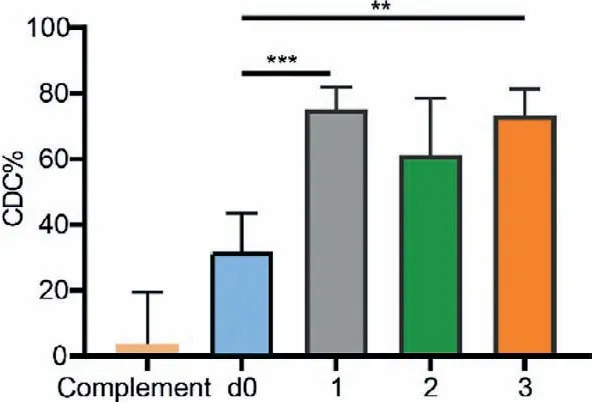
Fig.5 .Complement-dependent cytotoxicity (CDC) assay of MCF-7 with complement only, day 0 serum (d0), and antisera collected from mice of groups 1-3 on day 35.Data are presented as mean ± SD (n=5).∗∗∗P < 0.001, ∗∗P < 0.01.
In summary, we constructed MUC1 peptide vaccines using CSg-CD as carrierviahost-guest interaction between the adamantane moiety of MUC1 peptide andβ-CD of CS-g-CD.These novel vaccines based on non-covalent assembling could provoke strong immune responses and the induced antibodies could successfully recognize tumor cells and mediate cytotoxicity against tumor cells.The immunological studies indicated that the host-guest interaction could be employed for vaccine construct based on chitosan as carrier.Differing from a covalent conjugation, this simple manipulation of non-covalent assembling provides easier and higher loading ability and also a straightforward platform for other vaccine construction.Additionally, theβ-CD modified on chitosan can provide cavities for assembling antigens or other molecules, such as T help epitopes and the TLR agonists which could further improve the immune responses [27].Moreover, theβ-CD modification improves the solubility of chitosan which is critical for its application in medicines.Overall, the CS-g-CD is a promising carrier for vaccine construction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21907038 and 32000904), Natural Science Foundation of Jiangsu Province (No.BK20200601), National Postdoctoral Program for Innovative Talents of China (No.BX20200153),China Postdoctoral Science Foundation (Nos.2018M632227 and 2021M691293), the Social Development Key Project of Jiangsu Province (No.BE2019632), the Health and Family Planning Commission of Wuxi, China (No.Z202005), Suzhou People’s Livelihood Science and Technology Project, China (No.SYS2018100).This research was partly supported by the 111 Project (No.111-2-06).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.072.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
