Bromate formation during oxidation of bromide-containing water by the CuO catalyzed peroxymonosulfate process
Jingxin Yng, Hongrui M, Chun Wng,∗, Hong Liu
a Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Institute of Environmental Research at Greater Bay,Guangzhou University, Guangzhou 510006, China
b Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences, Chongqing 400714, China
Keywords:Bromate Peroxymonosulfate (PMS)CuO SO4•−Bromide Heterogeneous catalyst
ABSTRACT Bromate formation has been found in the SO4•−-based oxidation processes, but previous studies primarily focused on the bromate formation in the homogeneous SO4•−-based oxidation processes.The kinetics and mechanisms of bromate formation are poorly understood in the heterogeneous SO4•−-based oxidation processes, although which have been widely studied in the eliminations of micropollutants.In this work, we found that the presence of CuO, a common heterogeneous catalyst of peroxymonosulfate (PMS),appreciably enhanced the bromate formation from the oxidation of bromide by PMS.The conversion ratio of bromide to bromate achieved over 85% within 10 min in this process.CuO was demonstrated to play a multiple role in the bromate formation: (1) catalyzed PMS to generate SO4•−, which then oxidizes bromide to bromate; (2) catalyzed the formed free bromine to disproportionate to bromate; (3) catalyzed the formed free bromine to decomposed back into bromide.In the CuO-PMS-Br system, bromate formation increases with increasing CuO dosages, initial CuO and bromide concentrations, but decreases with increasing bicarbonate concentrations.The presence of NOM (natural organic matter) resulted in a lower formed bromate accompanied with organic bromine formation.Notably, CuO catalyzes PMS to transform more than 70% of initial bromide to bromate even after recycled used for six times.The formation of bromate in the PMS catalysis by CuO system was also confirmed in real water.
Bromate is a well-known disinfection byproduct in drinking water treatment [1].As a class 2B carcinogen stipulated by World Health Organization (WHO), bromate is regulated at a maximum contaminant level (MCL) of 10 μg/L in drinking water in many countries [2,3].It is typically detected during the ozonation of bromide-containing water [4,5].The kinetics and mechanisms of bromate formation during ozonation have been well understood previously [1,6,7].
In recent decades, bromate was also found to form in the bromide-containing water treated by sulfate radical-based advanced oxidation processes (SO4•−-based AOPs) [8–10].For example, significant bromate formation was observed in both UV/persulfate and Co(II)/peroxymonosulfate processes, where SO4•−is reported to play a critical role in the bromate formation[8,11].However, previous studies involving the bromate formation in the SO4•−-based systems almost focused in the homogeneous processes [12].Apart from homogeneous catalysts, heterogeneous catalysts can also activate peroxymonosulfate (PMS) or persulfate(PDS) to produce SO4•−[13–16].As compared to the homogeneous processes, the advantages of the heterogeneous process include applicability over a wide pH range, low toxicity, high stability and easy separation [17,18].However, little information is available on the bromate formation in the heterogeneous SO4•−-based systems in the bromide-containing water.
Cupric oxide (CuO) has been reported to be an effective heterogeneous catalyst of PMS for the generation of SO4•−to eliminate various emerging micropollutants in aqueous solution [19–20].More importantly, CuO was found to appreciably catalyze and accelerate the slow disproportionation of free bromine to form bromate and bromide during chlorination in a recent decade [21,22].Accordingly, the kinetics and mechanisms of bromate formation in the CuO/PMS process must be different from that in the homogeneous processes reported previously due to the involvement of CuO.Thus, the bromate formation in the CuO/PMS process should be comprehensively evaluated.To the best of our knowledge, however, the bromate formation in the CuO/PMS process still remains unexplored.
Therefore, the main objectives of this study are (1) to investigate the kinetics of bromate formation in the CuO/PMS process as a function of CuO, PMS, bromide, bicarbonate and humic acid concentrations and pH; (2) to elucidate the mechanisms of the bromate formation in the CuO/PMS process; (3) to evaluate the bromate formation in the real water by CuO/PMS process.
All chemical solutions in this work were prepared with reagent-grade chemicals and ultrapure water produced from the Millipore system (18.2 MΩcm).Peroxylmonosulfate (Oxone, KHSO5·0.5KHSO4·0.5K2SO4), potassium bromide (KBr), potassium bromte (KBrO3), copper nitrate trihydrate (Cu(NO3)2·2H2O),sodium hypochlorite solution (NaOCl, 6%−14% active chlorine), 2,6-dichlorophenol (99.0%),tert–butyl alcohol (t-BuOH, ≥99.8%) and sodium sulfite (Na2SO3, 98.0%) were obtained from Aladdin Industrial Corporation.Sodium bicarbonate (NaHCO3, ≥99.5%), sodium hydroxide (NaOH, ≥96.0%) and nitric acid (HNO3) were purchased from Sinopharm Chemical Reagent Co., Ltd.5,5-DimethylpyrrolidineN-oxide (DMPO) was purchased from Sigma-Aldrich.CuO particles were prepared by the previously reported method[18].The real water samples used in this work were characterized as follow: pH 8.0, UV254= 0.016, DOC = 3.8 mg/L, Br−was not detected for tap water, and pH 7.8, UV254= 0.057, DOC = 6.6 mg/L,[Br−] = 12.9 μmol/L for surface water.The real water samples were filtered through a 0.45-μm membrane before use.
All experiments were performed in a 500 mL batch-reactor covered with aluminum foil, which were under continuous agitation using a magnetic stirrer.The pH value of experimental solution was adjusted to the desired value with HNO3(0.1 mol/L) and NaOH(0.1 mol/L).Reactions were initiated by adding desired amounts of PMS and CuO into bromide-containing solutions.Water samples were withdrawn at predetermined reaction times and filtered through a 0.22 μm syringe filter, which were rapidly quenched with 2,6-dichlorophenol initially followed by adding 10 mmol/L sulfite for the analysis of reactive bromine, bromide and bromate.All experiments were conducted in duplicate and the results represent the average values.Standard deviations (± SD) were all within 10%.
Bromide and bromate were quantified using ion chromatography (IC, Thermo Dionex ICS 3000) with an eluent containing 30 mmol/L KOH.Reactive bromine was determined as 4–bromo-2,6-dichlorophenol, an intermediate product of reactive bromine with 2,6-dichlorophenol.It is measured using high performance liquid chromatography (HPLC) equipped with a C18 column(4.6 mm × 250 mm; 5 μm particle size), with an eluent containing 0.5% acetic acid, and acetonitrile (30:70, v/v) atλ= 252 nm.PMS was determined using spectrophotometric method.Total organic bromine (TOBr) was measured using a Multi 2500 TOX analyzer (Jena).Electron paramagnetic resonance (EPR) experiments were conducted on a Bruker A200 spectrometer.
Fig.1 shows the time-dependent evolution of bromide, free bromine, bromate and the total bromine from the CuO/PMS process in synthetic water, at initial concentration of bromide, PMS and CuO of 20 μmol/L, 1 mmol/L and 0.2 g/L, respectively.The concentration of bromide declined rapidly within the first 4 min of the reaction and then almost remained unchanged.The evolution of free bromine exhibited a sharp increase followed by a decrease as the reaction proceeded and peaked at 11.0 μmol/L within 2 min.The formed bromate increased rapidly within first 10 min and then reached a plateau, which accounted for 89.4% of the initial bromide.It indicated that PMS transforms bromide into bromate rapidly in the presence of CuO.The total bromine almost kept steady at 20 ± 0.5 μmol/L throughout the 30-min reaction, which implied that free bromine was a requisite intermediate during the bromate formation in the CuO/PMS process.
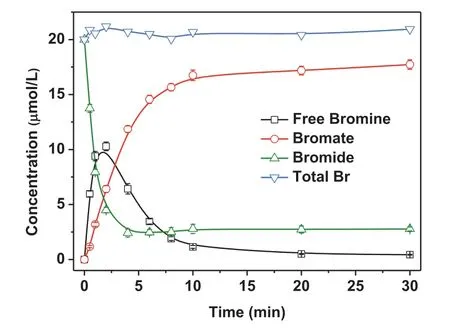
Fig.1 .Evolution of bromine species during the oxidation of bromide in the CuO/PMS process.Experimental conditions: [CuO] = 0.20 g/L, [PMS] = 1 mmol/L,[Br−] = 20 μmol/L, at initial pH 7, 20 ± 1 °C.
The evolution of PMS was measured simultaneously in the CuO/PMS process in this study.As shown in Fig.S1 (Supporting information), the concentration of PMS declined by around 96.9%within the first 10 min during the process.This trend was compatible to the bromate formation during the CuO/PMS process.It implied that bromate formation might be closely relative to the catalysis reaction of PMS by CuO in this process.
Based on the results mentioned above, free bromine is confirmed to be the main intermediate product for the bromate formation in the CuO/PMS process.Accordingly, the formation of bromate in the CuO/PMS process involved two steps: the oxidation of bromide to free bromine and the further oxidation of free bromine to bromate.The two-step process of bromate formation is commonly observed in other chemical oxidation processes as reported in previous literatures [6,23].
Fig.2 shows the bromide decay and free bromine formation in the CuO a, PMS alone, CuO/PMS, CuO/PMS/t-BuOH and CuO/PMS/MeOH processes at an initial bromide concentration of 20 μmol/L and pH 7.Bromide showed no obvious concentration change in the CuO alone, where no formed free bromine was detected.It implies that CuO alone has neither absorption nor transformation effect on bromide in aqueous solution.PMS alone transformed 28% of the initial bromide into free bromine within 30 min.The formed free bromine increased and accumulated throughout the reaction time.The loss rate of bromide by PMS alone is compatible to the second-order rate constant of PMS with bromide(k1= 0.16 L mol−1s−1) as Eq.1 [24].It indicates that PMS contributes partially to the free bromine formation in the CuO/PMS process.It should be noted that the concentration of leached Cu(II)from CuO was determined to be 76.3 μg/L.As shown in Fig.S2(Supporting information), Cu(II)/PMS and PMS alone shared a similar bromide transformation at pH 7.This result indicated that the leached Cu(II) played a negligible role in the transformation of bromide in the presence of PMS.Taking into account that over 86% of initial bromide was rapidly transformed within 4 min in the CuO/PMS process, the oxidants produced in the CuO/PMS process were assumed to contribute primarily to the bromide transformation.As has been reported in previous literature, sulfate radical (SO4•−) and hydroxyl radical (•OH) are generated from the CuO/PMS process.These two radicals are typically found to play essential roles in bromide transformation in other advanced oxidation processes [10,11].As shown in Fig.S3 (Supporting information), SO4•−and•OH were confirmed to generate in the CuO/PMS processviaEPR experiments using DMPO as a spin-trapping agent.Thus, radical scavenging experiments by spiking 2% alcohol(t-BuOH or MeOH) were conducted.Over 35% of the initial bromide was transformed within 4 min in the presence of excessivet-BuOH, while less than 3% of the initial bromide was converted in the presence of excessive methanol.These results fully convinced that SO4•−rather than•OH was the main reactive species responsible for the bromide transformation in the CuO/PMS process.
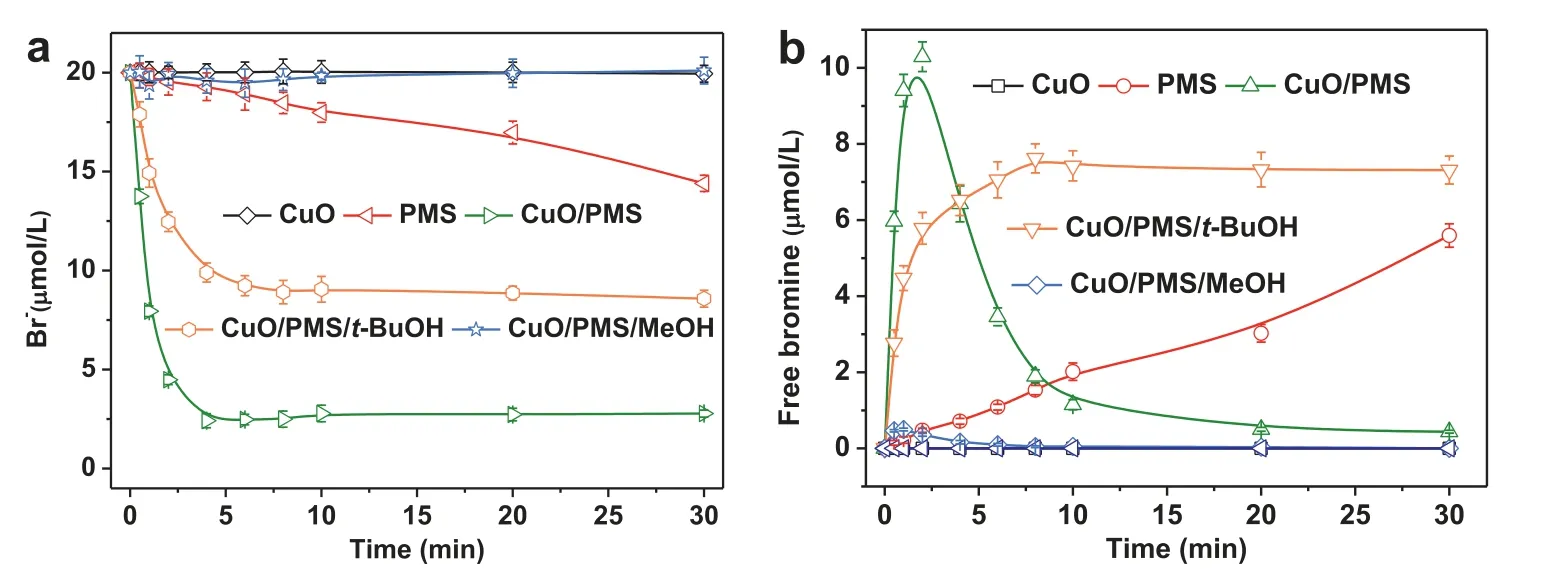
Fig.2 .(a) Bromide decay and (b) free bromine formation in the CuO, PMS, CuO/PMS, CuO/PMS/t-BuOH and CuO/PMS/MeOH processes.Experimental conditions:[CuO] = 0.20 g/L, [PMS] = 1 mmol/L, [Br−] = 20 μmol/L, 2% t-BuOH or 2% MeOH, at initial pH 7, 20 ± 1 °C.
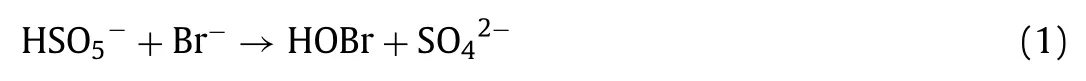
Fig.S4a (Supporting information) shows the time-dependent HOBr/−OBr decay in the HOBr/−OBr disproportionation, CuO alone,PMS alone and CuO/PMS processes at pH 7.In the absence of CuO, the loss of HOBr/−OBr was negligible within 30 min.It implied that the disproportionation of HOBr/−OBr without catalysis of CuO was an extremely slow process.In the presence of CuO, the HOBr/−OBr decay was accelerated appreciably.Approximately 69%of initial HOBr/−OBr was depleted within 30 min, suggesting that catalysis of CuO enhanced the decomposition of HOBr/−OBr.The HOBr/−OBr decay in the CuO/PMS process was the fastest among the four processes mentioned above, over 90% of HOBr/−OBr was depleted within 10 min.These results indicated that the SO4•−oxidation combined with the CuO catalysis was mainly responsible for further transformation of free bromine.
Fig.S4b (Supporting information) shows the time-dependent bromate formation from the oxidation of Br−and HOBr/−OBr at an initial Br concentration of 20 μmol/L in the CuO alone, PMS alone and CuO/PMS processes at pH 7.The initial rate of bromate formation during the oxidation of HOBr/−OBr was much higher than that during the oxidation of Br−by CuO/PMS process, although they have the same quantity of formed bromate after 30 min reaction.The CuO catalysis of HOBr/−OBr also formed bromate, revealing that the catalysis of HOBr/−OBr contributed partially to the bromate formation in the CuO/PMS process.In addition, it should be noted that the amount of formed bromate accounted for approximately 15% of the loss of free bromine from the CuO catalysis.It indicated that CuO primarily catalyzed HOBr/−OBr to decompose into bromide as Eqs.2 and 3.No bromate formation was observed in the CuO catalysis of Br−process.The oxidations of Br−and HOBr/−OBr by PMS in the absence of CuO did not form any bromate, suggesting that PMS fails to further oxidation of HOBr/−OBr to bromate although it is capable of converting Br−to free bromine.

All the above results imply that the bromate formation follows reaction pathways as presented in Fig.3.Bromide is initially oxidized to free bromine by SO4•−and PMS.The formed free bromine is further oxidized by SO4•−or catalyzed by CuO to form bromate.Some HOBr/−OBr can be converted back to Br−by CuO catalysis,which can then be re-oxidized to HOBr/−OBr by SO4•−and PMS until most of Br−is converted to bromate as long as PMS is present in excess.
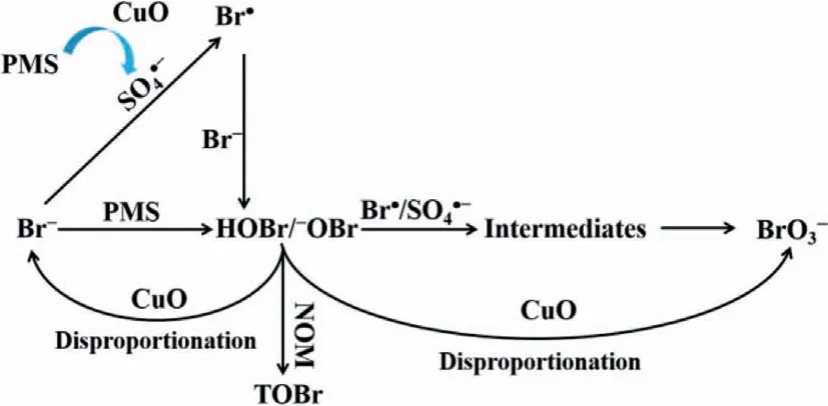
Fig.3 .Mechanism of the bromate formation in the CuO/PMS process.
The effect of CuO on the bromate formation in the CuO/PMS process is manifested in Fig.S5a (Supporting information).Results indicate that the formation of bromate increased as CuO dosage increased in the range of 0.05–0.2 g/L and almost remained steady in the range of 0.2–0.3 g/L in the CuO/PMS process.A lag-phase of bromate formation was observed at the low CuO dosage of 0.05 g/L.The conversion ratios of bromide to bromate by CuO/PMS process in 30-min time frame were 32.1%, 75.6%, 81.2%, 88.6%,88.3% and 88.6% at the CuO dosages of 0.05 g/L, 0.1 g/L, 0.15 g/L,0.2 g/L, 0.25 g/L and 0.3 g/L, respectively.It should be noted that the bromate formation generally followed pseudo-zero-order kinetics within initial reaction time, when both PMS and bromide were sufficient.Fig.S5b (Supporting information) shows the relationship between the initial formed rate of bromate and the CuO dosage of 0.05–0.3 g/L.The rate increased linearly with increasing CuO dosage, but the regression line did not go through the origin.This result confirmed the bromate formation is a two-step process again.Simultaneously, the evolutions of free bromine in the presence of different dosages of CuO were shown in Fig.S5c (Supporting information).The free bromine concentration exhibited a monotonous increase at a CuO dosage of 0.05 g/L, whereas increased sharply followed by declining gradually in the CuO dosage range of 0.1–0.3 g/L.Moreover, the formed free bromine after achieving the maximum depleted more rapidly at a higher CuO dosage.These results confirmed the essential role of CuO in the transformation of free bromine into bromate.
Fig.S6a (Supporting information) shows the bromate formation in the PMS dosage range of 0.01–1.0 mmol/L in the CuO/PMS process.The formed bromate increased with increasing PMS dosage.No bromate was detected at the PMS dosage of 0.01 mmol/L.The conversion ratio of bromide to bromate by CuO/PMS process in 30-min time frame were 1.5%, 4.7%, 10.5%, 25.8%, 52.1%, 69.5% and 88.6% at the PMS dosage of 0.02 mmol/L, 0.05 mmol/L, 0.1 mmol/L,0.2 mmol/L, 0.5 mmol/L, 0.8 mmol/L and 1 mmol/L, respectively.It should be noted that bromate formation exhibited two stages at different PMS dosages: (1) An initial rapid formation stage, and(2) a slower formation stage.For example, the formed concentration of bromate achieved 9.3 μmol/L within 10 min and further increased to 10.4 μmol/L in the following 20 min in the presence of 1 mmol/L PMS.As mentioned above, over 95% of initial PMS depleted within 10 min.It implies that SO4•−generated from the CuO catalysis of PMS is mainly responsible for the bromate formation in the initial phase.The CuO catalysis of HOBr/−OBr plays a critical role in the bromate formation in the following phase.As shown in Fig.S6b (Supporting information), the formed concentrations of free bromine increased to the maximum and then declined gradually for all the selected PMS dosages in CuO/PMS process.Interestingly, the maximum concentration of free bromine increased with the PMS concentration increasing from 0.01 mmol/L to 0.1 mmol/L, but decreased with the PMS concentration increasing from 0.1 mmol/L to 1 mmol/L.In the PMS concentration range of 0.01–0.1 mmol/L, most of SO4•−generated from the CuO catalysis of PMS oxidized bromide to free bromine, only few of them further oxidized free bromine to bromate.In the PMS concentration range of 0.1–1 mmol/L, an increasing number of SO4•−generated from the CuO catalysis of PMS further oxidized the formed free bromine to bromate.
The effect of bromide concentrations on bromate formation was also investigated in CuO/PMS process in the initial bromide concentration range of 2.5–80 μmol/L at pH 7.As shown in Fig.4a, the formed bromate was enhanced with the initial bromide concentration increasing from 2.5 μmol/L to 40 μmol/L after 30 min reaction.However, further increasing the initial bromide concentration from 40 μmol/L to 80 μmol/L did not significantly change the bromate formation in CuO/PMS process.It was also found that, in the initial bromide concentration range of 2.5–20 μmol/L, over 80% of the initial bromide has been transformed to bromate.But the conversion ratio of bromide to bromate reduced sharply to approximately 30% as the initial bromide concentration increased to 80 μmol/L in the CuO/PMS process.This observation was attributed to the fact that excessive bromide competes for SO4•−with the formed free bromine to inhibit the further oxidation of free bromine to bromate.
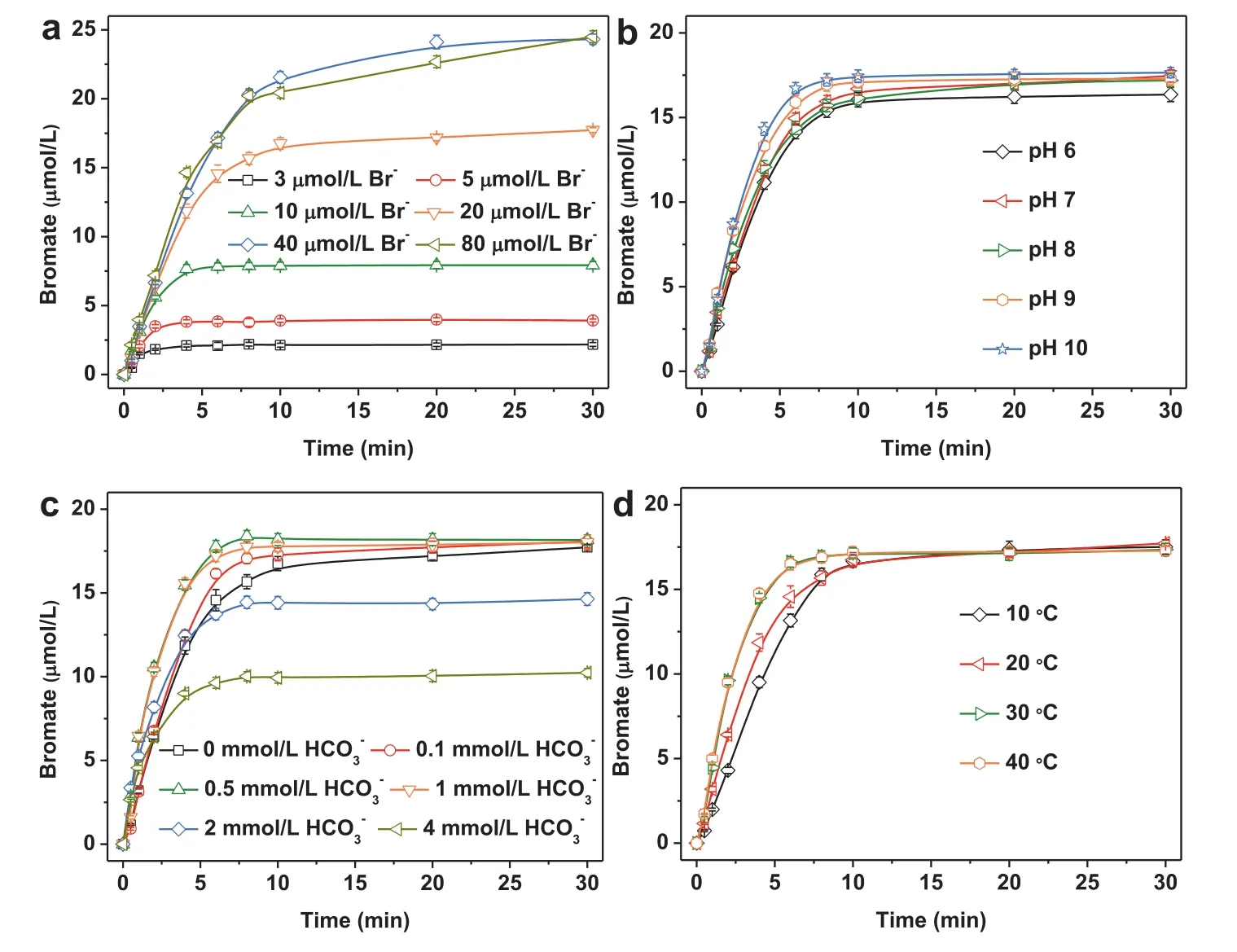
Fig.4 .Effect of bromide concentration (a), pH (b), HCO3− (c) and temperature (d) on the bromate formation in the CuO/PMS process.Experimental conditions:[CuO] = 0.2 g/L, [PMS] = 1 mmol/L; (a): [Br−] = 3–80 μmol/L, at initial pH 7, 20 ± 1 °C; (b): [Br−] = 20 μmol/L, at initial pH 6–10, 20 ± 1 °C; (c): [Br−] = 20 μmol/L,[HCO3−] = 0–4 mmol/L, at initial pH 7, 20 ± 1 °C; (d): [Br−] = 20 μmol/L, at initial pH 7, 20–40 °C.
The solution pH is a critical factor for the bromate formation in the oxidation of bromide-containing water [25].Fig.4b shows the bromate formation at different initial pH in the CuO/PMS process.The formed bromate increased rapidly and then reached a plateau at each initial pH.The steady concentrations of bromate were quite close to each other for different initial pH after 30 min.This observation can be ascribed to the variations of pH during reactions.As shown in Fig.S7 (Supporting information), the solution pH rapidly changed to be consistent for each initial pH.Moreover, the bromate formation exhibited less pH-dependent in the CuO catalyzed PMS oxidation process than in other homogeneous catalytic PMS oxidation processes.It implied that the bromate formation in the heterogeneous catalytic oxidation process might have a higher potential risk than in the homogeneous catalytic oxidation process.
The evolutions of bromate formed at bicarbonate concentrations of 0.1, 0.5, 1, 2 and 4 mmol/L were investigated and compared with that in the absence of bicarbonate in the CuO/PMS process.As shown in Fig.4c, the formed concentration of bromate decreased with increasing bicarbonate concentrations.For example, the conversion ratio of bromide to bromate reduced from 79.0% to 60.2% as the bicarbonate concentration increased from 0.1 mmol/L to 4 mmol/L after 30 min reaction.It was also found that the bromate formation in the absence of bicarbonate was higher than that in the presence of bicarbonate.It indicated that bicarbonate inhibited the bromate formation in the CuO/PMS process.The inhibiting effect was ascribed to the scavenge of SO4•−generated from the CuO catalysis of PMS by bicarbonate as Eqs.4 and 5.Although carbonate radical (CO3•−) formed from the reaction of bicarbonate with SO4•−can also oxidize bromide and free bromine [5], its oxidation rate of bromide by CO3•−(k(CO3•−,Br−) = 1.0 × 105L mol−1s−1) is four orders of magnitude lower than the oxidation rate of bromide by SO4•−(k(•OH, Br−) = 1.1 ×109L mol−1s−1) as Eqs.6-8 [26].
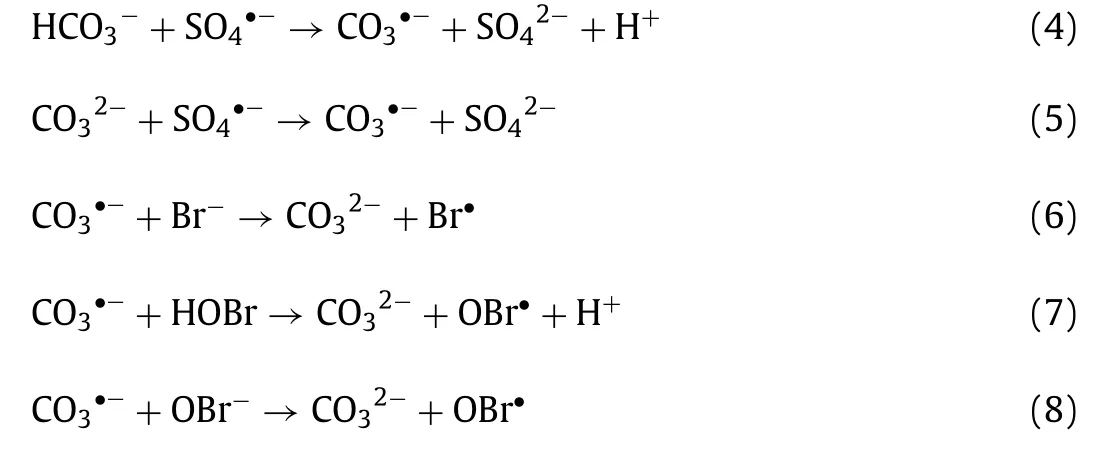
Fig.4 d shows the evolutions of bromate formation in the CuO/PMS process at 10 °C, 20 °C, 30 °C, and 40 °C, respectively.Results showed that the formed concentrations of bromate were stable in the temperature range of 10–40 °C in the CuO/PMS process after 30 min reaction.However, a higher temperature was favored to the initial formation rate of bromate.For example, the initial formation rate of bromate was 2.3 μmol L−1min−1at 10 °C,and 3.9 μmol L−1min−1at 40 °C.The rate at 40 °C is 71.3% higher than that at 10 °C.It indicated that high temperature accelerates the oxidation of bromide to bromate in the CuO/PMS process.
Humic acid as a significant constituent of NOM (natural organic matter) has been widely used as a surrogate of NOM in a considerable number of previous studies [27–29].In this study, a commercial humic acid purchased from Aladdin Industrial Corporation was chosen to investigate the effect of NOM on the bromate formation in the CuO/PMS process.Fig.S8 (Supporting information)shows the bromate formation in the NOM concentration range of 1–4 mg-C/L in the CuO/PMS process.The formed concentrations of bromate after 30 min reaction were 13.0 μmol/L, 9.2 μmol/L,5.5 μmol/L and 2.3 μmol/L in the presence of NOM concentrations of 1 mg-C/L, 2 mg-C/L, 4 mg-C/L and 8 mg-C/L, respectively.In contrast, the formed bromate reached 17.4 μmol/L in the absence of NOM.It implied that NOM inhibited the bromate formation in the CuO/PMS process, which was also observed in other oxidation processes treating bromide-containing water [6,30].The inhibiting effect of NOM on the bromate formation can be explained by three aspects: (1) NOM may consume the formed free bromine.The peak concentrations of accumulated free bromine decreased from 8.6 μmol/L to 2.0 μmol/L as NOM concentrations increased from 1 mg-C/L to 4 mg-C/L.It should be noted that the reaction of free bromine with NOM resulted in the generation of organic bromine as reported in previous literature.The formed concentrations of total organic bromine were 1.2 μmol/L, 2.7 μmol/L, 5.0 μmol/L and 7.8 μmol/L at the NOM concentrations of 1 mg-C/L, 2 mg-C/L, 4 mg-C/L and 8 mg-C/L after 30 min reactions, respectively.It meant that the conversion ratios of inorganic bromine to organic bromine increased from 5.7% to 38.9% as NOM concentrations increased from 1 mg-C/L to 4 mg-C/L.(2) NOM may compete with bromide for the reactive oxygen species (e.g., SO4•−and PMS).The conversion ratios of bromide after 30 min reaction were 77.1%, 66.4%, 53.2% and 46.5% at the NOM concentrations of 1 mg-C/L, 2 mg-C/L, 4 mg-C/L and 8 mg-C/L, respectively.It confirmed that the presence of NOM inhibits the transformation of bromide in the CuO/PMS process.In addition, NOM can also convert free bromine back to bromide, which results in a decrease in the bromide transformation as well.
The stability and recyclability of CuO were investigated in a series of continuous experiments.The bromate formation in the PMS combined with CuO recycled for 6 times is shown in Fig.S9 (Supporting information).After reusing CuO for twice, the formed concentration of bromate was lower than that using CuO for the first time.The decreased bromate formation could be attributed to the loss of the active site formed in the first run on the CuO surface.When CuO was further reused, the bromate formation fairly remained constant, which accounted for around 70% of the initial bromide.It indicated that the reused CuO can still result in the bromate formation that accounted for up to 70% of the initial bromideviaactivating PMS in the bromide-containing water.
To investigate the bromate formation in a more realistic water matrix in the CuO/PMS process, experiments were conducted in two real water samples including a tap water and a surface water spiked with certain volumes of bromide solutions to remain 20 μmol/L of the initial bromide.As shown in Fig.S10a (Supporting information), 73.2% of the initial bromide was decomposed in the tap water, while the formed bromate increased with increasing reaction time and reached 6.4 μmol/L after 30 min.The formed free bromine was rapidly accumulated and decomposed gradually after reaching its peak.Total organic bromine that accounted 7.7% of the initial bromide was observed in tap water treated by CuO/PMS process.As shown in Fig.S10b (Supporting information), the formed concentration of bromate was 0.7 μmol/L after 30 min reaction,while the total organic bromine reached 8.3 μmol/L that accounted for 41.3% of the initial bromide in the surface water.It means that the formed bromate in the tap water was higher than that in the surface water, while the total organic bromine in the tap water was much lower than that in the surface water.This phenomenon can be ascribed to a higher concentration of dissolved organic matter in the surface water (DOC = 12.6 mg/L) than in the tap water(DOC = 3.8 mg/L).Interestingly, the bromide concentration decomposed from 20 μmol/L to 4.4 μmol/L within first 6 min and then increased to 7.4 μmol/L in the following 24 min reaction in the surface water, while decomposed rapidly to 5.3 μmol/L and remained steady in the tap water.The difference in bromide conversion can be explained by the different concentration and nature of dissolved organic matter in two real water samples.The reaction mechanism of bromine varies for different organic compounds.One part of NOM and bromine undergo electron transfer reactions, while the other part of NOM and bromine proceed bromine atom transfer[31].Accordingly, the CuO/PMS process could transform bromide into bromate and brominated disinfection byproducts, when treating real water containing bromide.
In Summary, this study investigated the catalytic enhancement of CuO on the formation of bromate during the treatment of bromide-containing waters using PMS.Results showed that the presence of CuO resulted in appreciable yields of bromate formation from the oxidation of bromide by PMS, although PMS alone cannot transform bromide into bromate.Besides activating PMS to generate SO4•−to oxidize bromide to bromate, CuO was also demonstrated to catalyze the disproportionation of the formed bromine to bromate and bromide.The presence of NOM suppressed the bromate formation but increased the organic bromine.It should be noted that CuO still catalyzed PMS to transform bromide into bromate even after recycling several times.Therefore,CuO/PMS process has to be carefully examined before treating bromide-containing water or brominated organic contaminants.
Declaration of competing interest
We confirm this manuscript has not been published elsewhere and also not under consideration in any journal.All authors enclosed approve the submission.The authors declare no conflicts of interest.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (Nos.42077159 and 51978181).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.008.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
