Dimensionally confined nanosheets self-assembled through self-shielding multiple hydrogen bonding interactions in aqueous media
Jing Zhang, Shuaiwei Qi, Hao Yu, Ze Lin, Bao Li, Ming Wang, Zeyuan Dong
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China
Keywords:Hydrogen bonding Aqueous media Sandwich structure Self-shielding Two-dimension
ABSTRACT Herein, we adopt a simple supramolecular strategy to effectively control the tautomerism of ureidopyrimidinone (UPy) moiety and ultimately realize the complete arrangement of enol configuration.The obtained UPy derivatives containing self-complementary quadruple hydrogen bonding interactions can spontaneously self-assemble towards the formation of well-controlled, self-organized supramolecular nanostructure morphologies in both chloroform and water.The resulting aggregates had been fully characterized by various spectroscopy (absorption, emission) and microscopy (TEM, SEM and AFM) studies.It is anticipated that this study can provide an exact and excellent monomeric unit for controllable and precise supramolecular polymerization.The results achieved here also demonstrate the utility and feasibility of multiple hydrogen bonds to direct the self-assembly of small-molecule building blocks in aqueous media, which provides a strategy for the construction of well-defined and stable supramolecular architectures with chemical functionalities and physical properties as advanced materials for biological applications.
Hydrogen bonding is a highly directional and reversible intermolecular force, which is of great significance in determining the structures of significant compounds in biological system and plays a key role in maintaining their functions and properties [1–4].Most notable examples include theβ-sheet of proteins and the double helix of deoxyribonucleic acid (DNA) in nature.Inspired from nature, supramolecular self-assembly, which spontaneously in harness with the versatility of non-covalent interactions to associate simple building blocks into stable and well-defined architectures, has attracted considerable interest among academic researchers and industry over the last decade, and it is still a constantly growing research area [5–8].As one of the most important non-covalent driving forces, hydrogen bonding has seen considerable use in the construction of supramolecular polymers and various functional materials, molecular recognition, catalysis of organic reactions, and other fields [9–11].In fact, to date, considerable attention has been devoted to the development of well-defined supramolecular assemblies capable of undergoing self-association through hydrogen bonding interactions in organic solvents [12–14].However, in aqueous media, it remains a highly challenging issue to rationally exploit hydrogen-bond pairs to drive the hierarchical self-assembly of individual molecular building blocks into stable and orderly supramolecular structures, despite the omnipresent examples and their significance in biological assembly processes and molecular recognition.This is because water molecules, as a polar molecule and a strong hydrogen-bonding donor and acceptor, can effectively compete with directional polar interactions such as hydrogen bonds and strongly participate in non-covalent processes [15–18].More specifically, hydrogen-bonded mediated selfassembly in aqueous media has great advantages that are environmentally friendly and directly applicable for biological materials[19].
Non-covalent synthesis of supramolecular materials in the aqueous environment requires careful consideration of the molecular design of all components to produce stable materials [20].To realize precise structural control and high structural stability, many important biological processes (such as DNA assembly and protein folding) adopt a strategy based on shielding directional interactions into hydrophobic pockets, which is conducive to their complementary interactions [21–23].Inspired from nature, a possible strategy that may work well to drive and maintain the self-assembly of building blocks in aqueous environment is to ingeniously employ directional and complementary multiple hydrogen bonding interactions, in combination with the help of other non-covalent interactions.Further studies on the construction of supramolecular assemblies have shown that multiple hydrogen bonding interactions can be strengthened with the assistance ofπ-stacking [24–27].Among many multiple hydrogen bonding systems, ureidopyrimidinone (UPy)-based quadruple hydrogen bond self-complementary array with high directionality and strong binding capacity developed by the Meijer group in 1997 [28,29], is the most suitable H-bonding module to build unique architectures in aqueous media.It cannot be ignored that such systems based on UPy exist the equilibriums of proton tautomers (keto and enol) in solution[30].Unfortunately, there are various isomers in the supramolecular assembly based on this building block, which is unfavorable to the formation of well-defined architectures and essentially complicates the structures, properties and applications of the resulting supramolecular materials [31].In consequence, controlling over the keto-enol tautomerism of this kind of multiple hydrogen bond system to realize the specific arrangement of single quadruple hydrogen bonding dimeric array is an important prerequisite for designing building blocks to produce controllable and highly ordered artificial supramolecular assemblies in both organic and aqueous solvents.
Herein, we chose the self-complementary quadruple hydrogen bonding UPy motif with DADA array as the part of building block,which mimics nucleic acid base pair motifs found in DNA and provides four-fold hydrogen bonding interactions in the process of self-assembly.And, the easy synthesis, cheap raw materials and sizable association constant of ureido derivatives are attractive characteristics.Furthermore, in an effort to enhance hydrogen bonding interactions and build stable supramolecular structures in aqueous media, amphiphilic andπ-stacking strategies were also employed to increase the solubility of molecules in aqueous media and to provide nonpolar microenvironments to shield the multiple hydrogen bonding interactions from competitive water molecules.Following this general molecular design strategy, the building block designed here first forms dimers through the interactions of complementary quadruple hydrogen bonds, and further self-shields the directional hydrogen bonding interactions in the hydrophobic pockets of the aromatic rings by forming a characteristic sandwich structure, thus, one-dimensional linear growth along thex-axis is ensured, with concomitant parallel stacking along they-axis into a two-dimensional (2D) sheet structure (Fig.1a).The single crystal structures clearly demonstrate the formation of two-dimensional sheet structure along thex-axis andy-axis (Fig.1b).Besides, the driving force of aggregation along thez-axis comes from the weak intermolecular double hydrogen bonding interactions (Fig.S1 in Supporting information).
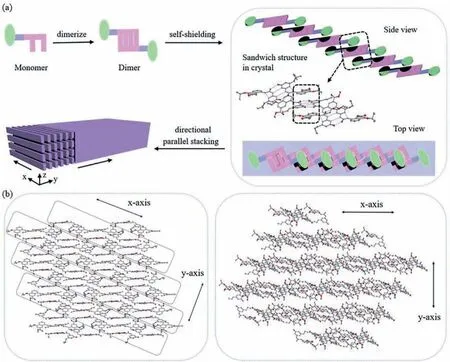
Fig.1 .(a) Schematic diagram of the dimerization of building block, further self-shielding into a one-dimensional linear chain along the x-axis, and finally parallel stacking into a two-dimensional (2D) sheet structure.(b) View of 2D structure in crystal along x-axis and y-axis (N, blue; O, red; C, gray).
Ureidopyrimidinone derivatives 1 and 2 were synthesized in a feasible way (Scheme S1 in Supporting information) and fully characterized by NMR spectroscopy and high-resolution ESI-MS (for details of synthesis and characterization, see Supporting information).Due to the inherent proton tautomerism of such system, firstly,we used a reasonable and simple supramolecular strategy to prevent the occurrence of tautomerism caused by intramolecular proton transfer, and the resulting derivatives 1 and 2 keeps only in the enol form (Fig.2a), which is expected to achieve sizable association constant and high stability.Within this molecule, an intramolecular six-membered conjugated H-bonded network and another five-membered hydrogen bond ring were introduced through urea group derivatization to generate a DADA-ADAD dimeric array with completely enol configuration.First of all, we need to determine the exact isomeric structure of building monomer 1, a single crystal X-ray diffraction study was carried out.The X-ray single crystal structure was obtained by the slow vapor diffusion at room temperature, in which 1 was dissolved in chloroform while methanol as antisolvent.In the crystal structure, it clearly demonstrates that the molecular structure of 1 adopts enol configuration in the solid state, with the formation of dimeric sequence DADA-ADADviafour intermolecular hydrogen bonds, which is in good agreement with our initial design (Figs.2b and c).Similarly,the NMR experiments were used to further investigate the formation of complete DADA-ADAD dimeric array from pyrimidin-4-ol monomer in deuterated chloroform (CDCl3) solution.1H NMR spectroscopy of 1 showed that there was only one set of NMR peaks, and the three NH proton signals between 9.5 ppm and 13.5 ppm in the down field area were the characteristic peaks of less strong hydrogen bonding interactions, corresponding to the formation of a pyrimidin-4-ol homodimersviaa quadruple DADAADAD array (Fig.S3 in Supporting information).On the other side,the existence of self-complementary DADA-ADAD dimers from 1 with enol form was also confirmed by the NOE effects between Haand Hbin two-dimensional NOESY spectrum (Fig.S4 in Supporting information).It is known that UPy is one of the most widely used units containing multicomponent hydrogen bond interactions due to its high association constants.Its derivative 1 has been successfully constructed without tautomerism that could be directly used as an excellent building monomer to further selfassemble into highly ordered supramolecular aggregates.Of special note, small variation to the structure of constituent molecules induces great effects on the solubility, and water-soluble molecular 2 was synthesized by the same method as 1.The difference between compounds 2 and 1 is that the side chain of quinoline was changed from isopropanol to solubilizing chain triethylene glycol,which provides solubility of molecules in water and is expected to form stable supramolecular assemblies in aqueous media through self-shielding directional multiple hydrogen bonds interactions into hydrophobic pockets.
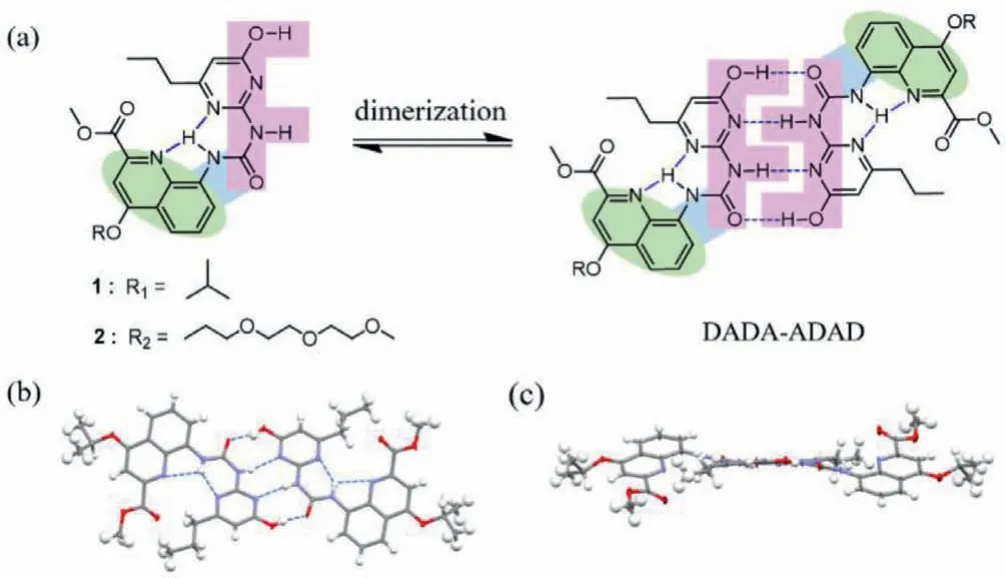
Fig.2 .(a) Molecular structure of compounds 1 and 2.(b) Single crystal X-ray structure of 1.(c) Side view of 1.
To provide information on aggregation behavior of hydrophobic association water-soluble molecule 2 in the occurrence of the intermolecular interactions, various spectroscopy measurements in aqueous solution were undertaken.As a reference, the aggregation behaviors of 2 in organic solvent were also characterized in detail by spectroscopy.Fluorescence titration experiments in chloroform showed that with increasing the concentration of 2, there was an obvious red shift from 420 nm to 460 nm (Figs.3a and b).Under the same conditions, a similar behavior was found for 2 in water, a significant red shift from 450 nm to 490 nm occurs in the fluorescence spectroscopy (Figs.3c and d).The ultravioletvisible (UV-vis) spectra in chloroform showed that, an absorption band around 370 nm increased nonlinearly with increasing the concentration of 2, and a deviated point at the concentration of approximate 7 μmol/L was observed (Fig.S5 in Supporting information).Whereas the deviated point of UV-vis spectra in water is at very low concentration, about 1 μmol/L (Fig.S6 in Supporting information).The critical concentrations of deviation of UV-vis absorption are essentially in agreement with those when the fluorescence emission spectrum is red shifted.From these, we conclude that under the synergistic effect of intermolecular four-fold hydrogen bonding andπ-stacking, self-assembly of 2 gave rise to the formation of aggregates in both organic and aqueous solvents.Yet it is worth noting that, in water, the transition concentrations of fluorescence and ultraviolet absorption spectrum of compound 2 are at lower concentrations when compared with 2 in chloroform,clearly indicating that water molecules here did not interfere with the self-assembly process driven by non-covalent interactions, and the aggregation ability of 2 in water became stronger than chloroform.
Scanning imaging technique is the most direct and definite way to evaluate the structural organization of these supramolecular aggregates.The self-assembled morphologies of 2 were investigated in aqueous solution, as revealed by transmission electron microscopy (TEM) and scanning electron microscope (SEM).Several microliters of aqueous solution of 2 were added dropwise on the freshly carbon support film copper surface, respectively, dried,and then observed under TEM.The stacked lamellar structures are visible for supramolecular assemblies based on building block 2 from TEM micrographs (Fig.4a).The same sample solutions were treated and further analyzed by using SEM.It could be seen in the SEM image that the aggregates of 2 formed flat 2D sheet-like structures, in good accordance with the results observed by TEM(Fig.4b).From the perspective of crystal structure, we analyzed the existence of sandwich structures with self-shielding function produces a hydrophobic microenvironment to allow the expression of directional multiple hydrogen bonding interactions, which plays a vitally important role for the generation of dimensionally confined nanostructures in aqueous media (Fig.1).To obtain more information about the topographical thickness of nanosheets, we also performed tapping mode atomic force microscopy (AFM) experiments, which has been an important tool for direct observing the height of samples on nanoscale.Bulk studies with AFM indicated that the nanosized sheets of 2 are very flat with an approximate thickness of 5.0 nm (Fig.4c).In addition, the thickness of self-assembled nanosheets of 1 in organic solution is about 4.1 nm (Fig.4d), which is consistent with the height of oligomeric tetramer formed by intermolecular double hydrogen bonding interactions along thez-axis (Fig.S2 in Supporting information).According to these findings shown here, the self-assembly of dimensionally confined nanosheets with uniform thickness was realized by self-shielding multiple hydrogen bonding interactions in aqueous media.
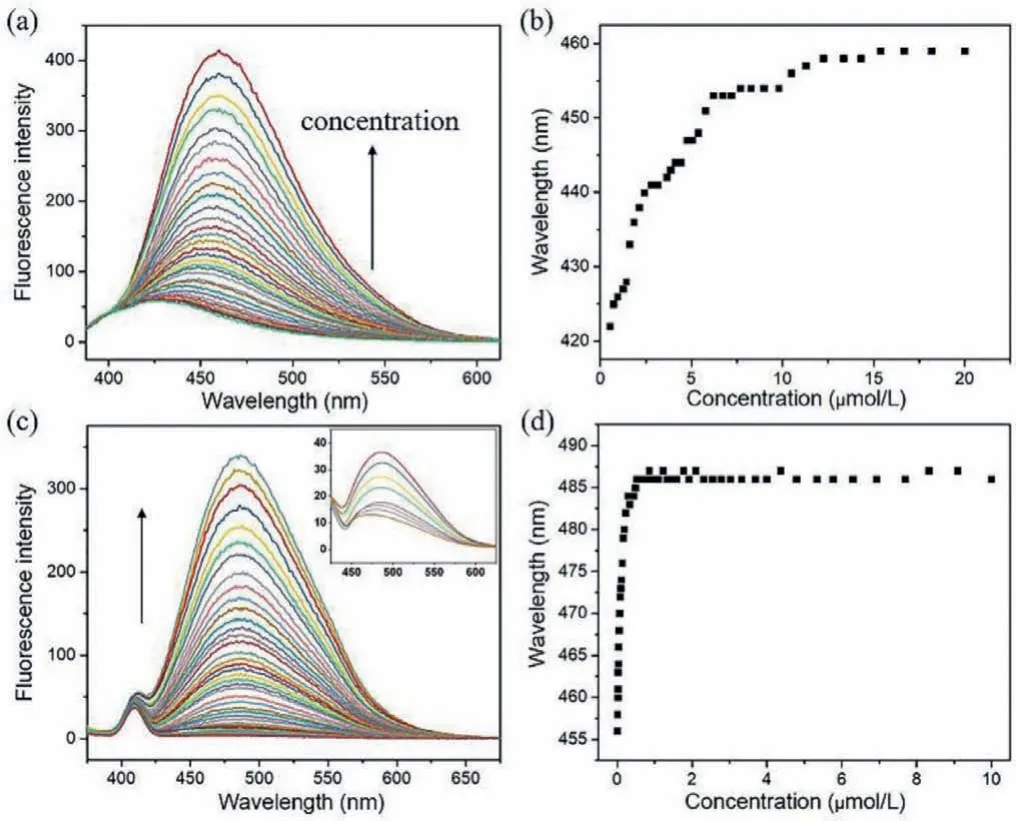
Fig.3 .(a) Fluorescence spectra of 2 at different concentrations (0–20 μmol/L) in CHCl3.(b) The maximum emission wavelength of 2 varied with the concentration.(c) Fluorescence spectra of 2 at different concentrations (0–10 μmol/L) in H2O.(d)Variation of the maximum emission wavelength versus concentrations.
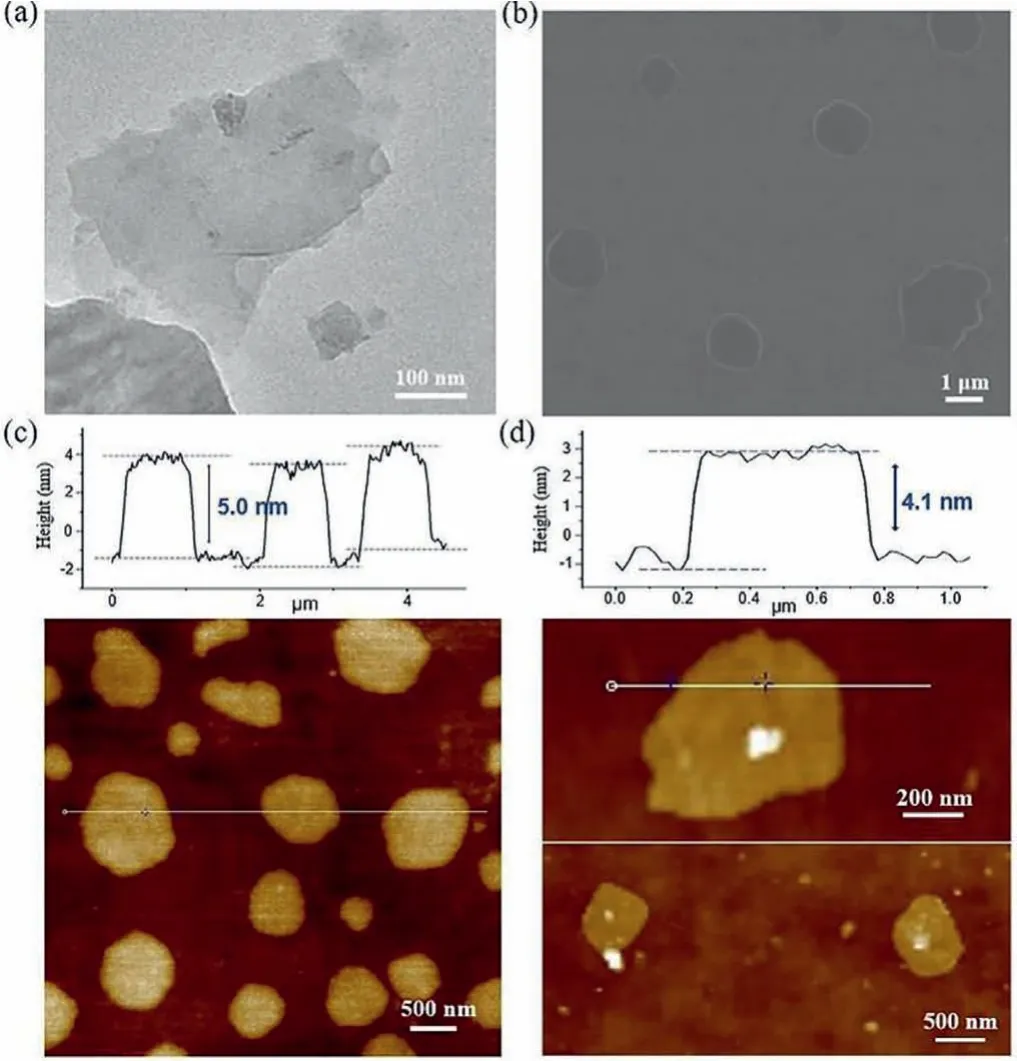
Fig.4 .(a) TEM, (b) SEM and (c) AFM images of 2 self-assembled into nanosheets in water.(d) AFM images of 1 self-assembled into nanosheets in chloroform.
In conclusion, we are able to design and synthesize ureidopyrimidinone derivatives 1 and 2 with complete enol configuration,which can be used as an excellent building unit to construct supramolecular assemblies with clear and definite structures.The solubility properties can be adjusted through subtle molecular design.By replacing the original hydrophobic side chain of quinoline moiety with triethylene glycol, water-soluble small molecule 2 was obtained.Just as hydrogen bonding in the interiors of watersoluble proteins is protected by the hydrophobic microenvironments, the self-complementary quadruple hydrogen bonds hidden inside of the hydrophobicπ-stacking pockets of quinoline are stabilized for the expression of hydrogen bonding interactions in the self-assembly, thus enabling the formation of highly stable and ordered sheet-structures in aqueous media.Nanosheets with different thicknesses can be obtained by tuning the length of the side chain of molecular monomer.Moreover, different functional groups could be incorporated at the end of assembled monomer, which may open the door to the new properties and functions of dimensional confined nanosheets.Additionally, our findings provide conformationally definite building block for accurately manipulating the self-assembly and self-organization process during the creation of highly ordered systems, and provide fundamental insights into understanding of the multiple hydrogen-bonding interactions in aqueous environment, with important implications on the design and fabrication of supramolecular assemblies for various bioengineering and engineering applications, such as novel self-organized nanostructures, and new functional materials with specific properties.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.22071078, 92156012 and 21722403) and the Program for JLU Science and Technology Innovative Research Team(JLUSTIRT, No.2019TD-36).We appreciate the staffs from BL17B beamline of National Facility for Protein Science in Shanghai (NFPS)at Shanghai Synchrotron Radiation Facility, for assistance during data collection.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.023.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
