A novel HNF4A mutation identified in a child with maturity onset diabetes of the young
Ming-Qiang Zhu·Yang-Li Dai·Xue-Feng Chen·Hu Lin·Jin-Na Yuan·Ke Huang·Wei Wu·Jun-Fen Fu·Guan-Ping Dong
Maturity-onset diabetes of the young (MODY) is a group of monogenic diabetes mellitus characterized by early-onset diabetes with dominant inheritance.To date,at least 14 different causative genes have been identified.HNF4A-MODY is relatively uncommon in China [1,2].We report a Chinese child with diabetes mellitus due to a heterozygousHNF4Amutation.
A 13-year-old girl visited the clinic due to overweight.Family history enquiry revealed that her father had a history of hyperglycemia,and it was improved after lifestyle changes.The father’s blood glucose was not monitored regularly.Her mother had type 2 diabetes (T2DM) and was treated with oral hypoglycemic drugs.Her sibling had been diagnosed type 1 diabetes mellitus (T1DM) and was treated with insulin.Laboratory examination revealed a higher glycated hemoglobin (HbA1c) (reference: 4.5–6.3%)of 14.2%;and all five pancreatic autoantibodies including glutamic acid decarboxylase antibody,islet cell antibody,insulin autoantibody,tyrosine phosphatase antibody and zinc transporter 8 antibody were negative.The insulin and C-peptide release test showed that the peak of serum insulin and C-peptide were delayed.Considering the possibility of T2DM,the patient was treated with insulin injection combined with oral metformin.This treatment modality was maintained for three months until genetic analysis results obtained.HbA1c was 9.5% after three months when treated with this modality.
Meanwhile,we made a genetic analysis for the family.The results of gene detection showed (Fig. 1) a heterozygous missense variant in exon 6 ofHNF4A(c.736G > T p.G246C) in the patient which was inherited from the father.Her sibling also carried the same mutation.This mutation has not been reported previously.The mutation was classified as a variant of uncertain significance according to the American College of Medical Genetics (ACMG) guidelines.It has been predicted as deleterious by all silico prediction algorithms.
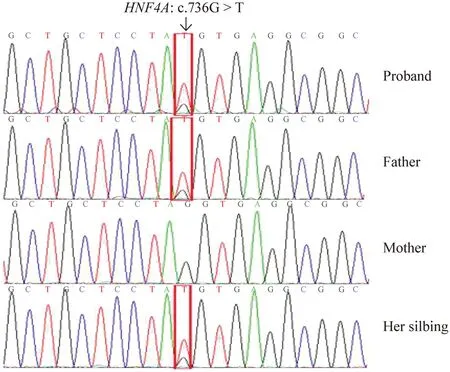
Fig.1 Genetic studies of the family.The proband had a heterozygous missense variant in exon 6 of HNF4A(NM_000457.4: c.736G > T p.G246C),inherited from the father,and her sibling also carried the same variant.The mother did not carry the same variant
According to ISPAD 2018 Consensus Guidelines,most patients withHNF4A-MODY are extremely sensitive to sulfonylureas,and the initial dose should be low (one-quarter of the normal starting dose in adults) to avoid hypoglycemia[3].With the informed consent by the child and the guardians,we changed the treatment modality from insulin injection and oral metformin to oral glibenclamide,with a dose of 0.625 mg/time,twice a day (one-quarter of the normal starting dose in adults).The entire process of drug adjustment was under intensive surveillance by continuous glucose monitoring (CGM) for nearly 72 hours (Fig. 2) as well as accurate fingerstick blood glucose measurements for 6 to 10 times per day.The transition to oral glibenclamide was achieved with uneventful glycemic control.CGM showed that the blood glucose was maintained at a stable level.Time in range (TIR) was 95%,and the patient had not complained of any adverse effects.Her HbA1c reduced to 6.5% after three months after administration of glibenclamide.Because the proband’s sibling carried the same variant,the sibling also changed to glibenclamide with a dosage of 0.625 mg/dose,twice a day.The sibling was successfully weaned offinsulin.The father’s blood glucose was monitored and was controlled well without pharmacologic treatment.
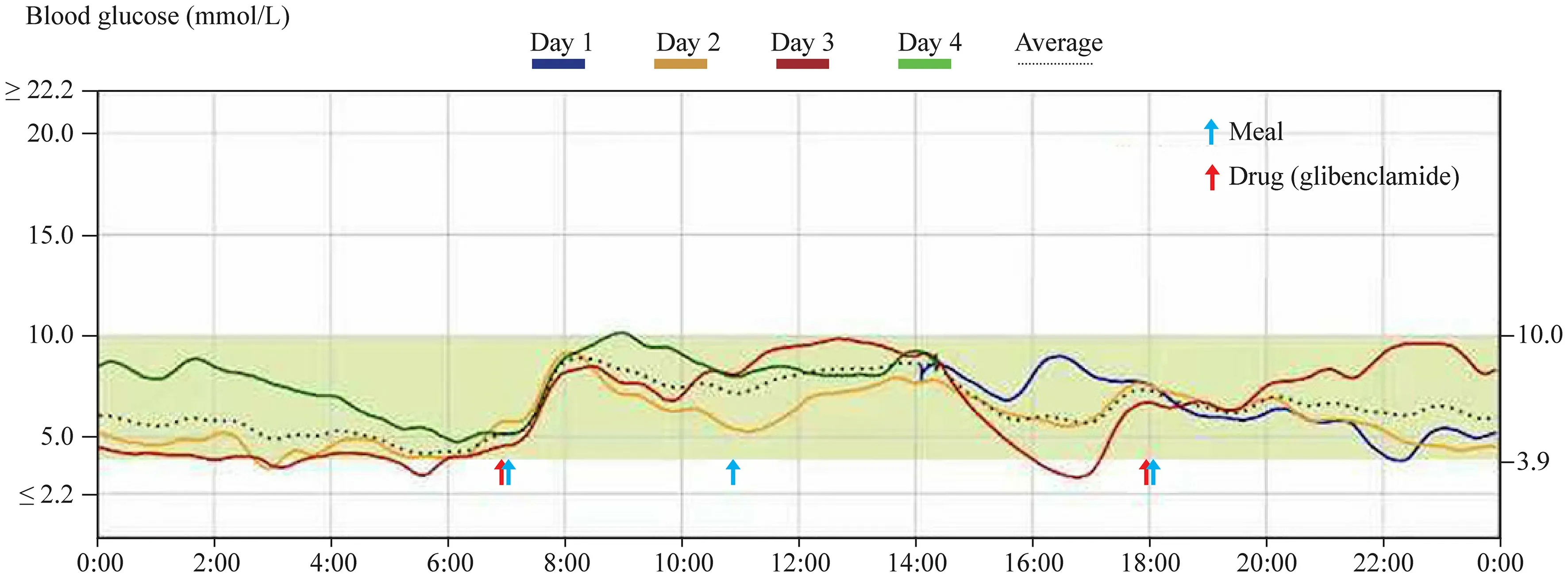
Fig.2 Continuous glucose monitoring of the patient on the first 72 hours after oral administration of glibenclamide
HNF4Ais a transcription factor primarily expressed in the liver but also in the pancreas and kidney.HNF4Aregulates the transcription of genes involved in glucose transport and metabolism.Variations ofHNF4Amutations have caused a wide spectrum of phenotypes.About 50% of theHNF4Amutation carriers are macrosomic at birth,and 15% have diazoxide responsive neonatal hyperinsulinemic hypoglycemia [3].Hyperinsulinism associated withHNF4AMODY generally remits during infancy,followed by a gradual decrease in endogenous insulin production and the emergence of diabetes in adolescence.Recent data showed that predisposition to hypoglycemia may persist into early adulthood [4].Moreover,HNF4Ahas been associated with triglyceride metabolism,and mutation carriers may exhibit reduced levels of apolipoproteins[5].
HNF4A-MODY can initially be treated with diet although the patients will have remarkable postprandial hyperglycemia with high carbohydrate food.Most patients need pharmacological treatment as they show progressive deterioration in glycemic control.They are extremely sensitive to sulfonylureas,which usually allow a better glycemic control than that achieved with insulin,especially in children and young adults [3].Glucagon-like peptide-1 (GLP-1)receptor agonists and dipeptidyl-peptidase-4 (DPP-4) inhibitors are alternative therapeutic options.However,similar to T2DM,HNF4A-MODY is a progressive form of diabetes,and insulin secretion reduces over time.Treatment with insulin may be needed in 30–40% of affected individuals.The frequency of microvascular complications (retinopathy,nephropathy,neuropathy) is similar to that of patients with T1DM and T2DM.Therefore,it is necessary to carry out intensive surveillance of the blood glucose and chronic complications.
The special features of this family are as follows:All the proband,her father and her sibling had the same heterozygous mutation ofHNF4Agene;the proband and her sibling had typical diabetes;the father had only hyperglycemia,which was well controlled without pharmacologic therapy.This may be attributed to the incomplete penetrance ofHNF4A.Unfortunately,the proband's father and sibling were not willing to do further tests on glucose metabolism.
Our case reported a novel mutation in theHNF4Agene.It has expanded the mutation spectrum ofHNF4Ain the Chinese population,providing important information for the molecular diagnosis and the screening ofHNF4A-MODY.A precise molecular diagnosis will lead to optimal treatment of the patient and will help the other family members to get early diagnosis and treatment.It greatly improves the patients’ clinical outcome and quality of life.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519-022-00512-w.
AcknowledgementsThe authors acknowledge the financial support from all the funders.The authors also want to thank the patient and their family who consented to participate in the study.
Author contributionsZMQ and DGP conceptualized and designed the clinical study.DYL,CXF,LH,YJN,HK,WW carried out the clinical assessments.ZMQ wrote the manuscript with critical input from FJF.
FundingZMQ is supported by the national multicenter cohort study on registration,precision medicine in pediatric diabetes and on phenotype and genotype in disorders of sex development in Chinese mainland(No.G20A0002).The effect of intestinal flora and its metabolites on the changes of islet function in children with type 1 diabetes mellitus during honeymoon (No.LGF21H070004).
Data availabilityNot applicable for a case report.
Declarations
Ethical approvalThis study was approved by the Medical Ethics Committee of the Children’s Hospital,Zhejiang University School of Medicine(No: 2019-IRB-124),and informed consent was obtained from a parent of the patient.All of the reported investigations were performed in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Conflict of interestThis was an investigator-initiated study.The funders had no role in study design,data collection and analysis,decision to publish,or preparation of this manuscript.No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.Author Jun-Fen Fu is a member of the Editorial Board for World Journal of Pediatrics.The paper was handled by the other Editor and has undergone rigorous peer review process.Author Jun-Fen Fu was not involved in the journal's review of,or decisions related to,this manuscript.
Informed consentConsent for publication of the details of this case and family has been obtained.
 World Journal of Pediatrics2022年11期
World Journal of Pediatrics2022年11期
- World Journal of Pediatrics的其它文章
- Pediatric dysphagia overview: best practice recommendation study by multidisciplinary experts
- Determinants of neonatal jaundice in Ethiopia: a systematic review and meta-analysis
- Comparison between hospital-and community-acquired septic shock in children: a single-center retrospective cohort study
- Pathogenic changes of community-acquired pneumonia in a children’s hospital in Beijing,China before and after COVID-19 onset:a retrospective study
- Effect of early atopic sensitization in children aged 0–2 years on the development of asthma symptoms at 9–11 years of age
- Integration of multiscale entropy and BASED scale of electroencephalography after adrenocorticotropic hormone therapy predict relapse of infantile spasms
