A cationic amphiphilic tetraphenylethylene derivative with hydrochromic sensitive property: Applications in anti-counterfeiting ink and rewritable paper
Qian Xu, Ziyu Qin, Yiling Bei, Shengyu Feng, Xing-Dong Xu
National Engineering Research Center for Colloidal Materials, Key Laboratory of Special Functional Aggregated Materials of Ministry of Education, Shandong Key Laboratory of Advanced Silicone Materials and Technology, School of Chemistry and Chemical Engineering, Shandong University, Ji’nan 250100, China
Keywords:Aggregation induced emission Amphiphilic compounds Hydrochromic responsive Tetraphenylethylene Green fluorescent ink
ABSTRACT Since the discovery of aggregation induced emission (AIE) phenomenon, various stimuli-responsive materials have been rapidly developed, but there are still great challenges in the application of ink printing due to the bad water solubility.In this research, a new cationic amphiphilic TPE-functionalized pyridine salt (TPE-OTs) was designed, which shows good water solubility and hydrochromic properties.The optical properties of the compound have been studied, which is equipped with the typical AIEE characteristics and TICT effect.The compound can self-assemble to form aggregates with a particle size of about 30 nm in water.What is more, the compound is responsive to the environmental humidity, whose fluorescent color changes from green to yellow as the humidity gradually increased.Based on this characteristic, we applied it to the fluorescent anti-counterfeiting ink, realizing the protection and encryption of information.
Information security is widely used in politics, military, economy, science and technology and other fields [1–5].With the continuous development of information technology, information security issues have become increasingly prominent, and various anti-counterfeiting technologies have gradually developed, which include texture anti-counterfeiting [6], invisible magnetic code technology [7,8], fluorescent anti-counterfeiting ink [9–12] and so on.Among them, fluorescent anti-counterfeiting ink is popular because of their ease of use and wide range of applications[13–16].The characteristic of anti-counterfeiting ink is to observe the color change of the ink print to achieve the purpose of anti-counterfeiting by implementing different external conditions,mainly using light, heat, and spectral detection.Consequently,various stimuli-responsive fluorescent materials provide more potential application prospects for anti-counterfeiting inks.
Unfortunately, most traditional fluorescent molecules lead to fluorescence quenching after aggregation [17].In 2001, Tang’s team discovered the aggregation-induced emission (AIE) and aggregation-induced emission enhancement (AIEE) phenomenon,which solved the problem in the aggregation process of traditional luminescent materials [18–22].Tetraphenylethylene (TPE) derivatives are hotly investigated in recent years for their broad applications in stimulus-responsive systems [23–30].Interestingly, besides AIE activity, TPE was found to be an electron donor, and has been utilized to construct luminogens exhibiting the twisted intramolecular charge transfer (TICT) behaviors by connecting the TPE group to an electron accepting group [31–33].Since the donoracceptor (D-A) structure of the TICT system is extremely sensitive to changes in environmental polarity, these luminogens characterized a strong response on environmental moisture changes and are commonly used as hydrochromic materials [34–37].For example, Tang and coworkers have developed an AIE sensor with good sensitivity and fast response/recovery time for humidity visualization [38].Using the TICT effect of AIEgens, the invisible information of humidity was converted into different fluorescent color signals that could be directly observed by the naked eye.However,these moisture-sensitive materials are rarely used for information encryption and most hydrochromic TPE derivatives used for encryption present poor solubility in water.Thus, the development of TPE derivatives with hydrochromic properties has pioneering significance for the application in information encryption and decryption.
Herein, based on our previous studies on luminescent materials and amphiphilic aggregates [39–46], we designed and synthesized a cationic amphiphilic TPE-functionalized pyridine salt (Scheme 1),named TPE-OTs and all of the desirable products were assigned using1H,13C NMR, mass spectroscopy analysis (see Supporting information for details).Appended to the fluorophore components were four pyridine-based receptors, which promoted the emission wavelength of the molecule to move to the long wavelength through D–A interactions.The pyridine salt formed by the protonation of the pyridine group and the four chains of triglycol monomethyl ether connected to the pyridine group endowed this molecule good solubility in water.
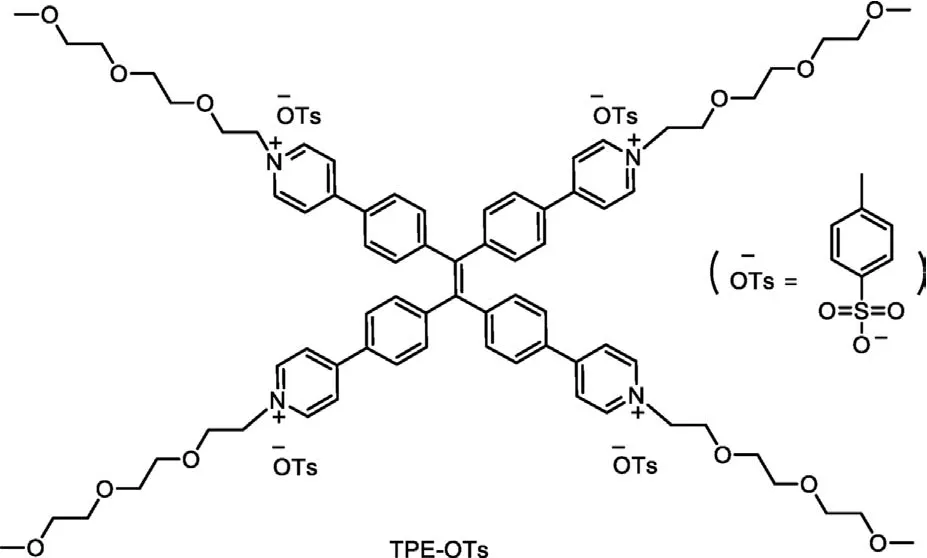
Scheme 1 .The chemical structure of TPE-OTs.
The ultraviolet absorption spectra and fluorescence spectra in dilute solution of different polar solvents were firstly studied in Fig.S4 (Supporting information).The absorption peaks (Fig.S4a)exhibited a blue shift from 372 nm (in DCM) to 358 nm (in H2O).From the fluorescence spectra (Fig.S4b), we could see a significant change from green (in DCM, 540 nm) to orange (in MeCN,582 nm) took place with an overall red shift of 42 nm as the polarity of the solvent increases.This phenomenon may be attributed to TICT effect.When the environmental polarity was increased, the luminogens became twisted and the charges were separated, leading to a red-shift in the light emission.To gain the better understand electronic structure, theoretical calculation of TPE-OTs was also conducted using B3LYP/6–311 G(d, p) basis set by Gaussian 09 program, which were simulated in gas and water phases environment with dielectric (ε) constants of 1.00 and 78.35, respectively.The optimized electronic structures in the absence of anions and the electron distributions of highest occupied molecular orbital (HOMO) and the lowest occupied molecular orbital (LUMO)were demonstrated in Fig.1.The electronic cloud of the LOMO is evenly distributed over the orbitals of the TPE unit and pyridinium moiety under two dielectric constants.Most of the electron cloud of the HUMO is transferred to the TPE unit in the aqueous phase while the HUMO in the gas phase is mainly located at the end of the alkoxy chain.Such distribution of HOMO and LUMO results in charge transfer and the formation of TICT.While in polar solvent,its bandgap becomes wider, which is consistent with the blue-shift in the absorption spectra.
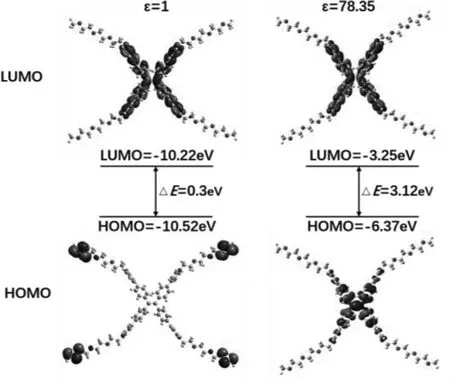
Fig.1 .Molecular orbital amplitude plots of the HOMO and LUMO of TPE-OTs calculated by using the B3LYP/6–31 G basis set in dielectric constants of 1.00 and 78.35,respectively.
Due to the AIEE properties of the TPE unit, we investigated the fluorescence spectra by employing DMSO as a good solvent and TOL as a poor solvent.As shown in Fig.2a, with adding toluene into the DMSO solution, the compound exhibited weak orange fluorescence and the emission intensity decreased slowly in the range of the toluene fraction (ft) from 0 to 80%.The fluorescence intensity slightly began to increase at 90% offt.When theftwas increased to 95% and 99%, the emission intensity was dramatically enhanced, and a bright yellow luminescence with a max at 541 nm could be observed.Moreover, the maximum emission wavelength showed a gradual blue-shift during the volume fraction of toluene increasing, which could be attributed to a reduced TICT caused by the decreased solvent polarity.The remarkable changes in color of TPE-OTs could be observed from the fluorescence photos in Fig.2c.In addition, TPE-OTs emitted orange fluorescence at 559 nm in DMSO/glycol binary solvents and the emission intensity increased gradually with the increasing of glycol component (Fig.S5 in Supporting information).These results suggest that the compound clearly featured typical AIEE characters.
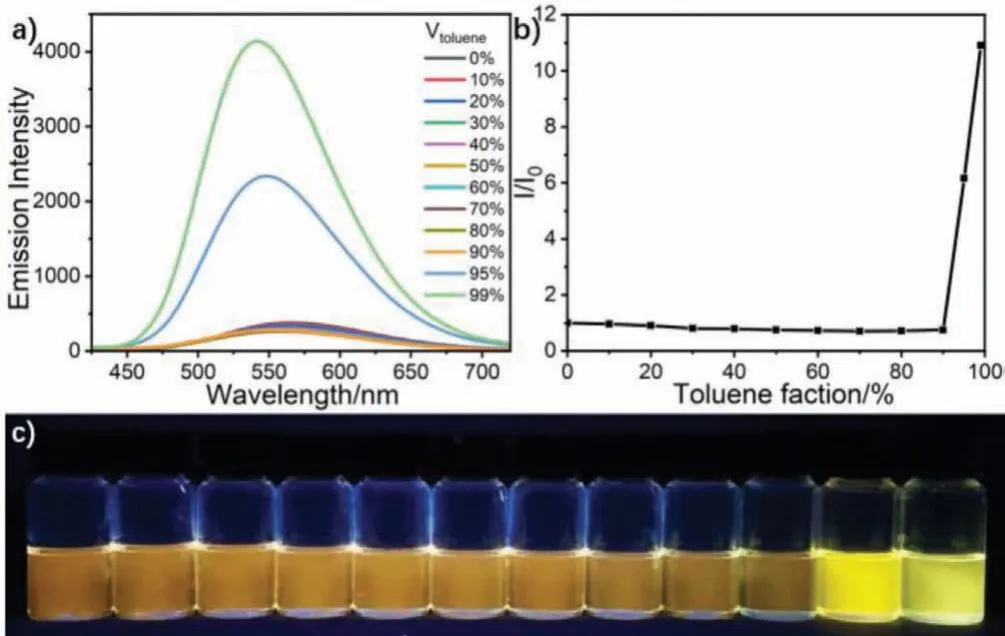
Fig.2 .(a) Fluorescence spectra of TPE-OTs (2.0×10−5 mol/L) in DMSO/toluene mixtures with different toluene volume fractions.(b) Plot of relative PL intensity (I/I0)versus the composition of the DMSO/TOL mixtures of TPE-OTs where I0 was the emission intensity in pure DMSO.Excitation wavelength: 365 nm.(c) Fluorescent photographs of TPE-OTs in mixed solvents with different toluene volume fractions taken under 365 nm UV irradiation.
In consideration of the amphiphilic nature and good water solubility of the compound, we determined to measure the selfassembled behavior and morphologies in pure water.The formation of irregular spherical aggregates with average hydrodynamic diameters of about 30 nm could be verified by transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) images in Figs.3a and b.Meanwhile, the gas phase environment morphology scan of atomic force microscopy(AFM) was also employed to further investigate the aggregation in Fig.3c, which were consistent with the TEM studies.Additional TEM images and AFM images of the compound were shown in Fig.S6 (Supporting information).Moreover, as presented in Fig.3d,dynamic light scatterings (DLS) reveals that the size distribution of nano-aggregates is concentrated around 30 nm.As presented in Fig.S7 (Supporting information) the absolute luminescence quantum yield (φ) of the compound in water is 2.93%.The successful formation of irregular spherical nanoparticles is likely due to the amphiphilic structure of the molecule.
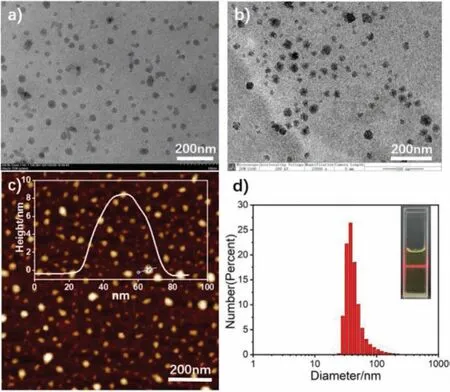
Fig.3 .TEM (a), HRTEM (b), AFM (c) images, DLS data and Tyndall effect (d) of TPE-OTs (2×10−4 mol/L) nanoparticles in water.
The material, being pyridine salt, is sufficiently hygroscopic in the solid state and thus this inspired us to study the sensitivity of the compound to relative humidity (RH) in the environment.A piece of conventional filter paper was soaked in the dichloromethane solution of TPE-OTs.The TPE-OTs-loaded paper after air-drying was served as a humidity probe to detect the humidity change.Different saturated salt solutions (LiCl, KAc, MgCl2,Mg(NO3)2, NaCl, KCl, KNO3) were prepared in bottles to simulate the atmospheric humidity (11.3%, 22.5%, 32.8%, 54.4%, 75.3%, 84.3%,93.6%), respectively (Fig.4a).The TPE-OTs-loaded paper was sealed in different humidity atmospheres for 1 h at 25°C.Subsequently the fluorescence spectra were detected in Fig.4b.With an increase in RH, the maximum emission wavelength was progressively redshifted from 511 nm to 530 nm.The CIE chromaticity diagram(Fig.4c) was used to show the variation in the fluorescence color from the initial green to yellow.The same change could be seen in the photographs (Fig.4a) of the TPE-OTs-loaded paper under UV lamp at 365 nm.What is more, to text the reversibility and repeatability of TPE-OTs-loaded paper, the RH was repeatedly varied from 11.3% (dry) to 93.6% (wet) andvice versa, and the emission center (λmax) was checked.Results suggest that there is little change of emission maximum even after 9 consecutive “wet”/“dry”cycles (Fig.4d).This demonstrates the excellent reversibility and stability of the hydrochromic effect in these materials.
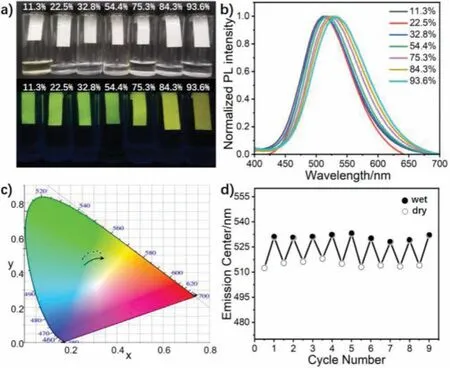
Fig.4 .(a) Comparison images of the TPE-OTs-loaded paper in different humidity under natural light and UV lamp at 365 nm.(b) PL spectra of the TPE-OTs-loaded paper in different humidity environments at 25°C.(c) CIE chromaticity diagram of the (x,y) color coordinates of the TPE-OTs-loaded varying with humidity.(d) Reversible cycling of the fluorescence emission center (λmax) by successive drying and wetting cycles for the TPE-OTs-loaded paper.
Fluorescent dyes have been used as anti-counterfeiting labels widely.According to the characteristics of the hydrochromic properties and good water solubility of the TPE-OTs, we designed and developed invisible anti-counterfeiting ink to increase the security of important information.As shown in Fig.5a, we dissolve the compound in water and injected it into a conventional inkjet print cartridge.Then draw the pattern that needed to be encrypted on the computer, and use the ink to print the information on nonfluorescent paper.The maple leaves can not be seen under the room lighting (Fig.5b), while emitted light-green fluorescence under 365 nm UV irradiation (Fig.5c).The maple leaves turn yellow after being fumigated for 30 s with water vapor (Fig.5d), which displayed good responsiveness to water vapor.What’s more, the fluorescent color changes were reversible, when the water evaporates, the maple leaves turn back to light-green.And the obvious color changes still exist after repeating 10 times, which meant that the reversible process had good stability.
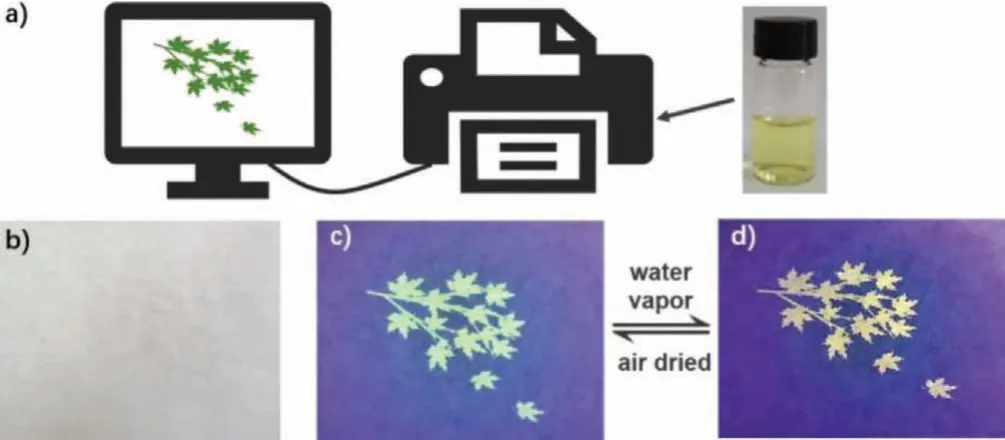
Fig.5 .(a) Schematic illustration of the inkjet printing process.(b) The paper after printing the patterns under natural light.(c) The pattern printed with TPE-OTs-ink under 365 nm UV lamp.(d) The pattern after steam fumigation.
Based on the above reversible fluorescence conversion characteristics of humidity response, a new technology for rewritable paper was designed.The TPE-OTs-loaded paper after air-drying(Fig.6b) did not differ much from conventional filter paper(Fig.6a) under natural light, while it appears green fluorescence(Fig.6c) under 365 nm UV lamp.When using pure water as ink to print the required information on the TPE-OTs-loaded paper, the information was not readable under sunlight, but appeared readable orange under ultraviolet light.With the volatilization of water, the orange information gradually disappeared, and the load paper returned to green for reuse.For example, the famous saying of the German chemist Mendeleev (Fig.6d) was first printed and gradually disappeared (Fig.6e) after volatilization of water.After the water evaporated, the chrysanthemum (Fig.6f) and pineapple(Fig.6h) were printed sequentially and disappeared in the same way (Figs.6g and i), respectively.There was little change of the TPE-OTs-loaded paper in color intensity after being rewritten many times, and the paper loaded with TPE-OTs could be stored for six months up to now or even longer.This indicated it was possible to develop ink-free rewritable paper by introducing fluorescent dyes.From a green perspective, water is a renewable resource and obviously poses no risk to the environment so that it would be ideal if water can be used as the trigger for the development of rewritable paper.And this rewritable printing is pointing a good direction on green life and will promote global sustainable development.
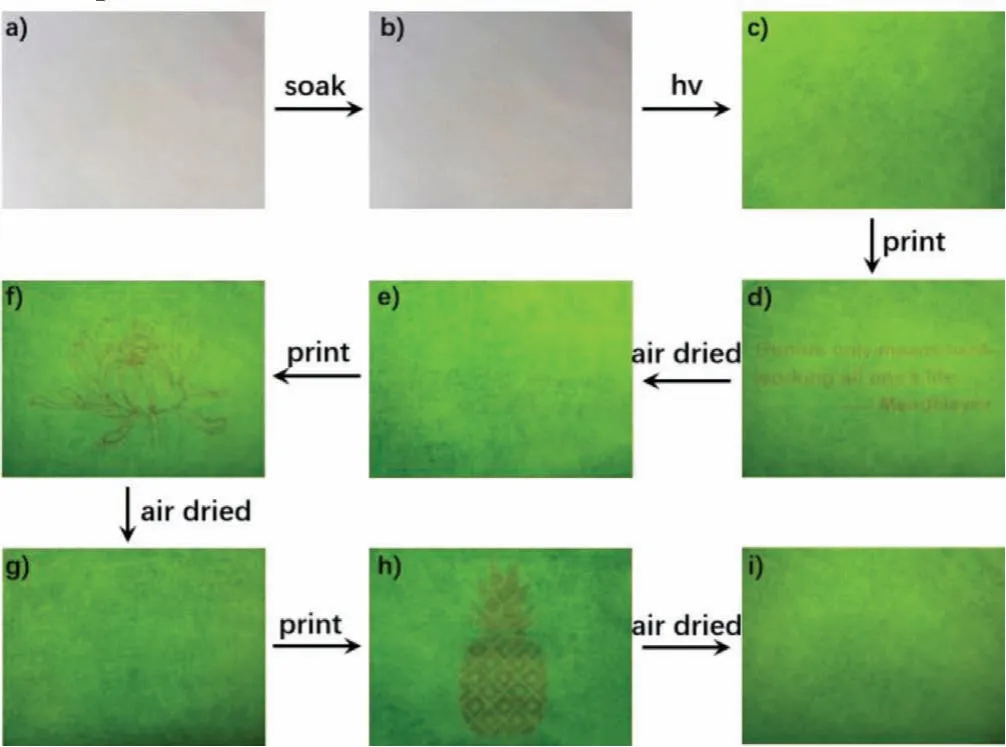
Fig.6 .(a) Paper before impregnation.(b) Paper after being immersed with TPE-OTs under natural light.(c) Paper after being immersed with TPE-OTs under 365 nm UV lamp.Patterns of famous saying (d), chrysanthemum (f) and pineapple (h) printed separately using water as ink under 365 nm UV lamp.Paper, respectively corresponded to the famous saying (e), chrysanthemum (g) and pineapple (i) after the water evaporated.
In summary, a cationic amphiphilic TPE-functionalized pyridine salt TPE-OTs decorated with triglycol monomethyl ether chains was successfully prepared.This compound possesses typical AIEE behavior in DMSO/TOL solvent mixture and DMSO/glycol binary solvent.What’s more, irregular spherical aggregates with average hydrodynamic diameters of about 30 nm could be formed in water.Because the compound is sensitive to the change of environmental humidity, we made the TPE-OTs-loaded paper as a humidity probe.With the increase of RH, the fluorescent color of paper changes from green to yellow.And the TPE-OTs-loaded paper is equipped with good reversibility and stability.In addition, taking into account of the hydrochromic properties, the TPE-OTs can be developed as anti-counterfeiting ink that exhibited good response to water vapor.A new technology for rewritable paper loaded with TPE-OTs is further designed, showing a wider application value in information security.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.52173102, 21774070 and 21602124), Fluorine Silicone Materials Collaborative Fund of Shandong Provincial Natural Science Foundation (Nos.ZR2021LFG001,ZR2021LFG007), and the Young Scholars Program of Shandong University (No.2018WLJH40).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.079.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
