Activation of sulfite by ferric ion for the degradation of 2,4,6-tribromophenol with the addition of sulfite in batches
Zongping Wng, Fn Bi, Lisn Co, Siyng Yue, Jingwen Wng, Songlin Wng,Jun M, Pengho Xie,∗
a School of Environmental Science and Engineering, Key Laboratory of Water & Wastewater Treatment (MOHURD), Hubei Provincial Engineering Research Center for Water Quality Safety and Pollution Control, Huazhong University of Science and Technology, Wuhan 430074, China
b School of Architecture & Urban Planning, Huazhong University of Science and Technology, Wuhan 430074, China
c State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China
Keywords:Fe(III)/S(IV)2,4,6-Tribromophenol (TBP)Sulfate radical (SO4•–)Batches Acute toxicity
ABSTRACT In this work, the removal of 2,4,6-tribromophenol (TBP) by ferric ion-activated sulfite [Fe(III)/S(IV)] process was systematically investigated with determining the intermediate products and evaluating the influences of some operational conditions and water matrices.Our results show that batching addition of S(IV) benefits the S(IV) utilization efficiency and TBP removal, with SO4•– being the primary reactive radical accounting for TBA degradation.The maximum TBP removal in the Fe(III)/S(IV) process was observed at pH 4.0 and oxygen is essential in this process.With increasing Fe(III) and S(IV) dosages from 0.05 and 0.1 mmol/L to 0.2 and 2.0 mmol/L, respectively, TBP removal followed trends of first increase then decrease.As the acute toxicity of the TBP solution was significantly reduced, the Fe(III)/S(IV) process was believed to be a good choice in the treatment of TBP.
Aromatic phenols are important environmental micro-organic pollutants and have attracted extensive attentions [1–3].2,4,6-Tribromophenol (TBP) which is commonly used as a pesticide,wood conservative and intermediate for brominated flame retardants has been widely studied in environmental field due to its worldwide use, environmental persistence and related health issues [3,4].The extensive application of TBP makes it easy to enter environmental matrices, such as surface water, landfill leachates,air, and sediment [4,5].As TBP that has a toxicological risk is difficult to be removed by conventional treatment processes [6,7], it is necessary to develop effective techniques to remove TBP from contaminated environments.
Due to the high degradation efficiency, advanced oxidation processes (AOPs) with producing high oxidative radicals have been widely applied for the treatment of organic pollutants [8,9].In comparison with hydroxyl radical (HO•) (2.4–2.7 V), sulfate radical (SO4•–) has a comparable redox potential (2.5–3.1 V), wider pH range, longer half-life and higher selectivity [10].Over the past decades, SO4•–-based advanced oxidation processes (SR-AOPs)have gained increasing attention [9,11-14].Due to the selectivity of SO4•–towards the bond of C–Br, SR-AOPs have been widely studied for the degradation of brominated organic pollutants such as bromophenols and tetrabromobisphenol A [15,16].Usually, SO4•–is obtained through the activation of persulfate (PS) including peroxydisulfate (PDS) and peroxymonosulfate (PMS) [11,12].However, the application of PDS or PMS in water/wastewater treatment might be limited due to their expensive price and the formation of bromate (BrO3–) in the presence of bromide (Br–) [15,17,18].Thus,it is meaningful to develop other economic SR-AOPs that can reduce the formation of BrO3–.
Recently, a promising SR-AOP through the activation of sulfite[S(IV)] has received increasing attention [19–21].Among the activated S(IV) processes, the iron-activated S(IV) is considered to have bright prospects [9,22].As for the iron-activated S(IV) process, a series of reactive radicals, such as SO3•–, SO5•–and SO4•–, are producedviathe reactions shown in Eqs.S6-S17 (Supporting information) [9,23].Additionally, HO•can also be produced through the reaction between SO4•–and OH–(or H2O) [10,20,21].As a strong reducing and complexing agent, S(IV) could transfer Fe(III) to Fe(II)and complex with iron ions, which can accelerate the Fe(II)/Fe(III)cycle, reduce the formed sludge, and broaden the adequate pH range [22,24,25].
In the traditional SR-AOPs, bromate (BrO3–) would be produced in the presence of bromide (Br–) with producing a sequence of intermediate oxidative bromine including Br•, Br2•–and HBrO[15,17,18].As a strong reducing agent, S(IV) can fast react with these oxidative bromine to re-convert them to Br–, which can inhibit BrO3–formation [23,24].As TBP can be fast degraded by SO4•–[26,27], the iron-activated S(IV) process would be a good choice in the treatment of TBP with simultaneous controlling BrO3–formation.However, S(IV) can also fast react with the oxidative radicals, which would cause the waste of S(IV) and lower the treatment efficiency [24,25].It is important to develop methods to increase the utilization efficiency of S(IV).As lowering the initial S(IV) concentration can slow down the rate of S(IV) reacting with the oxidative radicals, adding S(IV) in batches is proposed to increase the utilization efficiency, which still needs to be further studied.
The objectives of this study were to (1) determine the efficiency of iron-activated S(IV) process on TBP removal and disclose the roles of reactive radicals; (2) make clear whether the addition of sulfite in batches benefits TBP degradation; (3) investigate the influences of some typical operational parameters and water matrices; (4) and evaluate the transformation of bromine and the acute toxicity variation.
In this work, all solutions were prepared in ultra-pure water produced from a water purifier (MicroPure UV, Thermo Fisher Scientific, Germany), and the experimental procedures and analytic methods were offered in Text S1 (Supporting information).All the samples were replicated for two times at least and the errors stand for the standard deviations among the replicated samples.
As shown in Fig.1, negligible difference of TBP removal was observed in the presence of sulfite or Fe(III) compared to the control sample, meaning that the addition of sulfite or Fe(III) had no effect on TBP removal.It should be noted that the TBP concentration reduced by about 9% for the control sample, which might be due to that some TBP could be separated out from water solution during the continuous stirring, which was also observed in some previous studies focusing on the treatment of insoluble pollutants [22,24].In contrast, about 70.2% of TBP was removed by the combined process of Fe(III) and sulfite [Fe(III)/S(IV)] in 6 min at the selective experimental conditions, which was similar to the results obtained in other previous studies [22,28], suggesting that the iron-activated S(IV) process would be a good choice in the degradation of TBP.Additionally, different iron-activated processes including Fe(III)/S(IV), Fe(II)/S(IV), Fe0/S(IV), Fe2O3/S(IV)and Fe3O4/S(IV) were comparatively studied.As shown in Fig.S1(Supporting information), both Fe(III)/S(IV) and Fe(II)/S(IV) systems were observed to efficiently degrade TBP with similar removal efficiency at the same iron species concentration (0.1 mmol/L).However, negligible TBP degradation was observed in Fe0/S(IV),Fe2O3/S(IV) and Fe3O4/S(IV) systems with equivalent iron concentration (0.1 mmol/L).As ferric ion is much stable than ferrous ion,Fe(III)/S(IV) system was selected as a typical iron-activated process to evaluate the removal of TBP in the subsequent experiments.
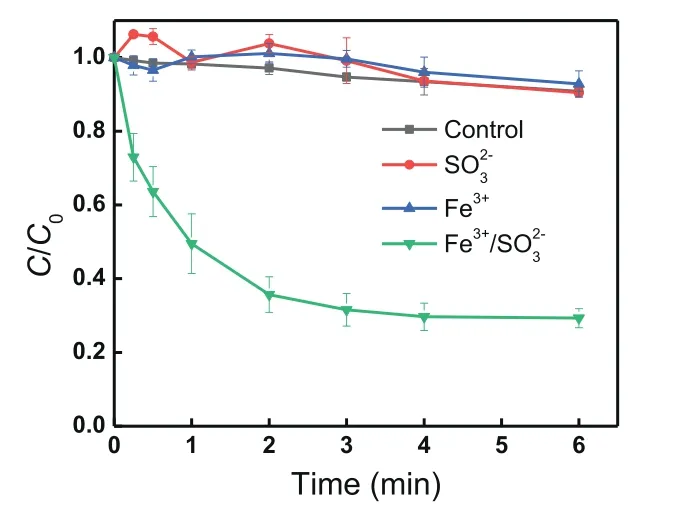
Fig.1 .TBP removal in different systems.Conditions: [TBP]0= 10 μmol/L, [Fe(III)]0=0.1 mmol/L, [S(IV)]0= 0.4 mmol/L, pH 4.0, temperature 25°C.
As for the Fe(III)/S(IV) process, fast TBP degradation was observed in the initial 3 min and the TBP degradation reached 68.6%at 3 min (Fig.1), while only a little removal was observed with further prolonging reaction time to 6 min, which might be due to the lack of S(IV) [23,25].To make clear the reason, the variation of S(IV) concentration was monitored.Fig.S2 (Supporting information) illustrates that S(IV) was fast consumed and reduced to nearly zero after reaction for 3 min due to the fact that S(IV) in the Fe(III)/S(IV) system can quickly complex with Fe(III) to form FeSO3+and can be further transformed to SO5•–and SO4•–in the presence of oxygen [29–31], which was in line with the variation of TBP concentration, evidencing that the lack of S(IV) stopped the further degradation of TBP from 3 min to 6 min.Our previous studies also found that the supplement of S(IV) can further increase the treatment efficiency of iron-activated S(IV) processes [20].As S(IV)can fast react with the reactive radicals, such as SO4•–, SO5•–and HO•, in the iron-activated S(IV) systems to consume both S(IV) and reactive radicals [23,30], lowering the initial S(IV) dosage and increasing the dose times would benefit the utilization efficiency of S(IV).
Fig.2 shows the effect of adding S(IV) in batches on TBP removal at 25°C with the initial reaction pH being 4.0.During the experiment, the total S(IV) dosages for all the samples were 0.4 mmol/L, and the three specific methods of adding S(IV) in batches are as follows: 0.4 mmol/L S(IV) was introduced into the system at 0 min in a time, adding S(IV) at 0 and 3 min separately with 0.2 mmol/L S(IV) each time, and adding S(IV) at 0, 2 and 4 min separately with 0.133 mmol/L S(IV) each time.With increasing S(IV) dosing times from 1 to 2 and 3, the removal of TBP was significantly increased from 70.8% to 87.5% and 91.5% after reaction for 6 min, respectively, evidencing that adding S(IV) in batches benefits the utilization efficiency of S(IV).Additionally, the variations of S(IV) concentration and solution pH during the reaction were monitored.As shown in Fig.S2, the S(IV) concentration decreased rapidly after the reaction started in all systems.As the reaction of Fe(III) and HSO3–could produce H+(Eq.S8 in Supporting information), the solution pH for all the samples also decreased at the beginning of the reaction (Fig.S3a in Supporting information),which was similar to other previous studies [22,24].From Figs.S2 and S3a, it is observed that the S(IV) was consumed completely in 6 min when adding S(IV) in a time, along with that the pH value gradually decreased to around 3.55 within 6 min.However, the pH values for the samples with multiple S(IV) addition suddenly increased after adding S(IV), which can be explained by that SO32−can react with H+to produce HSO3–.Along with the increase of TBP removal efficiency (Fig.2), the residual S(IV) after reaction for 6 min also gradually increased with increasing the S(IV) addition times from 1 to 3 (Fig.S2).The results suggest that adding S(IV)in batches was not only beneficial to increase the utilization effi-ciency of S(IV), but also can ease the pH reduction.
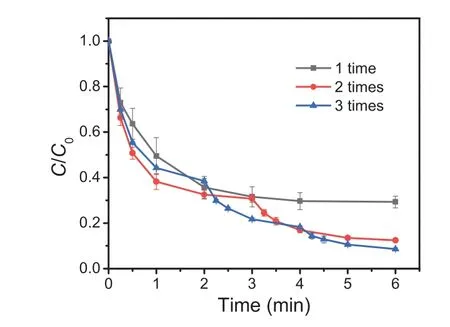
Fig.2 .Effect of sulfite dose times on TBP removal in the Fe(III)/S(IV) system.Conditions: [TBP]0= 10 μmol/L, [Fe(III)]0= 0.1 mmol/L, [S(IV)]total=0.4 mmol/L, pH 4.0,temperature 25 °C.
As the removal of TBP after reaction for 6 min was similar for the samples of adding S(IV) in 2 times and 3 times (Fig.2), the experimental condition with adding S(IV) in 2 times was selected for the next experiments in this study.The changes of Fe(III), Fe(II) and total Fe concentration in the system were also monitored when S(IV) was dosed in 2 times (Fig.S4 in Supporting information).In the initial 15 s, the rapid decrease of Fe(III) and increase of Fe(II)were observed, which could be attributed to the rapid formation and decomposition of FeSO3+viareactions shown in Eqs.S8 and S9 (Supporting information), respectively [29,31,32].According to the sequence reactions shown in Eqs.S6-S17 (Supporting information), Fe(II) and Fe(III) would circulate dynamically, and the concentration tends to be stable.However, due to the consumption of S(IV), the Fe(III) concentration gradually increased along with the gradual decrease of Fe(II) concentration with extending the reaction time from 15 s to 3 min.At 3 min, significant transformation of Fe(III) to Fe(II) was observed with adding S(IV), meaning that batching addition of S(IV) could elevate the utilization efficiency of iron too.It is worthy to note that the total concentration of dissolved iron ions gradually decreased, which can be explained by the hydrolysis of iron ions [24].
Previous reports have evidenced that SO3•–, SO4•–, SO5•–and HO•would be the main reactive radicals in the iron-activated S(IV)systems [24,28,33].SO4•–and SO5•–can be producedviathe sequence reactions shown in Eqs.S6-S17, and HO•can form through the oxidation of H2O and OH–by SO4•–[34].Due to the low oxidation ability of SO3•–, its role in the degradation of organic pollutants is always ignored in the activated S(IV) processes [20–23].However, the roles of SO4•–, SO5•–and HO•were different and controversial in different studies [22,24,28].In order to clarify which reactive species in the Fe(III)/S(IV) system contributed to TBP degradation, different radical inhibitors were used in this study.
Based on the data shown in Table S2 and Fig.S5 (Supporting information), the reaction rate constants of TBP reacting with HO•and SO4•–were determined to be 5.51× 109L mol−1s−1and 1.36× 109L mol−1s−1, respectively, using a relative rate technique[10].Generally, TBA is considered as a good quencher for HO•(6×108L mol−1s−1) but not for SO4•–(9.1× 105L mol−1s−1) [35,36].As EtOH can fast react with SO4•–(7.7× 107L mol−1s−1) and HO•(1.9× 109L mol−1s−1) but is difficult to react with SO5•–(<1×103L mol−1s−1), EtOH can be used to scavenge both SO4•–and HO•in the Fe(III)/S(IV) process [35,37].Then the TBP removal efficiencies among the samples with adding different scavengers can tell the roles of different radicals on TBP degradation.Based on the discussion in Text S2 (Supporting information), the initial TBA and EtOH concentrations were set at 1 and 8.65 mmol/L, respectively.As shown in Fig.3a, in comparison with the control sample, the removal ratio of TBP decreased from 88.6% to 80.5% with the addition of TBA, suggesting that HO•played a small role in TBP degradation.However, TBP removal ratio significantly declined to 23.5%in the presence of EtOH, meaning that SO4•–played the dominant role in the system.Combing the results shown in Figs.1 and 3a,the removal efficiency of TBP in the Fe(III)/S(IV) system with the addition of EtOH was still higher than the sample without S(IV) or Fe(III) (around 9%), which means that SO5•–would also take part in the treatment of TBP in the Fe(III)/S(IV) system.
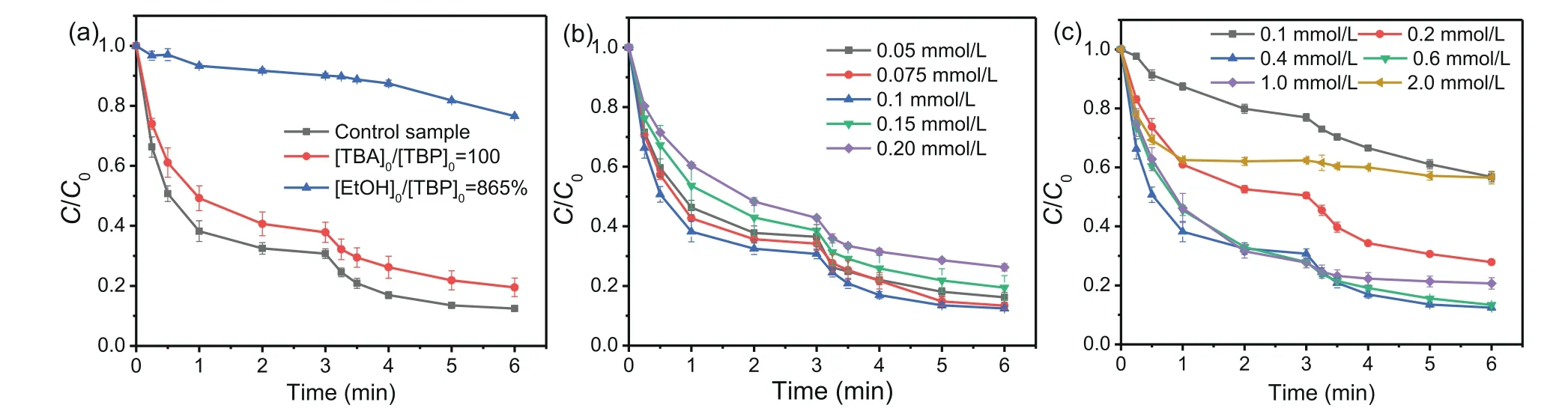
Fig.3 .Effect of TBA or EtOH (a), initial Fe(III) concentration (b), and initial S(VI) concentration (c) on TBP degradation in the Fe(III)/S(IV) system with dosing S(IV) in two times.Conditions: [TBP]0= 10 μmol/L, [Fe(III)]0= 0.1 mmol/L (except b), [S(IV)]0= 0.4 mmol/L (except c), pH 4.0, temperature 25 °C.
Fig.3 b shows the effect of different Fe(III) concentrations on TBP removal in the Fe(III)/S(IV) system.With 0.4 mmol/L S(IV)dosage and pH 4.0, the removal efficiencies of TBP only slightly increased from 83.8% to 87.5% after reaction for 6 min with the initial Fe(III) dosages increasing from 0.05 mmol/L to 0.1 mmol/L.However, when the initial Fe(III) dosage was further increased to 0.2 mmol/L, the removal efficiency dropped to 73.8%.The production of SO3•–increased with Fe(III) dose increasing from 0.05 mmol/L to 0.1 mmol/L, which would benefit the formation of SO5•–and SO4•–, causing the slight increase of TBP removal.However, with further elevating Fe(III) concentration, excessive Fe(III)would produce a large amount of Fe(II) to consume the formed SO4•–, which might be the reason for the inhibition of TBP removal [38,39].In addition, the formation of Fe(OH)3colloids at high Fe(III) concentration might also inhibit the degradation of TBP[23,24].
Fig.3 c shows the effect of different S(IV) dosages on TBP degradation in the Fe(III)/S(IV) system, and a similar trend to the Fe(III)dosages was observed.When the initial Fe(III) dosage and pH were 0.1 mmol/L and 4.0, respectively, the TBP removal efficiencies increased rapidly from 43.3% to 87.5% after 6 min with S(IV) dosages increasing from 0.1 mmol/L to 0.4 mmol/L.As S(IV) dose was further elevated to 2.0 mmol/L, the TBP removal efficiency dropped rapidly from 87.5% to 43.5%, indicating that high concentration of S(IV) had an adverse effect on the removal efficiency, which was also reported in some previous studies [22,24].Although the supplement of S(IV) can affect the solution pH (Fig.S3a), the solution pH kept in the range of around 3.5–3.9 for all the samples with the addition of S(IV) at different concentration, meaning that the different TBP degradation efficiencies would not be due to the solution pH.It is expected that more radicals would be generated in the Fe(III)/S(IV) system with increasing the initial S(IV) concentration, which was beneficial to the removal of TBP.However, as a strong reducing agent, S(IV) could also fast consume the formed SO4•–, HO•and SO5•–at the meantime.Therefore, excess S(IV) dose played adverse role in the degradation of TBP.pH directly affects the distribution of Fe(III) and S(IV) species, which can significantly influence the treatment efficiency of pollutants in the Fe(III)/S(IV)system [23,29].Fig.S6a (Supporting information) presents the effect of different initial pH values on the degradation of TBP in the Fe(III)/S(IV) system with adding S(IV) in two times.The removal efficiencies initially increased from 25.9% to 87.5% as the pH varied from 2 to 4, then gradually decreased to 6.0% with further elevating the initial pH to 6.
Although iron ions in the solution were the most at pH 2 and 3, SO2(aq) which is the dominant S(IV) species could not be combined by iron ions [22,23,29].In addition, the reactionviaEq.S8 would be inhibited because of the H+at pH 2 and 3 [20,29].HSO3–, the dominant S(IV) species at pH 4–6, could complex with Fe(III) to produce FeSO3+, which benefit the removal of TBP in the iron-activated processes.However, significant decline of TBP removal efficiency was observed with the initial solution pH increasing from 4 to 6, which was different from the results obtained in previous studies that high treatment efficiency was maintained in the initial pH range of 4–6 when the S(IV) was added in one time[20,22,23].To make clear the reason, the variations of solution pH were monitored.As shown in Fig.S6b (Supporting information), insignificant variations of solution pH were observed for the samples whose initial solution pH were 2, 3, and 6.While small decrease of solution pH was obtained for the samples whose initial solution pH were 4, 4.5 and 5, but the final solution pH after reaction for 6 min gradual increased with elevating the solution pH, which was different from some previous studies that similar final solution pH of around 3.5–3.8 was achieved in an initial solution pH range of 4–6 with dosing S(IV) in a time [22,23].Following the reactions shown in Eqs.S6-S17, lots of H+can be produced in the iron-activated S(IV) system, causing the decrease of solution pH[24,29].However, aforementioned discussion has proven that the addition of S(IV) consumed H+to efficiently ease the pH decline(Fig.S3 in Supporting information).Therefore, the significant decrease of TBP removal in the pH range of 4–6 would be because of the precipitation of Fe(III).
The oxidation of SO3•–by oxygen to form SO5•–viathe reaction shown in Eq.S10 was an essential step for the subsequent production of SO4•–[22,29].In order to investigate the effect of O2on the degradation of TBP, experiments with no purging (control sample), continuous purging air and continuous purging N2were carried out with simultaneous monitoring the dissolved oxygen (DO) concentration.As shown in Fig.S7a (Supporting information), there was negligible difference of the removal efficiencies between the control sample and the sample of purging air because of the enough oxygen in the two systems (Fig.S7b in Supporting information), which was also obtained by some previous studies[23,24].However, the removal efficiency of TBP significantly decreased to 17.5% with continuous purging nitrogen, which would be due to that the lack of O2inhibited the transformation of SO3•–to SO5•–[20,24].The explanation was supported by the low DO concentration for the sample of purging N2(Fig.S7b).
As shown in Fig.S8 (Supporting information), the removal effi-ciency of TBP significantly decreased from 97.6% to 32.9% when the TBP concentration increased from 5 μmol/L to 50 μmol/L.Although the overall removal efficiency decreased, the total removal amounts of TBP increased with the increase of initial TBP concentration.As the concentration of TBP increased, its ability to compete for radicals could be enhanced, resulting in the reduced concentrations of reactive radicals [22].While the stronger competition ability of reactive radicals by TBP elevated the total amounts of reduced TBP.
Humic acid (HA) is a common seen representative substance of natural organic matter (NOM) which always shows negative effect on the degradation of pollutants in AOPs through the competition of reactive radicals [40,41].Fig.S9a (Supporting information) shows the effect of HA on the degradation of TBP in the Fe(III)/S(IV) system with adding S(IV) in two times.When the initial HA concentration increased from 0 mg/L to 10 mg/L, the removal efficiencies of TBP gradually decreased from 87.5% to 73.7%,which was also reported in iron-activated S(IV) systems in previous studies [20,22,24].Generally, the reaction rate constants of NOM reacting with HO•and SO4•–were at the levels of 108(L mol−1)carbons−1and 107(L mol−1)carbons−1, respectively [10,42].Although the reaction rate constants were slower than that of TBP,the high HA concentration made sure it could compete for reactive radicals to decrease TBP removal.Additionally, the carboxylic functional groups in HA would complex with Fe(III) and inhibit the formation of FeSO3+, which would also contribute to the decrease of TBP degradation [24].Fig.S9b (Supporting information) shows the effect of HCO3–on the degradation of TBP in the Fe(III)/S(IV) system.When the concentration of HCO3–increased from 0 mmol/L to 2 mmol/L, the removal efficiency of TBP decreased from 87.5%to 76.1% within 6 min.HCO3–could react with HO•and SO4•–to produce CO3•–which had much weaker oxidation ability than HO•and SO4•–[22,34], causing the decrease of TBP removal in the Fe(III)/S(IV) system.In addition, HCO3–could act as solution buffer and increase the solution pH to some extent during the reaction,which would also lower the removal of TBP.Fig.S9c (Supporting information) shows the effect of Cl–on the degradation of TBP in the Fe(III)/S(IV) system.When the concentration of Cl–increased from 0 mmol/L to 2 mmol/L, the removal efficiencies of TBP decreased from 87.5% to 74.0% after reaction for 6 min.SO4•–and HO•could be consumed through the reaction with Cl–, which would produce a sequence of reactive chlorine species, such as Cl•and Cl2•–[34,43].As these reactive chlorine species can also selectively degrade organic pollutants, the influence of Cl–on organic pollutants degradation in AOPs always depends on the reaction rate constants of these chlorine species reacting with organic pollutants [17,23].In this study, the inhibition of TBP degradation in the Fe(III)/S(IV) system would be because that the reaction rate constants between these chlorine species and TBP were lower than that of SO4•–and HO•.
Although HA, HCO3–and Cl–could retard TBP degradation in the Fe(III)/S(IV) process with adding S(IV) in batches, good treatment efficiency can always be achieved for all the samples (>70%), suggesting that the Fe(III)/S(IV) process with adding S(IV) in batches might perform well for the treatment of TBP in natural water.
The degradation of TBP might form 2,4-dibromophenol, an intermediate product that is more toxic than TBP [44–46].In addition, the presence of bromine atoms in TBP might produce a series of other toxic brominated intermediates and BrO3–.Therefore, inorganic bromine including Br–and BrO3–were monitored and shown in Fig.S10 (Supporting information).No BrO3–was detected during the treatment of TBP in the Fe(III)/S(IV) system, which was also observed when applying Fe(III)/S(IV) system to degrade TBBPA [23].However, the concentration of Br–gradually increased to around 20 μmol/L with extending the reaction time to 6 min, which means that about 67% of bromine atoms in the system were converted to nontoxic Br–.Additionally, two kinds of brominated intermediates including 2,6-dibromo-1,4-benzenediol and dibromophenol(2,4-dibromophenol or 2,6-dibromophenol) were detected by an ultimate 3000 complete ultra high performance liquid chromatography system (U-HPLC-Q-Exactive Orbitrap HRMs, Thermo Fisher Scientific, Waltham, MA) (Table S3 in Supporting information), suggesting that debromination and hydroxylation might be the initial reaction in the system.Combining with the result that the remained TBP was about 13% (4 μmol/L bromine atoms), it is calculated that only around 20% (6 μmol/L) of bromine atoms were contained in the brominated organic intermediates based on mass balance.As brominated organic matters usually contain higher toxicity [47], the treatment of TBP by Fe(III)/S(IV) process is expected to reduce the toxicity of TBP solution.To comprehensively evaluate the safety of applying Fe(III)/S(IV) system to degrade TBP, the variation of the acute toxicity during the reaction was investigated by luminescent bacteria [48].As shown in Fig.4, the inhibition ratio of the original reaction solution was 27.3%, which indicated that TBP had a certain toxic effect on luminescent bacteria.Then the inhibition ratio rapidly decreased to 17.6% within 1 min, and then gradually stabilized to 16.1% at 3 min, which was coincided with the disappearance of S(IV) (Fig.S2).After the addition of S(IV) again, the inhibition ratio gradually decreased to 0 in 6 min.The results reflect that adding S(IV)in batches can not only greatly increase the removal efficiency of TBP but also can greatly reduce the acute toxicity of the reaction solution.
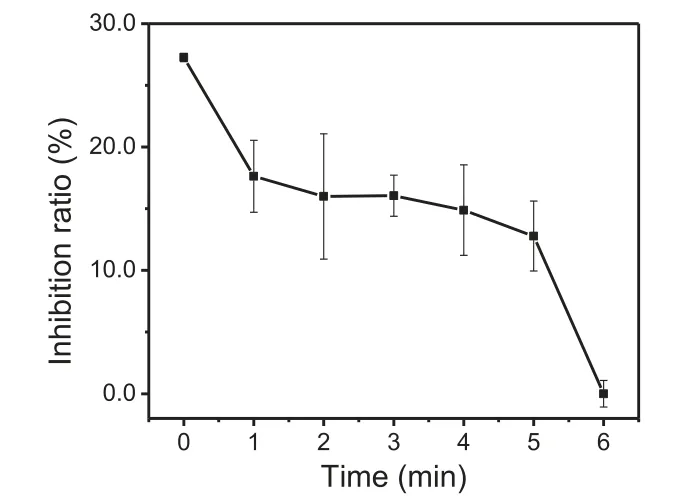
Fig.4 .The inhibition of luminescent bacteria during the treatment of TBP in the Fe(III)/S(IV) system with dosing S(IV) in two times.Conditions: [TBP]0= 10 μmol/L,[Fe(III)]0= 0.1 mmol/L, [S(IV)]0= 0.4 mmol/L, pH 4.0, temperature 25 °C.
This study comprehensively evaluated the degradation of TBP in the Fe(III)/S(IV) system with adding S(IV) in batches, evidencing that the addition of S(IV) in batches could effectively increase the utilization efficiency of S(IV), which improved the removal efficiency of TBP.The oxidation of TBP was attributed to SO4•–, HO•and SO5•–as SO4•–was the primary radical.Highest TBP removal in the Fe(III)/S(IV) system was achieved at pH 4.0 in the system.In the selected experimental conditions, the treatment efficiencies followed trends of initial increase then decrease with increasing initial Fe(III) and S(IV) dosages from 0.05 and 0.1 mmol/L to 0.2 and 2.0 mmol/L, respectively.Additionally, the increase of initial TBP concentration lowered TBP removal efficiency, and oxygen has been found to be necessary for the degradation of TBP in the Fe(III)/S(IV) system.The typical water matrices including HA,HCO3–and Cl–performed negative impacts on the degradation of TBP.The significant decrease of acute toxicity after the treatment of TBP by the Fe(III)/S(IV) system with adding S(IV) in batches further suggests that this system would be a good AOP in the degradation of TBP.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The support by the National Natural Science Foundation of China (No.51878308) and the Young Top-notch Talent Cultivation Program of Hubei Province are appreciated.We also thank the Analytical and Testing Center of Huazhong University of Science and Technology for the related measurements.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.003.
 Chinese Chemical Letters2022年11期
Chinese Chemical Letters2022年11期
- Chinese Chemical Letters的其它文章
- Zeolite-based Fenton-like catalysis for pollutant removal and reclamation from wastewater
- 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents
- Degradation of florfenicol in a flow-through electro-Fenton system enhanced by wood-derived block carbon (WBC) cathode
- Simultaneous determination of indole metabolites of tryptophan in rat feces by chemical labeling assisted liquid chromatography-tandem mass spectrometry
- Self-powered anti-interference photoelectrochemical immunosensor based on Au/ZIS/CIS heterojunction photocathode with zwitterionic peptide anchoring
- The role of Cs dopants for improved activation of molecular oxygen and degradation of tetracycline over carbon nitride
