Iron-catalyzed hydroaminocarbonylation of alkynes:Selective and efficient synthesis of primary α,β-unsaturated amides
Zijun Hung, Ji Tng, Xiongwei Jing, Tinle Xie, Minmin Zhng, Donghui Ln,Shofeng Pi, Zhengde Tn, Bing Yi,∗, Yuehui Li
a Hunan Province Key Laboratory of Environmental Catalysis and Waste Rechemistry, College of Materials and Chemical Engineering, Hunan Institute of Engineering, Xiangtan 411104, China
b State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou 730000, China
Keywords:Iron-catalyzed Alkynes Ammonium bicarbonate Aminocarbonylation Linear α,β-unsaturated amides
ABSTRACT α,β-Unsaturated primary amides are important intermediates and building blocks in organic synthesis.Herein, we report a ligand-free iron-catalyzed hydroaminocarbonylation of alkynes using NH4HCO3 as the ammonia source, enabling the highly efficient and regioselective synthesis of linear α,β-unsaturated primary amides.Various aromatic and aliphatic alkynes are transformed into the desired linear α,βunsaturated primary amides in good to excellent yields.Further studies show that using NH4HCO3 as the ammonia source is key to obtain good yields and selectivity.The utility of this route is demonstrated with the synthesis of linear α,β-unsaturated amides including vanilloid receptor-1 antagonist TRPV-1.
α,β-Unsaturated amides are important structural motifs ever present in wide range of natural products and materials science frameworks [1–8].For instance,α,β-unsaturated amides derivatives display biological activities applied in the treatment of psychological diseases and showed remarkable potential for the treatment of cancer (Fig.1) [9–11].Besides,α,β-unsaturated amides are versatile building blocks in organic synthesis [12–17].Synthetic methods forα,β-unsaturated amides preparation has attracted broad interest due to important applications in medicine and organic synthesis.The development of greener, low-cost, energyefficient and selective synthetic methods forα,β-unsaturated amides preparation has attracted broad interests.
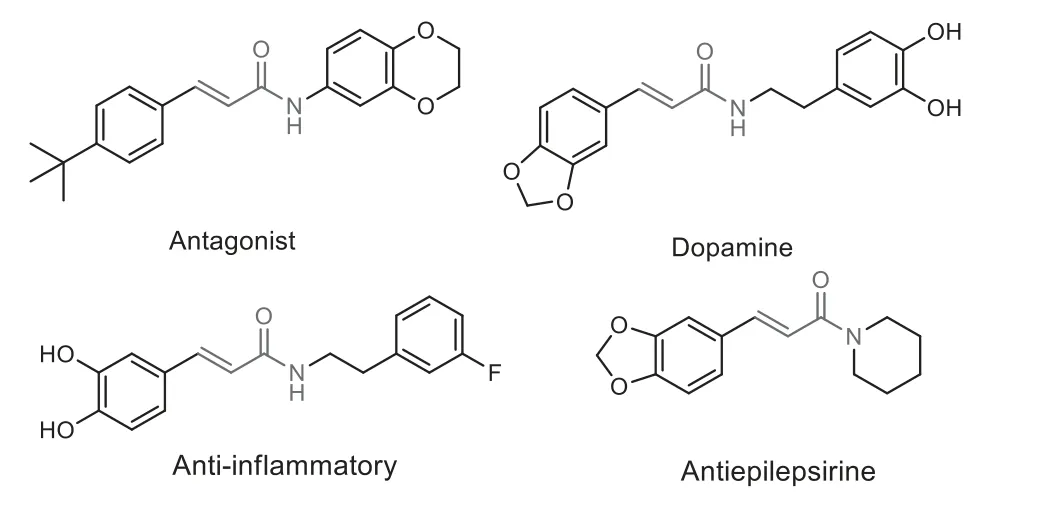
Fig.1 .Selective examples of bioactive linear α,β-unsaturated amides.
Traditional synthetic methods for unsaturated amides are generally, based on nucleophilic substitution of amines with carboxylic acids derivatives and acyl chlorides or cyclization with amic acids in the presence of activating reagents (Scheme 1a) [18–23].Transition metal catalyzed hydroaminocarbonylation provides a high atom- and step-economy pathway for producing high value-added unsaturated amides [24–37].Reports on catalytic hydroaminocarbonylation of alkynes have been documented in literature by reacting primary and secondary amines for the preparation of the correspondingα,β-unsaturated amides with high chemo- and regionselectivity (Scheme 1b) [38–46].However, noble metals, costly ligands or additives were necessary to achieve reasonable yields and good regioselectivity [27,38,39,41-43].The development of more efficient carbonylation methods for the synthesis of unsaturated primary amides remains desirable.
Solid ammonium salts are cheaper and easy-to-handle ammonia source widely applied in aminocarbonylation reactions to produce various amide compounds [47–56].Recently, we have developed the efficient iron-catalyzed aminocarbonylation of alkynes to produce succinimides with NH4HCO3[54].Huang and co-workers developed a palladium-catalyzed aminocarbonylation method for transforming alkenes with NH4Cl into the corresponding amides [55].Besides, Huanget al.reported a selective palladium-catalyzed hydroaminocarbonylation reaction between alkynes and NH4Cl to branchedα,β-unsaturated primary amides[56].Liu’s group reported a hydroaminocarbonylation reaction with alkynes and NH4HCO3to generate branchedα,β-unsaturated primary amides (Scheme 1c) [37].Nevertheless, developing non-noble metal-catalyzed hydroamin-ocarbonylation with NH4HCO3for the preparation linearα,β-unsaturated primary amides has not been achieved up to date.Herein, we report the first example of ligandfree iron-catalyzed hydroaminocarbonylation of alkynes to generate linearα,β-unsaturated primary amides using NH4HCO3as ammonia source (Scheme 1d).
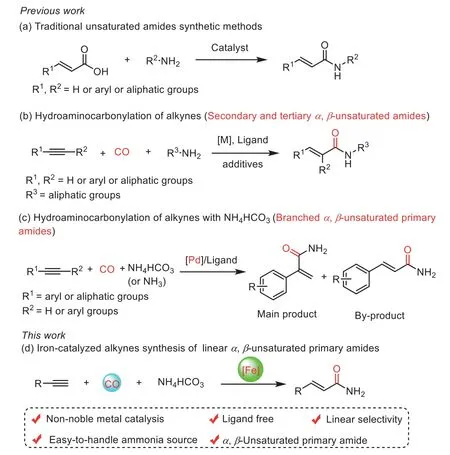
Scheme 1 .Synthetic strategy of α,β-unsaturated amides.
Initially, phenylacetylene 1a was selected as the model substrate for this hydroaminocarbonylation reaction.A series of commercially available catalysts were examined with NH4HCO3used as ammonia source in the presence of CO.When iron salts such as FeCl3and FeCl2were used, no desiredα,β-unsaturated amide product 2a could be observed.To our delight, when Fe3(CO)12was used as catalyst the hydroaminocarbonylation reaction proceeded successfully and gave the desired cinnamamide 2a in 46% yield.The use of Fe2(CO)9instead of Fe3(CO)12as catalyst afforded the desired product in only 13% yield for 2a (Table 1, entries 1–4).In addition, other metal carbonyls were found inefficient for this transformation, with no desired product could be detected(Table S1 in Supporting information).Moreover, carrying out the reaction without Fe3(CO)12resulted in undetected product, while adding PPh3as ligand was ineffective for hydroaminocarbonylation reaction (Table 1, entries 5 and 6).We observed that reaction temperature and CO pressure played important role in this reaction.Cinnamamide 2a was prepared in 81% yield when the reaction was performed at 140°C under 30 bar CO (Table 1, entries 7–11).The yield was slightly decreased to 62% at 160°C (Table 1, entry 12).Increasing the amount of NH4HCO3to 5.0 mmol improved the yield significantly, and the desired product 2a was obtained in 91% yield(Table 1, entries 13 and 14).Further screening of other reaction parameters such as solvent and the catalyst loading did not improvethe reaction yield (Table 1, entry 15 and Table S2 in Supporting information).We also examined the effect of the ammonium salts and found (NH4)2CO3and HCOONH4proved to be effective as ammonia source.However, NH4Cl or NH4OAC were found inefficient for the hydroaminoca-rbonylation reaction (Table S3 in Supporting information).
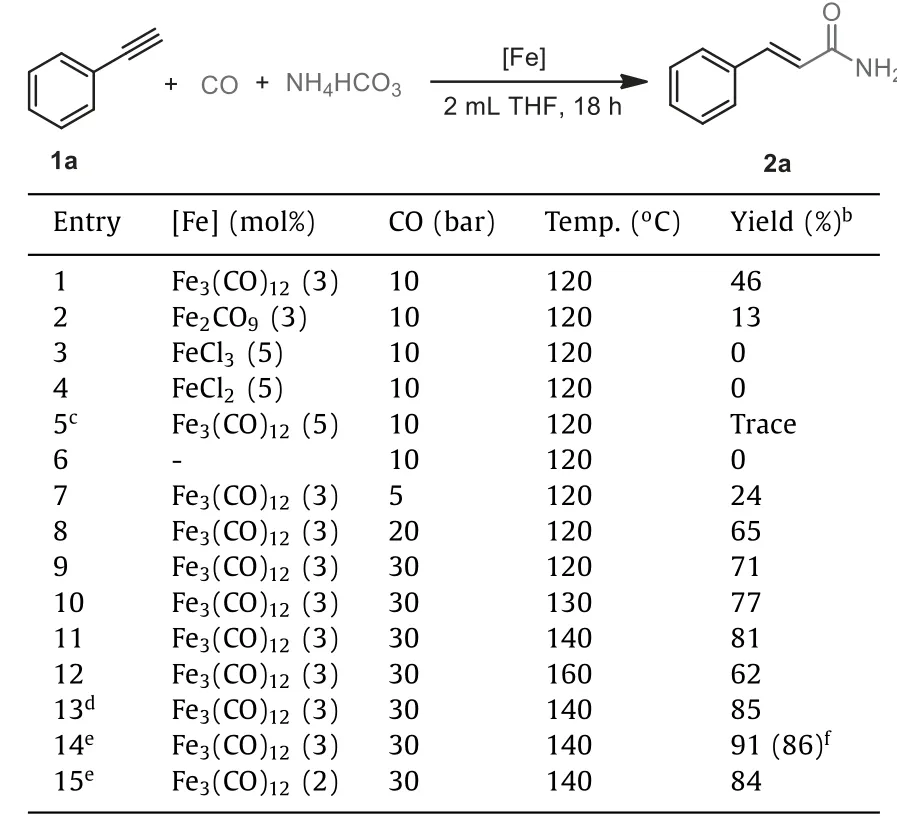
Table 1 Hydroaminocarbonylation of phenylacetylene.a
With the optimized reaction conditions in hand, we explored the scope of alkyne substrates for the synthesis of linearα,βunsaturated primary amides (Scheme 2).Gratifyingly, a range of alkynes are suitable substrates to react with NH4HCO3and CO under the optimized reaction conditions.The desired linearα,βunsaturated primary amides 2a-2q were obtained in 46%−91%yields.The electronic properties of the substituents on the aromatic ring of the aromatic alkynes have weaker influence on the reactivity and selectivity.The results ofmeta- andparasubstituted aromatic alkynes showed insignificant electronic effects.And surprisingly, the reaction of sterically hindered 1-ethynyl-2-methoxybenzene (1b) provided the desired linearα,βunsaturated primary amides in good yield (2b, 88%).Moreover,aromatic alkynes bearing electron-withdrawing substituents such as fluoro- and chloro– groups have less influence on the reactivity(2c, 84%; 2d, 83%; 2g, 81%; 2k, 83%).Similarly, aromatic alkynes with strong electron withdrawing groups like trifluoromethyl and esters substitution afforded linearα,β-unsaturated primary amides in moderate yields (2l, 79%; 2m, 81%).To our delight, aliphatic alkynes were also transformed in moderate to good yields,e.g., 2n,2o and 2p.Trace amounts of succinimide was detected under the optimized reaction conditions.The method could be applied for the internal alkyne 1,2-diphenylethyne (1q) to give moderate yield(46%).
To demonstrate the synthetic utility of this method, vanilloid receptor-1 antagonist TRPV-1 was prepared.3-(4-(tert–Butyl)phenyl)acrylamide 2j preparation proceeded smoothly at gram-scale and 1.68 g of 2j was obtained under the slightly modified reaction conditions in 83% yield (Scheme 3a).The subsequent coupling reaction of 3-(4-(tert–butyl)phenyl) acrylamide afforded vanilloid receptor-1 antagonist TRPV-1 4jr on a gram scale (Scheme 3b) [57].
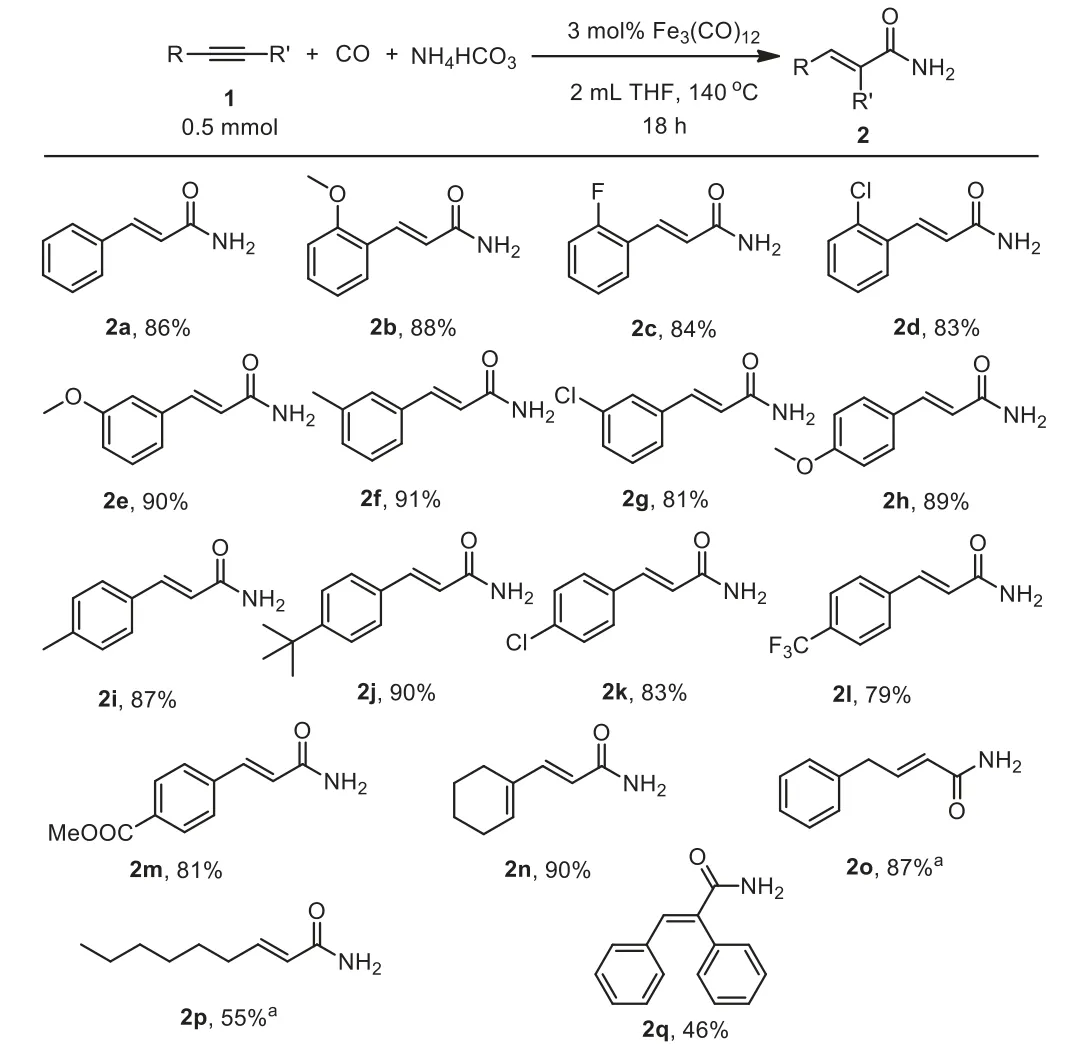
Scheme 2 .Substrate scope for hydroaminocarbonylation of alkynes using NH4HCO3.Reaction conditions: alkyne (1, 0.5 mmol), CO (30 bar), NH4HCO3(5.0 mmol), Fe3(CO)12 (0.015 mmol), THF (2.0 mL), 140°C, 18 h, isolated yield.a Yield was determined by GC using dodecane as an internal standard.
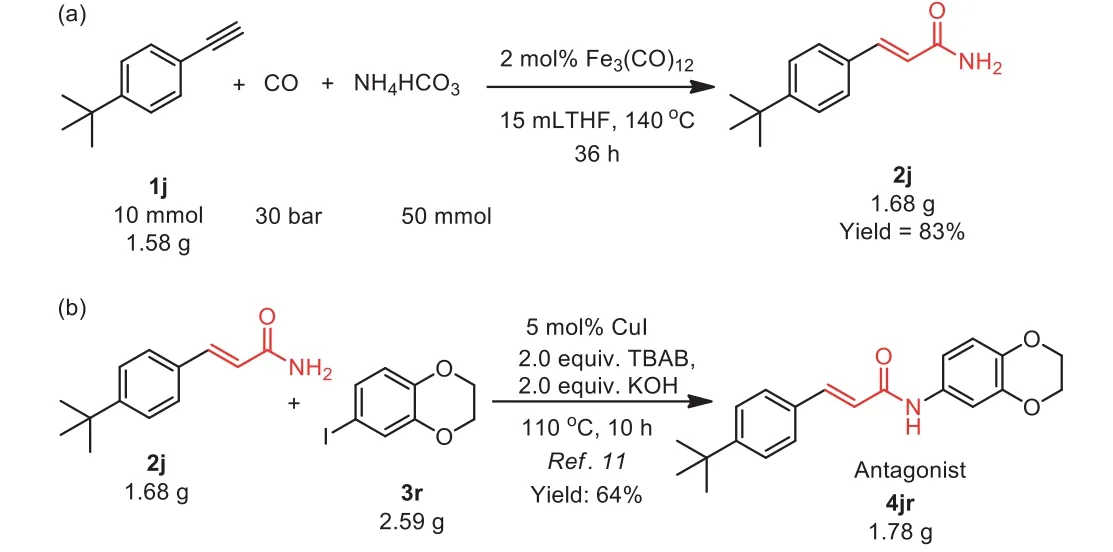
Scheme 3 .Scale-up reaction.
In order to better understand the mechanism of iron-catalyzed hydroaminocarbonylation of alkynes using NH4HCO3as the ammonia source, a series of experiments were performed (Scheme 3 and Fig.S5 in Supporting information).Firstly, hydroaminocarbonylation reaction in the absence of Fe3(CO)12of phenylacetylene 1a with NH4HCO3using CO was unable to provide the desired cinnamamide 2a (Scheme 4a).Furthermore, carrying out the reaction without CO resulted in undetected cinnamamide 2a (Scheme 4b).Cinnamamide 2a was detected when the reaction was performed with Fe3(CO)12serving as catalyst and CO source (Schemes 4c and d).Interestingly, cinnamamide 2a and succinimides 3a were detected when the reaction was performed with gaseous NH3(6 bar) serving as ammonia source instead of NH4HCO3under the optimized reaction conditions (Scheme 4e).Various solid ammonium salts used as ammonia source provided different results and showed clear influence on the reactivity (Table S3 in Supporting information).It was indicated that NH4HCO3promoted the formation [Fe]-H species responsible for the efficient hydroaminocarbonylation of alkynes, enabling the highly efficient and regioselective synthesis of linearα,β-unsaturated primary amides [26–28].We established that Fe(CO)5is formed from Fe3(CO)12reaction with NH4HCO3based onin situ13C NMR experiments using NH4HCO3and Fe3(CO)12[54].
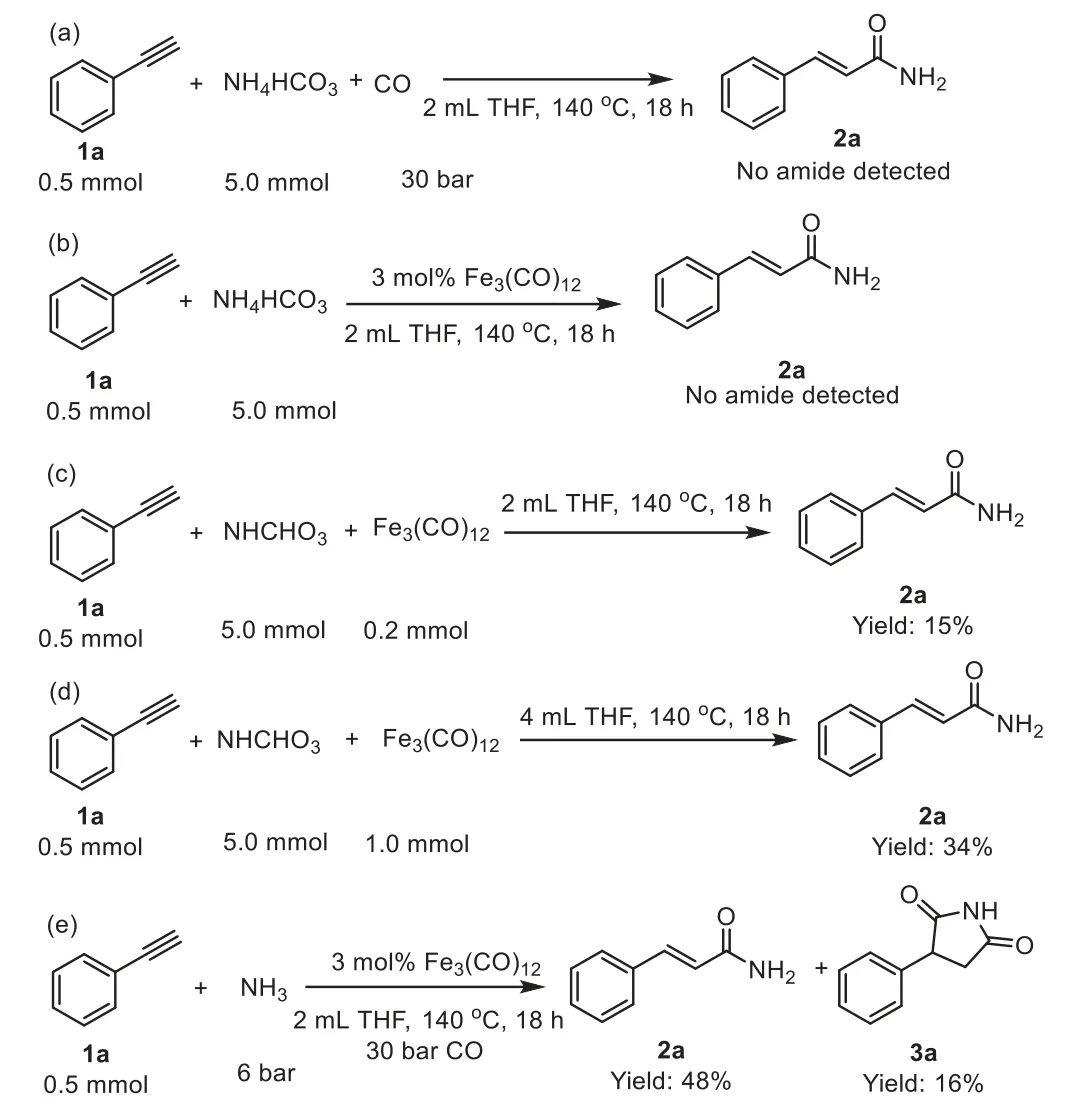
Scheme 4 .Control experiments.
Based on the experimental results and recent experimental data on the hydroaminocarbonylation of alkynes and NH4HCO4[24-28,54-56,58-62], we propose a possible mechanism pathway as shown in Fig.2.Initially, the active mononuclear iron carbonyl Fe(CO)5was formedin situthrough interactions of Fe3(CO)12with NH4HCO3.Meanwhile, NH3and H2CO3is released throughin situdecomposition of NH4HCO3.Then, Fe(CO)5, NH4HCO3and CO generate the intermediate A.Intermediate A reacts with alkyne to produce intermediate B or B’.The steric hindrance of the terminal alkyne plays the major role for the formation of the kinetically favored terminal alkenyl-iron intermediate B.Subsequent CO insertion forms intermediate C and C’, which then affords the final carbonylation product D and D’with the presence of NH3released from NH4HCO3.Besides, the coordination of alkyne substrates and NH3with Fe center makes CO insertion and product D formation much more accessible, which is similarly observed in Beller’s work using organic amines as the substrates [26].
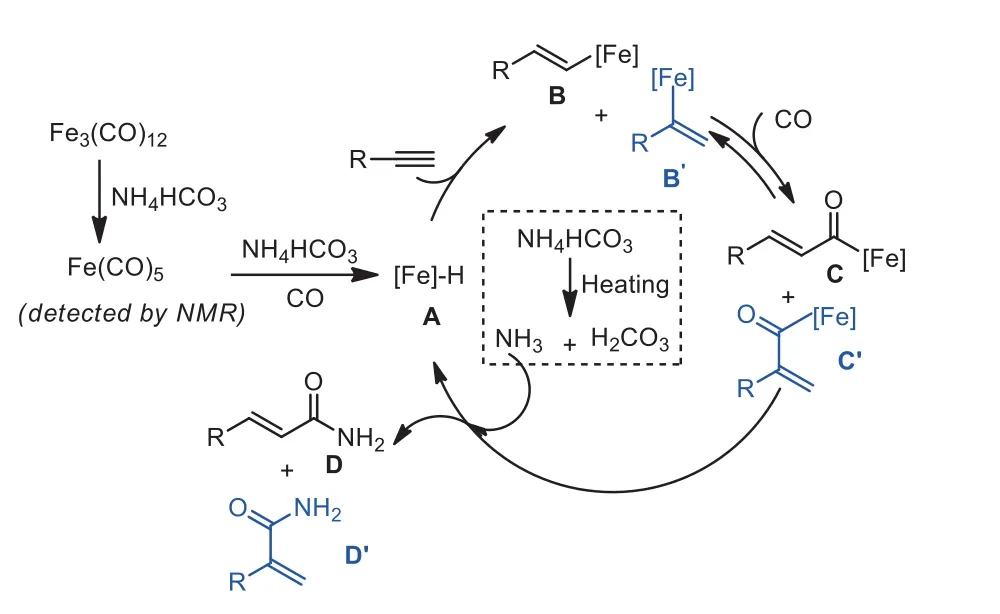
Fig.2 .Proposed reaction mechanism.
In summary, we have demonstrated the first example of ligandfree iron-catalyzed hydroaminocarbonylation of alkynes synthesis of linearα,β-unsaturated primary amides using NH4HCO3as the ammonia source.In the presence of NH4HCO3and nonnoble Fe3(CO)12serving as catalyst, a variety of alkynes, including aromatic alkynes, aliphatic alkynes, terminal alkynes, internal alkynes, were transformed into the desired linearα,β-unsaturated primary amides in good to excellent yields.The applicability of this methodology has been demonstrated by synthesis ofα,βunsaturated amides bio-active compound.Preliminary mechanistic studies reveal the activation model involving interactions of NH4HCO3with Fe3(CO)12.Further investigations are currently underway to apply the method to other reactions.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We are grateful for the financial supports from the National Natural Science Foundation of China (Nos.21772035, 22022204,22072167, 21202206) and Natural Science Foundation of Hunan Province (Nos.2021JJ40147).The authors would like to dedicate this work to Professor Matthias Beller (LIKAT) on the occasion of his 60thbirthday.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.080.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Distinct structural characteristics define a new subfamily of Mycoplasma ferritin
- Insight into the in vivo fate of intravenous herpetrione amorphous nanosuspensions by aggregation-caused quenching probes
- A TICS-fluorophore based probe for dual-color GSH imaging
- Toward accurate and efficient dynamic computational strategy for heterogeneous catalysis: Temperature-dependent thermodynamics and kinetics for the chemisorbed on-surface CO
- Ruthenium-modified porous NiCo2O4 nanosheets boost overall water splitting in alkaline solution
- Oral colon-targeted mucoadhesive micelles with enzyme-responsive controlled release of curcumin for ulcerative colitis therapy
