Randomized controlled trial to evaluate the efficacy and safety of fexuprazan compared with esomeprazole in erosive esophagitis
Kang Nyeong Lee,Oh Young Lee, Hoon Jai Chun, Jin Il Kim, Sung Kook Kim, Sang Woo Lee, Kyung Sik Park,Kook Lae Lee, Suck Chei Choi,Jae-Young Jang, Gwang Ha Kim, In-kyung Sung, Moo In Park, Joong GooKwon, Nayoung Kim, Jae Jun Kim, Soo Teik Lee, Hyun Soo Kim, Ki Bae Kim,Yong Chan Lee,Myung-GyuChoi, Joon Seong Lee, Hwoon-Yong Jung, Kwang Jae Lee, Jie-Hyun Kim, Hyunsoo Chung
Abstract
Key Words: Gastroesophageal reflux; Esophagitis; Proton pump inhibitors; Heartburn; Quality of life
INTRODUCTION
Gastro-esophageal reflux disease (GERD) is characterized by heartburn and acid regurgitation symptoms resulting from abnormal gastric reflux into the esophagus[1]. GERD prevalence is increasing in Asian and Western countries[2]. A recent report documented the worldwide prevalence of GERD as 13.3% (10.0% in Asia, 15.4% in North America, and 17.1% in Europe)[3]. The percentage change in agestandardized GERD prevalence in South Korea was 7.6% between 1990 and 2017[2]. GERD is classified as erosive esophagitis (EE) or non-erosive reflux disease (NERD) based on the presence of esophageal mucosal breaksviaendoscopic examination[4]. Approximately one-third to half of the patients with EE complain of the typical symptoms of GERD[5]. In addition to typical symptoms, atypical and extraesophageal symptoms in patients with EE may impair health-related quality of life (HRQL)[6,7]. However,a poor HRQL is more likely associated with symptom frequency and severity rather than the presence or absence of EE[8].
A main treatment of GERD has been the use of proton pump inhibitors (PPIs). Current practical guidelines recommend PPIs as the first-line therapy for patients with EE[9]. PPIs irreversibly inhibit (H+/K+)-ATPase within the parietal cells of the gastric mucosa[10]. Studies have demonstrated that PPIs are 40%-50% more effective than placebo in healing of EE and resolving GERD symptoms[11,12].Furthermore, complete healing of EE has been reported in 80% to 90% of patients after four and eight weeks of PPI treatment, respectively[11]. However, there are shortcomings of PPIs in GERD treatment,including unsatisfactory efficacy in atypical and extraesophageal symptoms and typical symptoms[13].This might be due to the pitfalls of PPIs: The variability in PPI metabolism based on cytochrome P450(CYP) 2C19 polymorphisms and the delayed onset of PPIs owing to their slow absorption associated with enteric coating to prevent degradation by acid.
As an alternative to PPI in GERD treatment, a novel potassium-competitive acid blocker (P-CAB),fexuprazan (Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea), was developed[14]. In contrast to PPIs, metabolism of fexuprazan is independent of CYP 2C19; enteric coating is not needed because of acid stability. While PPIs bind irreversibly to only the active forms of the proton pump, fexuprazan can bind to both the active and inactive forms of the proton pump competitively and reversibly. A previous study on healthy individuals demonstrated the effect of fexuprazan’s acid suppression and tolerability,observing that gastric pH > 4 was reached within 2 h and maintained for 24 h in a dose-dependent manner[14].
Nevertheless, the effectiveness and safety of fexuprazan compared to esomeprazole, one of the most widely used PPIs in GERD, have not been confirmed among patients with EE. Therefore, this phase III,double-blind, randomized, active-controlled, multi-center study was conducted to compare the efficacy and safety between fexuprazan and esomeprazole in patients with EE.
MATERIALS AND METHODS
Study design and treatments
This randomized, double-blind, parallel-group, multicenter, and phase III trial was performed in 25 institutions in South Korea between December 2018 and August 2019. Adult patients provided written informed consent prior to enrolment, and then screening test including the endoscopy was performed.Eligible participants were randomized 1:1 to receive either fexuprazan 40 mg or esomeprazole 40 mg following the screening test. At this point, participants were stratified according to Los-Angeles (LA)Classification Grade classified by the result of upper gastrointestinal endoscopy.
To ensure the double-blinded nature of the study, patients were administered once daily with two tablets of the study medication (fexuprazan 40 mg or esomeprazole 40 mg with its matching placebo in the study and control groups, respectively) for eight weeks.
Compliance of the study medication was ascertained at each visit by participants returning the unused portion and empty packaging, and was calculated using the total numbers of tablets to be taken,of tablets actually taken, and of returned and unreturned tablets in each participant.
This study was approved by the institutional review boards of each institution, conducted in compliance with the relevant ethics guidelines, and registered at ClinicalTrials.gov (NCT03736369). All the study medications and procedures performed were in accordance with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards.
Participants
Eligible participants were male or female patients (20-75 years old) with EE (LA Classification Grades A to D) confirmed by endoscopy at the same institution within 14 d of study treatment initiation. The major exclusion criteria were Barrett’s esophagus (> 3 cm); esophageal stricture; active peptic ulcers;ulcer-related stenosis; gastrointestinal bleeding; eosinophilic esophagitis; Zollinger-Ellison syndrome;inflammatory bowel diseases; irritable bowel syndrome; pancreatitis; psychiatric disorders; acquired immune deficiency syndrome (AIDS); viral hepatitis; history of gastric acid suppression surgery;significant morbidities in the cardiovascular, respiratory, hepatic, renal, neurologic, endocrine,hematologic, and urologic systems; history of malignancies within five years; drug or alcohol abuse; and hypersensitivity to drugs containing active constituents of esomeprazole or other similar drugs (benzimidazoles and antibiotics). Also excluded were those who had abnormal laboratory values, including alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), total bilirubin, creatinine, and blood urine nitrogen > 2′ upper limits of the normal range, and women with child-bearing potential who did not consent to appropriate contraceptive methods use during the study.
Protocol
Endoscopy was performed at the start of the screening period and at weeks 4 and 8. EE healing was defined as the complete absence of mucosal breaks. If mucosal breaks did not heal at week 4, the patients continued to receive the study drug until week 8, when endoscopy was performed again. Two weeks after the confirmation of EE healing from the centralized endoscopic evaluation, the patients were evaluated for safetyviatelephone interviews, and where applicable, they underwent additional tests and procedures (Figure 1).
The primary efficacy endpoint was the proportion of patients with endoscopically confirmed EE healing at week 8. The secondary efficacy endpoints were EE healing rate at week 4; the patients’reported symptom outcomes, symptom assessment by reflux disease questionnaire (RDQ), and GERDhealth related quality life (GERD-HRQL). Symptoms were evaluated based on patients’ symptom diaries. Symptom severity in the daytime and at night were measured according to the five-point scale(0: none, 1: mild, 2: moderate, 3: severe, 4: very severe).
The parameters for assessing symptom responses were the first day of the complete resolution of symptoms (heartburn, acid regurgitation, and heartburn/acid regurgitation) after treatment, the proportion of patients without symptoms in the first 7 d and through the 8 wk of treatment, and the proportion of symptom-free days in the first 7 d and through the 8 wk of treatment. Changes in symptoms and GERD-HRQL from baseline at weeks 4 and 8 were evaluated using the RDQ and GERDHRQL scales, respectively. The RDQ is a self-administered questionnaires comprising of 12 items to assess the frequency and severity of heartburn, acid regurgitation, and dyspepsia. Each item for frequency and severity was scored from 0 to 5; the higher score, the more severe or frequent symptoms[15]. The RDQ demonstrated the validity and reliability for diagnosis of GERD in primary care and community settings[16]. The GERD-HRQL scale comprises 11 items for the symptoms of heartburn and dysphagia, medication effects, and the patients’ health conditions[17]. Each item was scored from 0 to 5;the higher the score, the lower the quality of life. The GERD-HRQL was validated and considered as an appropriate instrument to evaluate typical GERD symptoms[18]. Therefore, previous clinical studies performed in South Korea have used the RDQ and GERD-HRQL to evaluate the therapeutic effect in patients with GERD[19,20].
Additional analyses included heartburn and extraesophageal symptoms of GERD (chronic cough and throat irritation) in terms of the proportion of patients without symptoms and the proportion of symptom-free days in the first 3 d, 7 d and through the 8 wk of treatment. Patients with moderate/severe heartburn (RDQ ≥ 3) were also compared between the groups in terms of the proportion of patients without symptoms and the proportion of symptom-free days in the first 3 d, 7 d and through the 8 wk of treatment.
The patients’ baseline characteristics included age, sex, body mass index (BMI), smoking history,drinking history, LA grade,Helicobacter pylori(H. pylori)infections, and CYP2C19 extensive metabolizer(EM)/poor metabolizer (PM) status. Serum gastrin levels were measured at weeks 4 and 8. Safety outcomes were measured by the analysis of adverse events (AEs), vital signs, physical examination,electrocardiogram (ECG) findings and laboratory tests. Adverse events (frequency, severity and seriousness) and concomitant medications were monitored throughout the study. Treatment-emergent adverse event (TEAE) was defined as an AEs newly occurred after the randomization and the first administration of study medication, and adverse drug reaction (ADR) was defined as any untoward and unintended response to the study medication of which causal relationship cannot be excluded.
Sample size
Based on previous studies, we estimated the sample size, assuming that the complete healing rate of mucosal breaks was 94.8% at week 8 after treatment with fexuprazan 40 mg and esomeprazole 40 mg[21,22]. Based on this threshold parameter, the sample size was 104 patients per treatment group, using the following conditions of the PASS program: non-inferiority margin of 10%[23], a one-sided significance level of 2.5%, 90% statistical power, and 1:1 randomization.
Randomization
This study was used stratified block randomization method base on LA grades (A/B/C/D) by the result of upper gastrointestinal endoscopy. An independent statistician generated a randomization list based on stratification factor (LA grades) using the PLAN (Proc Plan) procedure of SAS (ver. 9.4, SAS Institute, Cary, NC, United States). Eligible participants were randomly assigned by the investigators in a ratio of 1:1viaan interactive web-response system (IWRS). Neither participants nor relevant investigators were aware of these assignments.
Statistical analysis
Efficacy was evaluated by both the full analysis set (FAS) and per-protocol set (PPS), and PPS findings were interpreted as the main results. For the safety assessment, statistical analysis was performed on the safety set (SS). The FAS, based on the intention-to treat principle, included patients who received at least one dose of the study drug after randomization and had at least one primary efficacy assessment. The PPS included patients in the FAS who completed the study without any major protocol deviation. The SS group included all patients who received the study drug at least once after randomization.
For symptoms responses daily (day-time and night-time) assessment in the efficacy analysis, missing symptom in day-time or night-time was imputed using the last observation carried forward. Except for this, missing value was set to missing without imputation, and the results of patients who were completed the study early as mucosal breaks were completely healed up to week 4 were used as the results of the week 8.
Summaries of baseline characteristics of patients were presented in FAS. To assess the difference between the treatment group, the two sample t-test or Wilcoxon rank-sum test were used after normality evaluation in continuous baseline characteristics variables, and the chi-square test or Fisher’s exact test were used in categorical baseline characteristics variables.
民营企业没有各种发展的支持,拥有的只是野蛮生长的力量和一颗不断向上的心,而这正是晟图机械能够逐步走向成功、推动行业不断前进的内生动力。
The primary objective of the study was to demonstrate the non-inferiority of fexuprazan 40 mg compared with esomeprazole 40 mg. The cumulative healing rate of EE and corresponding two-sided 95% confidence interval (CI) were presented for visit (up to week 4 or week 8) by treatment group. The common risk difference of the healing rate of EE up to week 8 between the treatment groups(fexuprazan 40 mg group - esomeprazole 40 mg group) and corresponding two-sided 95% CI using the Cochran-Mantel-Haenszel method adjusted by a stratification factor (baseline LA grade) were presented in the PPS. The non-inferiority of fexuprazan 40 mg to esomeprazole 40 mg was determined the lower limit of its two-sided 95%CI is larger than the non-inferiority margin of -10%. The same analyses were performed for the non-inferiority of healing rate of EE up to week 4. Furthermore, continuous data were analyzed using an analysis of covariance (ANCOVA) model, including treatment group as treatment effect, and baseline score (included if evaluation data were changed from baseline) and baseline LA grade as covariates. The changes from baseline within-treatment group were used the paired t-test or Wilcoxon signed rank test after normality evaluation as a post hoc analysis. Categorical data were analyzed using the Cochran-Mantel-Haenszel method adjusted by baseline LA grade. For the safety analysis, the chi-square test or Fisher’s exact test was used to compare the difference in the incidence of AEs between the treatment groups. All statistical analyses were performed using SAS (ver. 9.4, SAS Institute, Cary, NC, United States) with a two-sided significance level of 5% for all tests.
RESULTS
Baseline characteristics of the participants
Of the total of 470 patients screened, 263 patients with EE were randomized to receive either fexuprazan 40 mg or esomeprazole 40 mg (Table 1). In total, 231 patients [152 men (65.8%) and 79 women (34.2%);54.4 ± 12.7 mean age] were included in the FAS and completed the study (n= 116 and 115 in the fexuprazan and esomeprazole groups, respectively). Thirteen patients with study medication-related deviation, visit window deviation and consent withdrawal were excluded from the FAS (9 and 4 in the fexuprazan and esomeprazole groups, respectively), and 218 patients completed the study on the PPS (n= 107 and 111 in the fexuprazan and esomeprazole groups, respectively). The SS included 131 patients each in the fexuprazan and esomeprazole groups (Figure 2).
There were no significant differences in baseline characteristics between both groups, except CYP2C19 genotypes (EM or PM). A statistically significant difference was seen in the classification of CYP2C19 genotype (P= 0.007), but the result was obtained from only some of the participants who agreed to genotyping (n= 51 and 56 in the fexuprazan and esomeprazole groups, respectively).
The mean compliance rates were 98.6% ± 8.1% and 99.0% ± 2.6% at weeks 4 and 8, and the overall compliance rate with study medication exceeded 95% in all treatment groups without between-group differences.
Efficacy
Healing rate of EE: In the PPS, the proportions of patients with complete absence of mucosal breaks at week 8 were 99.1% (106/107) and 99.1% (110/111) in the fexuprazan and esomeprazole groups,respectively. The difference in proportions of patients with complete absence of mucosal breaks adjusted by baseline LA grade [fexuprazan 40 mg group - esomeprazole 40 mg group] was 0.9%(95%CI, -0.9 to 2.6) (Figure 3). The lower limit of two-sided 95%CI at week 8, -0.9%, was greater than the non-inferiority margin of -10%, indicating the non-inferiority of 8-week treatment of fexuprazan 40 mg to esomeprazole 40 mg in EE healing in GERD. At week 4, the healing rates of EE were not different between the two groups [90.3% (93/103) in the fexuprazan group and 88.5% (92/104) in the esomeprazole group, respectively] with a difference of 2.6% (95%CI: -5.7 to 10.9). The lower limit of 95%CI, -5.7%, was also greater than the non-inferiority margin of -10%. These results demonstrate that fexuprazan 40 mg was non-inferior to esomeprazole 40 mg in EE healing in GERD at weeks 4 and 8.
As the results in the exploratory analysis, healing rates of EE were not statistically significantly different according to CYP2C19 genotype (EM or PM) andH. pyloriinfection (positive or negative).Healing rates of EE at weeks 4 and 8 in EM participants (n= 88) were not different between fexuprazn and esomeprazole groups [91.7% (33/36)vs89.4% (42/47) at week 4; 100.0% (36/36)vs98.1% (51/52) at week 8]. EE healing rates at weeks 4 and 8 in PM participants (n= 14) were not different between the treatment groups [70.0% (7/10)vs100.0% (3/3) at week 4; 90.9% (10/11)vs100.0% (3/3) at week 8].Healing rates of EE inH. pylori-positive participants (n= 47) were 100.0% (17/17) and 88.46% (23/26) in the fexuprazan and esomeprazole groups at week 4, and all ofH. pylori-positive participants were completely healed at week 8. Healing rates of EE inH. pylori-negative participants (n= 169) were 88.2%(75/85)vs88.3% (68/77) at week 4, and 98.9% (87/88)vs98.8 (80/81) at week 8 in the fexuprazan and esomeprazole groups.Symptom response: Fexuprazan exhibited an overall symptom relief comparable to esomeprazole. The differences between the groups were not significant with respect to the first day of the complete resolution of symptoms (resolution of typical symptoms for 7 d) after treatment: the median values of days to complete resolution for heartburn, acid regurgitation, and heartburn/acid regurgitation were 13, 8, and 18 dvs10, 6, and 16 d in the fexuprazan and esomeprazole groups, respectively. There were no statistically significant differences in the proportions of patients without symptoms in the first 7 d(26.2%, 25.2%, and 15.0%,vs21.6%, 27.9%, and 11.7%, for heartburn, acid regurgitation, and heartburn/acid regurgitation, in the fexuprazan and esomeprazole groups, respectively) and through the 8 wk (20.6%, 21.5%, and 10.3%,vs17.1%, 27.0%, and 9.9%). Similarly, there were no statistically significant differences in the proportion of symptom-free day/night-time in the first 7 d and through the 8 wk between both groups. (Supplementary Tables 1-4).

Table 1 Baseline characteristics of the patients (full analysis set)
In the RDQ and GERD-HRQL, the frequency and severity of heartburn and acid regurgitation improved in both groups, with no significant difference in changes from baseline at weeks 4 and 8(Tables 2 and 3).
In the results of subgroup analyses, fexuprazan demonstrated better heartburn relief in patients with moderate-to-severe symptoms who had experienced heartburn for 2 or more days in the week before treatment: the proportions of those without heartburn on the first day 3 were significantly greater in the fexuprazan group than in the esomeprazole group (22.4%vs7.9%,P= 0.026 at the day/night-time;29.3%vs12.7%,P= 0.037 at the day-time; 34.5%vs17.5%,P= 0.035 at the night-time) (Supplementary Table 5). The extraesophageal symptom of chronic cough improved better with fexuprazan:the least squares (LS) means of days without chronic cough were significantly greater in the fexuprazan group than in the esomeprazole group on the days 3, 7, and week 8 (P= 0.006,P= 0.003, andP= 0.002,respectively). The extraesophageal symptom of throat irritation improved in both groups on days 3, 7,and week 8 without significant differences between the treatment groups (Supplementary Table 6).
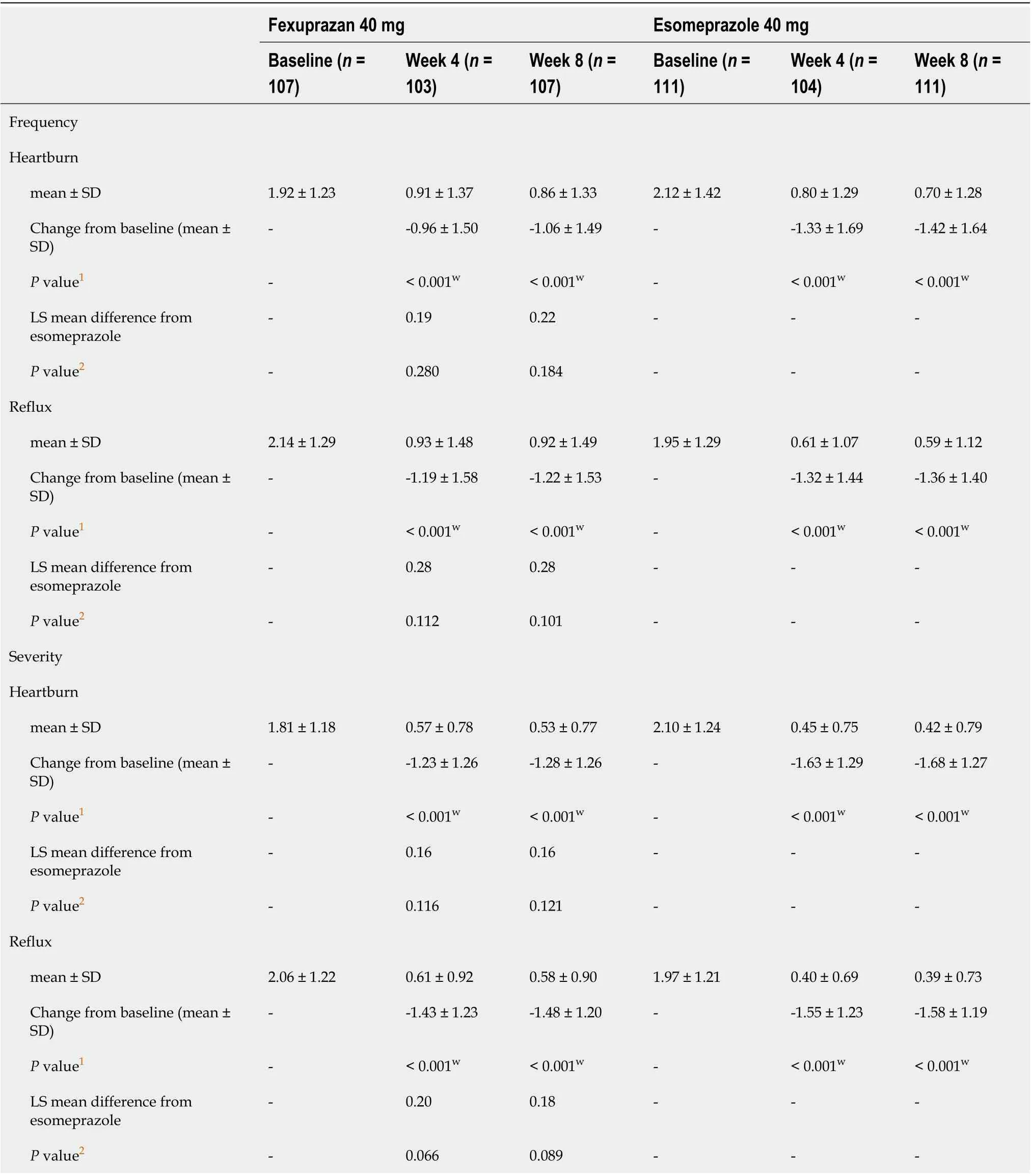
Table 2 Change in reflux disease questionnaires symptom scores from baseline at weeks 4 and 8 (per protocol set)
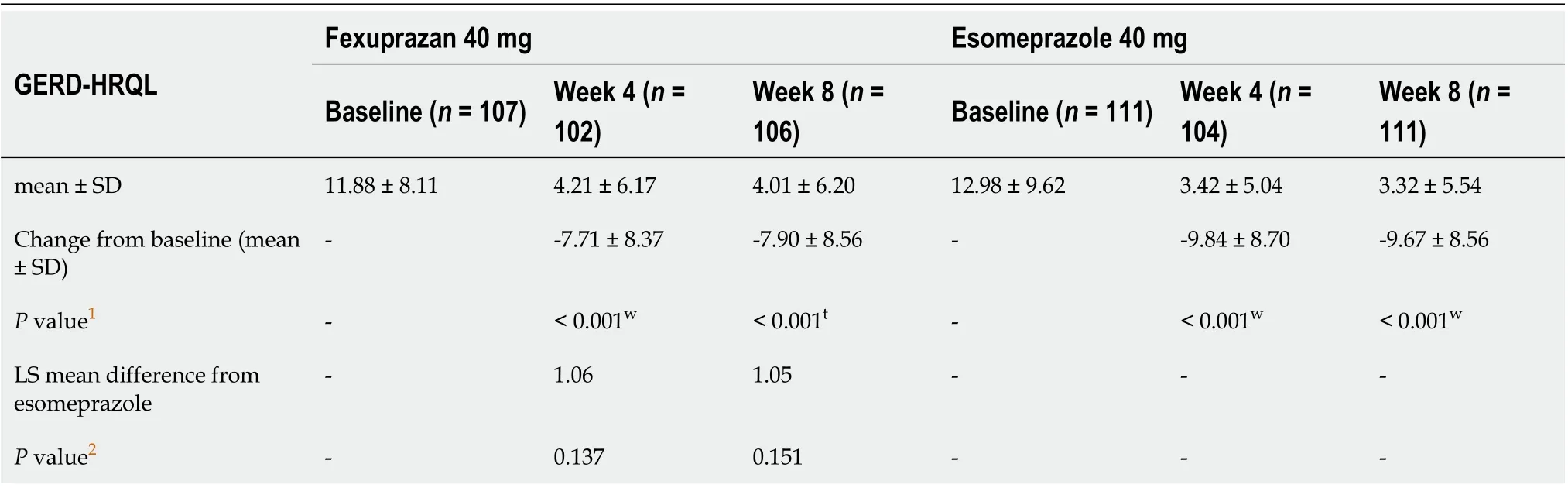
Table 3 Change in gastroesophageal reflux disease-health related quality of life score from baseline at weeks 4 and 8 (per protocol set)
Safety
Safety analyses were performed for 262 patients who received at least one dose of the study meditation.The overall incidences of TEAEs and ADRs were not significantly different between the treatment groups; TEAEs were reported by 22 patients (16.8%) and 25 (19.1%), and ADRs were reported by 9 patients (6.9%) and 7 patients (5.3%) in the fexuprazan and esomeprazole groups, respectively (Table 4).The severity of TEARs was mostly mild (61 events), with six moderate events (diarrhea, nausea,dysgeusia, pruritus, pain, and cystitis) and only one severe event (influenza). All ADRs were either mild(21 events) or moderate (3 events). There were 2 patients (1.5%) with ADRs (diarrhea and pruritus)leading to discontinuation of the study medication in the fexuprazan group, not esomeprazole group.However, there was no statistically significant difference in the incidence of ADRs leading to discontinuation between both groups. No serious TEARs or ADRs were reported in either group of patients(Supplementary Table 7). The most frequently occurring (≥ 2%) TEAEs were shown in Table 4.
The serum gastrin levels intended to increase, and their differences between the treatment groups were not significant at weeks 4 and 8 (Figure 4). There were no clinically significant changes in the laboratory test, vital signs, physical examination and ECG findings, and no liver enzyme elevations were reported.
DISCUSSION
This study demonstrated the non-inferior efficacy and safety of fexuprazan 40 mg once daily to esomeprazole 40 mg once daily in the healing of EE at week 8 in patients with EE. The rates of healing EE were not different between the two groups at week 4. No differences between the groups were found in the secondary endpoints regarding symptom responses, including the first day of the complete resolution of symptoms (heartburn and acid regurgitation) and the proportions of patients without symptoms along with the proportions of symptom-free days in the first 7 d and throughout 8 wk of the treatment period. Furthermore, the two groups did not differ in the changes in RDQ and in GERDHRQL from baseline at weeks 4 and 8. Serum gastrin levels and safety-related TEAEs and ADRs did not differ.
Our results were consistent with those of other P-CABs (tegoprazan and vonoprazan) in comparison with PPIs. Studies in patients with GERD and healthy volunteers have revealed the efficacy and safety of tegoprazan and vonoprazan, to be similar to those of PPIs. In a phase I study of tegoprazan, which has been used since 2018 after approval in South Korea, it safely inhibited acid secretion compared to esomeprazole[24]. In a multicenter, randomized, double-blind, and parallel-group non-inferiority study on 302 patients with endoscopically confirmed EE[19], tegoprazan 50 mg and 100 mg indicated cumulative healing rates of 98.9% and 98.9% at week 8, respectively, compared to the 98.9% healing rate of esomeprazole 40 mg. Regarding vonoprazan, its efficacy has been identified in clinical and pharmacological factors, including healing EE, symptom responses, maintenance treatment effect after healing EE, efficacy in refractory GERD, the effect of intermittent therapy, and the pH 4 holding time ratio[25-28]. A study of short-term symptom response at week 4 was similar: 88.0% and 81.8% in the esomeprazole 20 mg and vonoprazan 20 mg groups, respectively[29]. In a dose-ranging study,vonoprazan 5, 10, 20, and 40 mg exhibited non-inferior efficacy to lansoprazole 30 mg in the healing rates of EE at week 8[30]. In those with severe grades of EE and extensive metabolizers, treatments with vonoprazan 20 mg and lansoprazole 30 mg for 8 wk did not differ in the rates of EE healing[23].Additionally, the recurrence rates of EE were significantly lower after a 24-wk treatment using 10 mg and 20 mg vonoprazan than with lansoprazole 15 mg[31]. Regarding the effect of vonoprazan on gastric acidity, the pH 4 holding time ratio significantly increased from 73.21% to 96.46% and from 69.97% to 100.00% in the 20 mg and 40 mg groups, respectively[26].

Table 4 Overall Summary of treatment-emergent adverse events (safety set)
In this study, fexuprazan led to rapid treatment response in patients with moderate-to-severe heartburn. The proportion of patients without heartburn on day 3 who had moderate-to-severe symptoms was significantly higher with fexuprazan than with esomeprazole, in both day-time and night-time, and also at night-time only. Nocturnal heartburn was reportedly presented in approximately 80% of patients with frequent heartburn and impaired sleep quality and daytime HRQL[6,32].Moreover, the continuous use of PPIs was not effective for nocturnal heartburn in 30% of patients with reflux esophagitis[33], and in over 50% of patients with symptomatic EE[34]. Thus, this study suggests that fexuprazan may provide rapid symptom resolution in patients with nocturnal heartburn and refractory response to PPI treatment. The rapid response of symptoms to P-CABs was identified in another study revealing that vonoprazan 20 mg relieved heartburn symptoms on day 1 in more patients than lansoprazole 30 mg[35]. Although the present study did not demonstrate faster healing of EE, there have been studies showing rapid healing of EE after 2-week treatment of vonoprazan than PPIs[23,36].Accordingly, in conjunction of this faster healing in EE with our finding of rapid symptom response by fexuprazan, it is cautiously suggested that patients with EE may need a relatively short-term treatment period by fexuprazan than PPIs. Further studies on shorter treatment in EE by fexuprazan are needed.
The pharmacodynamic and pharmacokinetic profiles of fexuprazan explain the rapid onset and sustained inhibition of acid secretion in GERD. Studies of fexuprazan in healthy individuals revealed that the mean percentage of time of gastric pH > 4 was achieved in 80% of 24 h and even at night.However, esomeprazole achieved a lower mean percentage time of gastric pH > 4, which was also lower at night[14]. With regard to the pharmacokinetic parameters, Cmaxwas reached within 1-4 h after dosing,and the mean elimination half-life was approximately 9 h. Fexuprazan also exhibited dose-response relationships. Plasma concentrations increased proportionately with the doses ranging from 10-320 mg,whereas multiple doses did not cause significant accumulation. The elimination pathway of fexuprazan was not a renal route but probablyviathe liver or gut. Furthermore, in contrast to PPIs, food intake was not necessary for optimal action, as the parameters of gastric pH and plasma concentrations of fexuprazan did not change with a high-fat diet. Adverse drug effects on the liver were not higher with fexuprazan than with placebo, in contrast to the 0.2% potential liver toxicity in the pre-clinical experiment of vonoprazan[37]. Moreover, the gastrin-increasing effect of fexuprazan was similar to that of other PPIs, and was less frequent than that of vonoprazan[38]. Furthermore, the effects on gastric acid suppression, serum gastrin elevation, and dose response relationship were also consistent in different populations including Korean, Caucasian, and Japanese ethnicities[39].
In our study, fexuprazan improved one of the extraesophageal symptoms of GERD better than esomeprazole. Despite its unknown pathophysiology, patients with GERD-related chronic cough have been treated with PPIs with unsatisfactory symptom control. The superior efficacy of PPIs over placebo has not been confirmed in patients with GERD-related chronic cough in recent randomized controlled trials (RCTs)[40,41]. In addition, a meta-analysis of 5 RCTs did not suggest any evidence in favor of PPI therapy[42]. Taken the effect of fexuprazan in this study with the overall inadequate efficacy of PPIs in chronic cough, we suggest that fexuprazan could provide a better solution than PPIs for GERD-related chronic cough.
This study revealed elevated serum gastrin levels, but these were not significantly different between both groups. Previous reports have revealed higher serum gastrin levels in the P-CAB group than in the PPI group[37,43]. In the study of 212 outpatients, the serum gastrin in the P-CAB group had 2-3 fold and 1-2 fold increases than the normal and PPI groups, respectively[43]. However, increased serum gastrin levels were limited, particularly in patients with normal mucosa or mild atrophic gastritis. Additional limitations were the treatment periods of less than one year and the sampling time at pre-meal rather than at the peak level of 30 min after meals.
This study had some limitations. First, the number of patients classified as LA grade C/D was small.Actually, those with LA grades C/D accounted for only 6.2% of the fexuprazan and 7.0% of the esomeprazole groups. Therefore, it was difficult to confirm the advantage of fexuprazan, better clinical performances due to unique pharmacokinetics and pharmacodynamics of fexuprazan in severe EE than PPIs, as in other P-CAB studies[30]. Future fexuprazan studies need to be focused on significantly larger number of patients with severe EE (LA grades C/D). Second, the treatment period was only eight weeks, and studies on the long-term safety or recurrence rates after EE healing are required in the future, considering the insufficient data regarding the long-term safety of P-CABs. Third, when evaluating symptom severity, the possible effects of comorbidities such as chronic obstructive pulmonary disease were not considered[44].
CONCLUSION
We concluded that fexuprazan 40 mg once daily has non-inferior efficacy and safety to esomeprazole 40 mg once daily in healing EE at weeks 4 and 8. From the symptom evaluation through the symptom diary, RDQ and GERD-HRQL, it was confirmed that fexuprazan improved symptoms of heartburn and acid regurgitation and quality of life similarly to esomeprazole. The increase in serum gastrin levels by fexuprazan was not different from that of esomeprazole. Future research on fexuprazan is needed to evaluate the long-term efficacy and safety of fexuprazan in GERD including EE, PPI-refractory GERD,and other acid-related diseases along with the long-term maintenance therapy including on demand or intermittent treatment.
ARTICLE HIGHLIGHTS
FOOTNOTES
Author contributions:Lee OY contributed to study design, acquisition and interpretation of data, and critically reviewed and edited the manuscript; Lee KN contributed to the data interpretation, and drafting and editing the manuscript; All authors contributed to enrolment of patients, agreed to be responsible for every aspect of this work,reviewed and finally approved the manuscript.
Institutional review board statement:This study was approved by the institutional review boards (IRBs) of each institution, conducted in compliance with the relevant ethics guidelines. All the study medications and procedures performed were in accordance with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards.
Clinical trial registration statement:This study is registered at ClinicalTrials.gov. The registration identification number is NCT03736369.
Informed consent statement:All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement:The corresponding author, Lee OY, had been a member of outside directors at the Daewoong Pharmaceutical Co., Ltd, form the period March 242018 to Nov 22021. All other authors declare no conflict of interests regarding this study.
Data sharing statement:Data are available upon reasonable request.
CONSORT 2010 statement:The authors have read the CONSORT 2010 Checklist, and the manuscript was prepared and revised according to the CONSORT 2010 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:South Korea
ORCID number:Kang Nyeong Lee 0000-0002-3728-8672; Oh Young Lee 0000-0002-6025-530X; Hoon Jai Chun 0000-0002-5539-361X; Jin Il Kim 0000-0001-6801-6891; Sung Kook Kim 0000-0002-2861-8123; Sang Woo Lee 0000-0003-3491-0371;Kyung Sik Park 0000-0003-1874-9936; Kook Lae Lee 0000-0001-6676-9451; Jae-Young Jang 0000-0002-7930-1468; Gwang Ha Kim 0000-0001-9721-5734; Nayoung Kim 0000-0002-9397-0406; Jae Jun Kim 0000-0002-0226-1330; Soo Teik Lee 0000-0002-2975-053X; Hyun Soo Kim 0000-0003-4834-0496; Ki Bae Kim 0000-0001-6372-432X; Yong Chan Lee 0000-0001-8800-6906; Myung-Gyu Choi 0000-0003-4083-5187; Hwoon-Yong Jung 0000-0003-1281-5859; Kwang Jae Lee 0000-0002-8534-0850; Jie-Hyun Kim 0000-0002-9198-3326; Hyunsoo Chung 0000-0001-5159-357X.
Corresponding Author's Membership in Professional Societies:Korean Society of Gastointestinal Endoscopy; Korean Society of Neurogastroenterology and Motility; The Korean Society of Gastroenterology; and Korean Association of Internal Medicine.
S-Editor:Gong ZM
L-Editor:A
P-Editor:Gong ZM
 World Journal of Gastroenterology2022年44期
World Journal of Gastroenterology2022年44期
- World Journal of Gastroenterology的其它文章
- Comment on “Prognostic value of preoperative enhanced computed tomography as a quantitative imaging biomarker in pancreatic cancer”
- Virological and histological evaluation of intestinal samples in COVID-19 patients
- Postoperative outcomes and recurrence patterns of intermediate-stage hepatocellular carcinoma dictated by the sum of tumor size and number
- Glucagon-like peptide-2 analogues for Crohn’s disease patients with short bowel syndrome and intestinal failure
- Development of Epstein-Barr virus-associated gastric cancer: Infection, inflammation, and oncogenesis
- Machine learning insights concerning inflammatory and liver-related risk comorbidities in noncommunicable and viral diseases
