Catalytic and highly stereoselective β-mannopyranosylation using a 2,6-lactone-bridged mannopyranosyl ortho-hexynylbenzoate as donor
Yingle Feng, Jie Yng, Chenglin Ci, Toto Sun, Qi Zhng, Yonghi Chi
a Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education and School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi’an 710119, China
b School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi’an 710119, China
Keywords:β-Mannanoside 2,6-Lactone Catalytic glycosylation Ortho-hexynylbenzoates 1,2-cis Glycosylation
ABSTRACT An efficient and catalytic protocol for highly stereoselective construction of β-mannopyranosylation has been developed.Glycosylation of 2,6-lactone-bridged mannopyranosyl ortho-hexynylbenzoate with various acceptors proceeded smoothly in the presence of 5% Hg(II) at room temperature, resulting in the corresponding β-mannosides in high yield and exclusive β-stereoselectivity.
β-Mannopyranosyl units are important components of numerous natural bioactive products.High stereoselectiveβmannopyranosylation has been considered one of the most challenging problem in the field of glycochemistry, due to the anomeric effect and steric hindrance from the 1,2-cisconfiguration [1,2].In the past several years, a variety of strategies have been reported to solve this problem, including intramolecular aglycon delivery (IAD)[3,4], hydrogen-bonding aglycone delivery (HAD) [5,6], 4,6-tethered strategy [7–11], C-5 carboxylate strategy [12], 2,6-lactone-bridged methods [13,14] and other approaches [15–19].Among these methods, the 4,6-O-benzylidene tethered strategy and C-5 carboxylate strategy have been widely used in the synthesis of natural products and oligosaccharides containingβ-mannanoside [20–24].In this paper, we reported a catalytic, efficient and concise strategy to constructβ-mannosidic linkages with a donor which could be readily prepared.
In 2019, a novel protocol for stereoselective construction ofβ-mannosides was developed by our group using a 2,6-lactonebridged thiomannosyl donor through the remote acyl-group participation as well as the steric effect ofO-4 substituent (Fig.1a)[14].In that paper, we demonstrated that 2,6-lactone-bridged mannosyl imidate donors were difficult to be prepared in large scale,due to the sensitivity of the bridged lactone to bases and therefore the formation of a tricyclic product containing 1,3-dioxolane ringviaintramolecular reaction.Thus, thioglycosyl donors were used for the stereoselectiveβ-mannosylation.However, stoichiometric NIS (N-iodosuccinimide) and subcatalytic amount of AgOTf are required to activate the thioglycosyl donor due to decreased activity of the bridged lactone motif.Considering that the 2,6-lactone-bridged mannosyl donor possess superiority in high stereoselectiveβ-mannosylation, we envision that the glycosylation of 2,6-lactone-bridged mannosyl donor with proper leaving group could realize mild, catalytic and highβ-stereoselective mannosylation (Fig.1b).Given that the alkynoates [25–27], especially theortho-alkynylbenzoate developed by Yu and co-workers [26,27]have been widely used in glycosylation in recent years, we explore 2,6-lactone-bridged mannosyl donors with an alkynoate as leaving group forβ-mannopyranosylation (Fig.1).

Fig.1 .2,6-Lactone-bridged mannopyranosyl donor for glycosylation.
The glycosyl donors were prepared on the base of our reported work [14].The building block 1 was first prepared through 3 steps from mannose (Scheme 1).Then, the hydroxyl group at the C-4 was protected with bulky Piv group for its best performance in remote participation in our previous work [14].After treatment of compound 2 with Pd(PPh3)4in AcOH, the hemiacetals 3 was afforded.And then, the 2,6-lactone-bridged mannosyl alkynoates 4-1 and 4-2 were readily afford by one-step esterification of 3 with the corresponding acyl chlorides in about 90% yield.
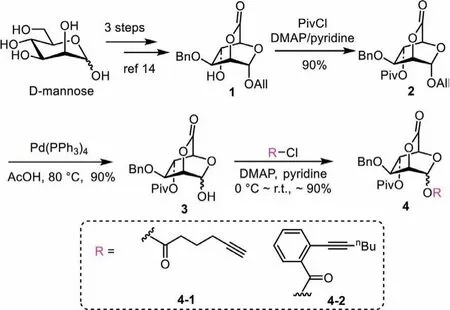
Scheme 1 .Preparation of glycosyl donor 4-1 and 4-2.
With the above glycosyl donors in hand, we initiated our studies by investigating the coupling reaction between 4-1 and acceptor 5a (Table 1).At first, the reaction was optimized in the presence of the Au(I) or Au(III) which are typically used to activate alkynyl group in organic synthesis [28] and glycosylation [27].Unfortunately, only minor or even no product was afforded (Table 1,entries 1–6).Considering that Hg(II) catalysts have been successfully explored to catalyzen–pentenyl-type glycosides for glycosylation in our previous work [29], Hg(II) catalysts were thus examined in this work (Table 1, entries 7 and 8).The results showed that both Hg(NTf2)2and Hg(OTf)2can catalyze the reaction withhighβ-selectivity and Hg(OTf)2(Table 1, entry 8) exhibited better catalytic activity than Hg(NTf2)2(Table 1, entry 7).However, owing to incomplete consumption of acceptor 5a in the presence of both Hg(NTf2)2and Hg(OTf)2, the glycosylation provided the corresponding product only in 33% and 45% yield respectively.When 10% Hg(OTf)2was used and the reaction concentration was increased from 0.1 to 0.5 mol/L, no obvious improvement was observed (Table 1, entry 9).It’s speculated that the strong electronwithdrawing character of bridged lactone may decrease the activation of the glycosyl donor.Thus, Yu’s ortho-alkynylbenzoate method [26,27] was chosen for the next investigation.
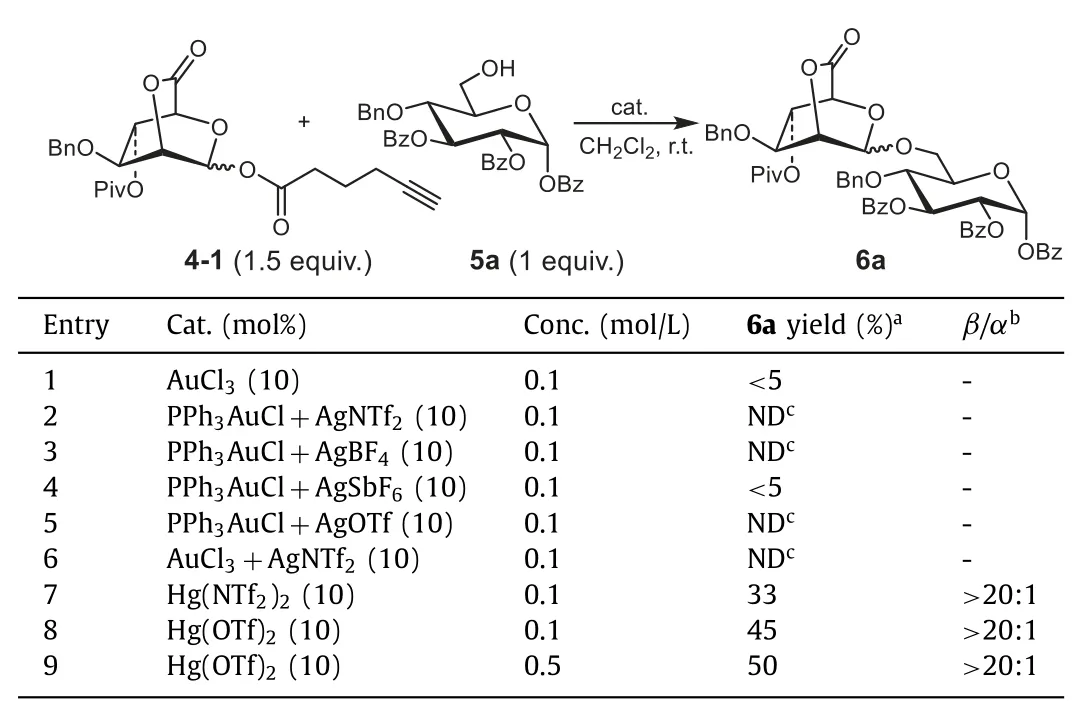
Table 1 Optimization of reaction conditions for glycosylations of donor 4-1 with acceptors 5a.
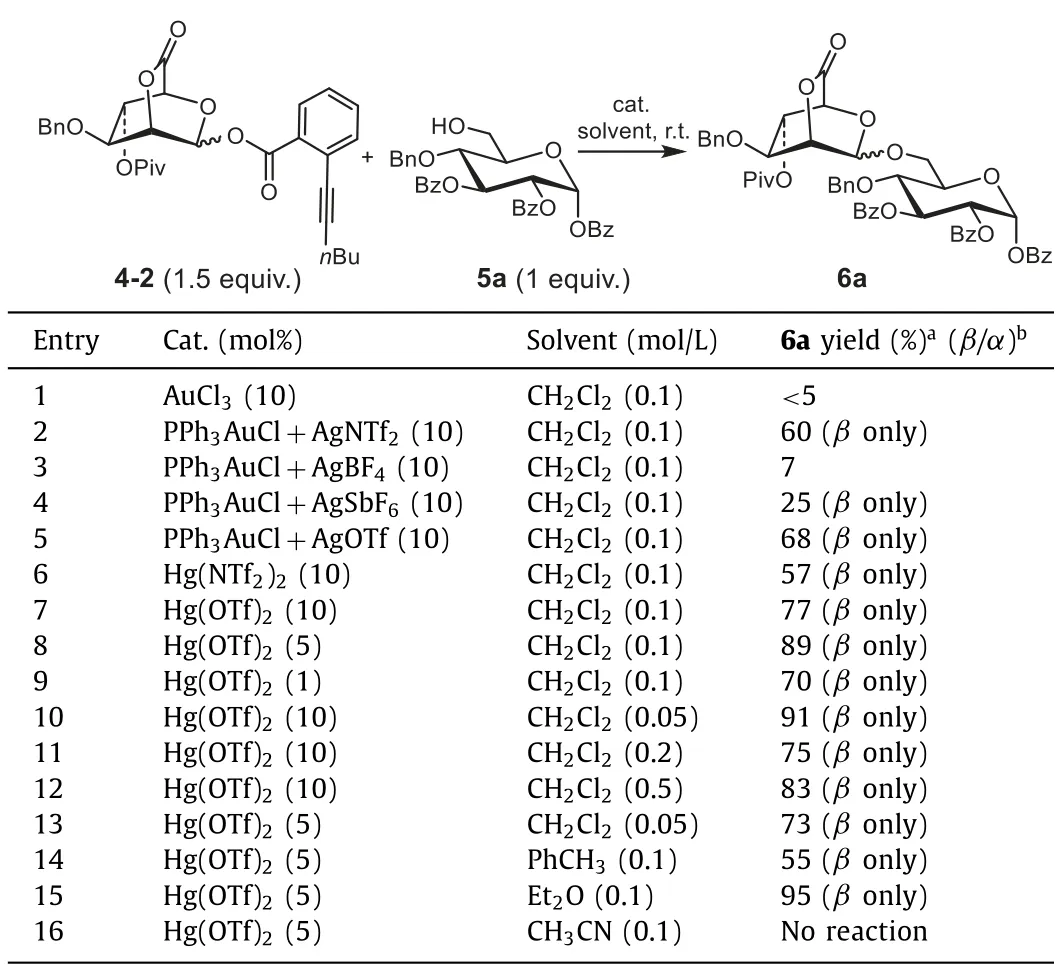
Table 2 Optimization of reaction conditions for glycosylations of donor 4-2 with acceptors 5a.
Initially, coupling reaction betweenortho-alkynylbenzoate donor 4-2 and acceptor 5a was conducted using the typical Au catalyst as promotor.Gratifyingly, 6a with exclusive stereoselectivities was afforded when the reaction was catalyzed by Ph3PAuCl(0.1 equiv.) and AgNTf2(or AgSbF6) (0.1 equiv.) (Table 2, entries 2 and 4).However, the acceptor 5a was not yet completely consumed, leading to the formation of the glycosyl product in 60% yield for AgNTf2and in 25% yield for AgSbF6respectively.Furthermore, when Ph3PAuCl (0.1 equiv.) and AgOTf (0.1 equiv.)(Table 2, entry 5) was used to calalyze the reaction, the yield for the target disaccharide was only increased to 68%.According to the aforementioned results, Hg(II) (Table 1, entries 7–9) exhibited better activity than Au(I) or Au(III) (Table 1, entries 1–6) for the activation of the alkynoate.Therefore, Hg(NTf2)2and Hg(OTf)2were applied to catalyze the reaction (Table 2, entries 6 and 7).Again, Hg(OTf)2displayed better catalytic activity in comparison with Hg(NTf2)2, and 77% yield was afforded when the reaction was conducted in CH2Cl2at concentration of 0.1 mol/L (Table 2, entry 7).Thus, Hg(OTf)2was considered as the optimal catalyst for the further studies of glycosyl condition.Then, the amount of catalyst,reaction concentration and solvents were screened for the optimal glycosylation condition.It was shown that 89% yield was obtained for using 5% catalyst (Table 2, entry 8) at a reaction concentration of 0.1 mol/L, and 91% yield for using 10% catalyst (Table 2, entry 10) at a reaction concentration of 0.05 mol/L.To reduce the usage of Hg(II), the amount of catalyst was determined as 5% for thefollowing studies.At last, different solvents (including toluene,ether, and acetonitrile, Table 2, entries 14–16) were also examined,and the yield was further increased to 95% when using Et2O as the solvent (Table 2, entry 15).Generally, Et2O was considered to be helpful for construction ofα-configuration glycosides.However,the selectivity was not influenced in this work, demonstrating the strong tendency toβ-configuration of 2,6-lactone-bridged mannosyl donor.
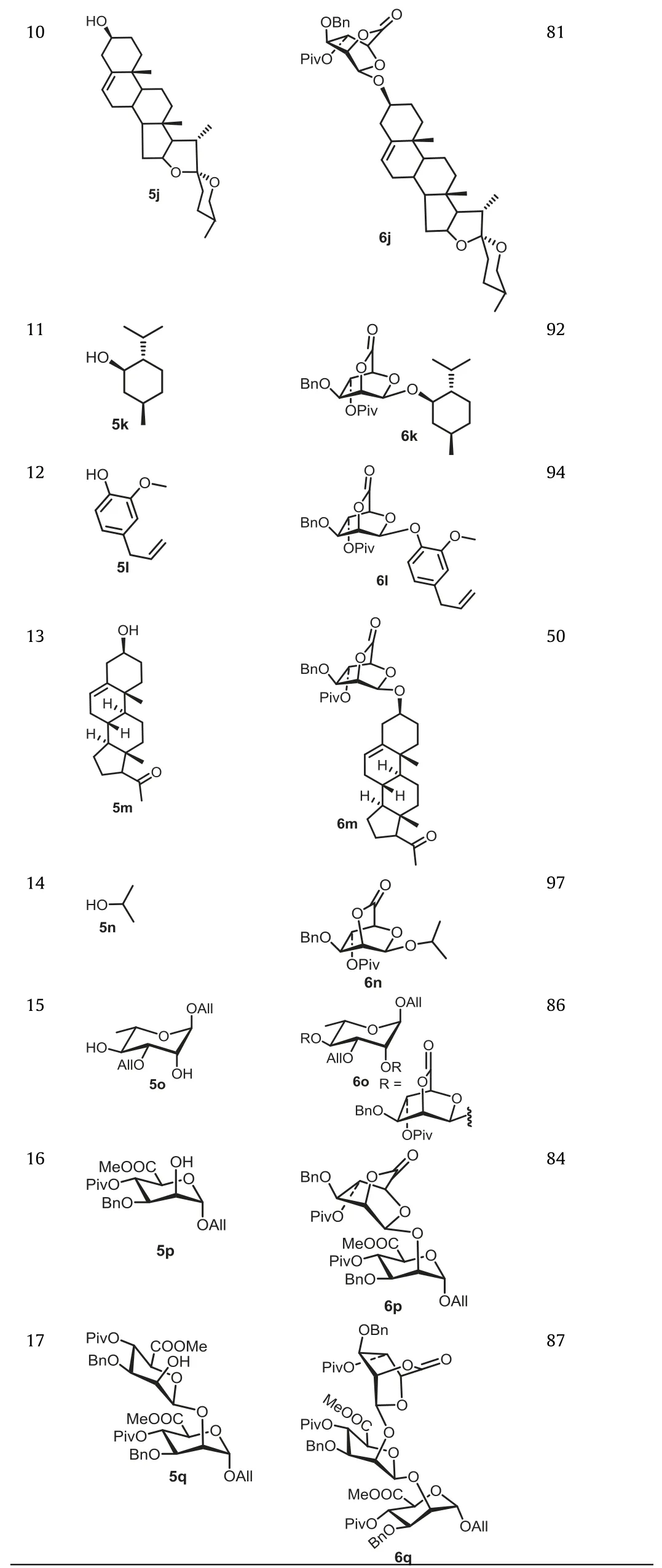
Table 3 (continued)
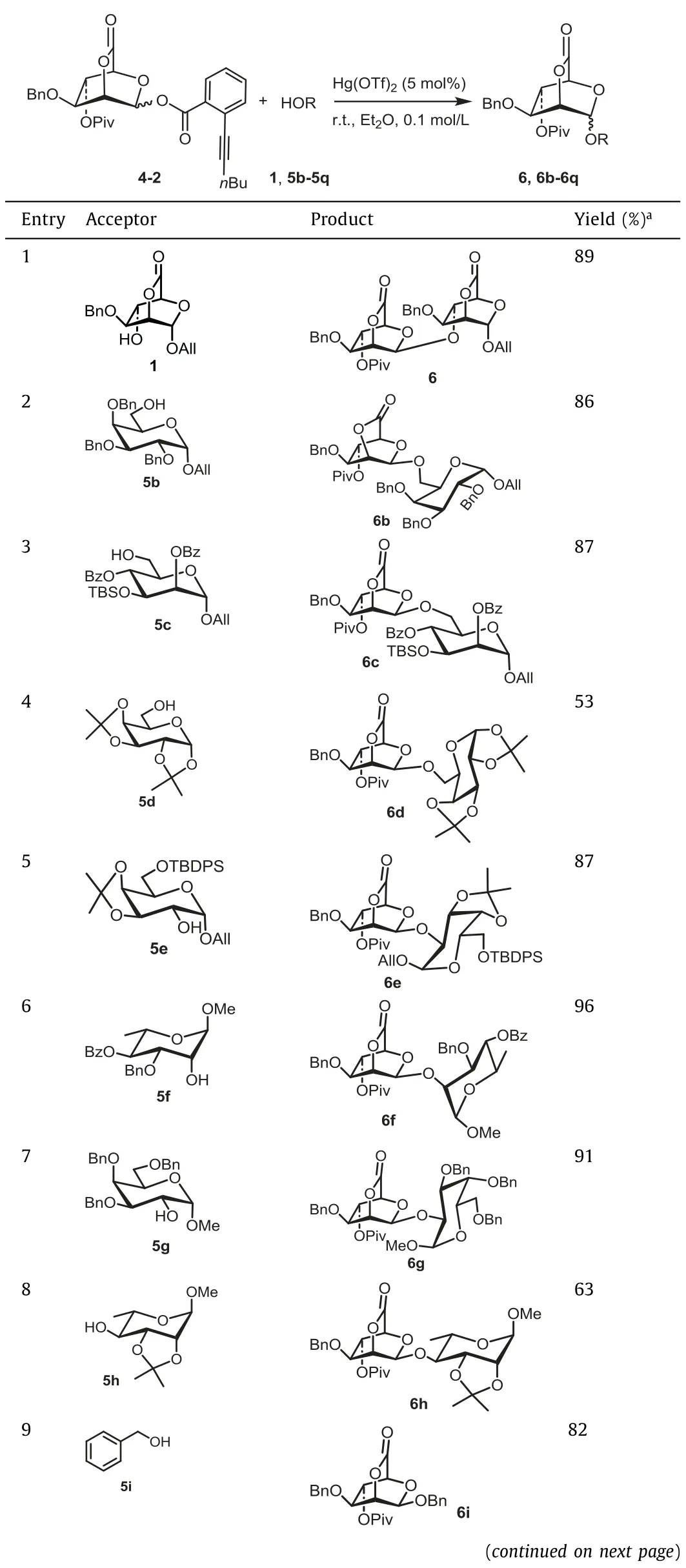
Table 3 Glycosylations of donor 4-2 with acceptors 1 and 5b-5q.
With the optimized condition in hand, the scope of this reaction was then explored.With 4-2 as the donor, glycosylation with primary and secondary hydroxyl acceptors was conducted.To our delight, the glycosylation of 4-2 with all acceptors proceeded smoothly, providing the corresponding glycosides withβconfiguration in good yield (Table 3).Although it was reported that acceptor with less reactivity usually led to increase inα-selectivity[31,32], the highly stereoselectiveβ-mannopyranosylation performance remained, when the alcohol with less nucleophilicity (represented by 1) was used as acceptor.Interestingly, when acceptor 5o with two free hydroxyl groups was used, the catalytic dose (5% Hg(OTf)2) was still sufficient to ensure the smooth glycosylation (Table 3, entry 15), indicating the strong efficacy of Hg(OTf)2for activatingortho-hexynylbenzoate.In addition, the glycosylation condition was also suitable for saponins (Table 3, entry 10), demonstrating the potential of this protocol for the synthesis ofβ-mannosyl saponins.However, when the acid-sensitive protecting group ketal was used (in 5d, 5e, 5h), the corresponding yield (Table 3, entries 4, 5, 8) was slightly affected due to the Lewis acidic character of Hg(II) catalysts.It should be emphasized,β−1,6-,β−1,4-linked mannobioses units, which are enormously existed in nature [30], can be readily constructed in high yield(Table 3, entries 1 and 3).More importantly, the lactone can be feasibly attacked by base with high nucleophilicity (CH3COO−Na+)and the resulting product (such as 5p, 5q) with free hydroxyl groups at C-2 can be used as acceptor for further glycosylation.Thus, theβ−1,2-linked oligomannosyl moiety, which is an important component of the cell wall of Candida albicans [33], could be readily constructed as 6p and 6q (Table 3, entries 16 and 17).After several manipulations (including reduction and deprotection) of 6p and 6q, the resultant products could be used as antigens for investigation of anti-bacterial vaccine.All of those results demonstrated that our strategy has a wide range of substrate applicability.
In conclusion, a mild and catalytic method for highly stereoselectiveβ-mannosylation of various alcohols with a 2,6-lactonebridged mannopyranosylortho-hexynylbenzoate as donor has been developed.The reaction proceeded smoothly to give the corresponding product in high yield with exclusive stereoselectivities,demonstrating its potential application in the synthesis of natural products containingβ-mannosyl structural unit.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos.21472119, 22107061), the Science and Technology Program of Shaanxi Province, China (No.2018ZDXM-GY-152).All of the financial supports are gratefully acknowledged.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.071.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
