Pediatric dysphagia overview: best practice recommendation study by multidisciplinary experts
Ebru Umay·Sibel Eyigor·Esra Giray·Evrim Karadag Saygi·Bulent Karadag·Nihal Durmus Kocaaslan·Deniz Yuksel·Arzu Meltem Demir ·Engin Tutar·Canan Tikiz·Eda Gurcay·Zeliha Unlu·Pelin Celik·Ece Unlu Akyuz·Guven Mengu·Serkan Bengisu·Sibel Alicura·Necati Unver·Nida Yekteusaklari·Cuma Uz·Merve Cikili Uytun·Fatih Bagcier·Elif Tarihci·Mazlum Serdar Akaltun·Iclal Ayranci Sucakli·Damla Cankurtaran·Zeynep Aykın·Resa Aydın·Fat ma Nazli
Abstract Background Currently,there is no comprehensive and multidisciplinary recommendation study covering all aspects of pediatric dysphagia (PD).This study aimed to generate PD management recommendations with methods that can be used in clinical practice to fill this gap in our country and in the world,from the perspective of experienced multidisciplinary experts.Methods This recommendation paper was generated by a multidisciplinary team,using the seven-step process and a threeround modified Delphi survey via e-mail.First,ten open-ended questions were created,and then detailed recommendations including management,diagnosis,treatment,and follow-up were created with the answers from these questions.Each recommendation item was voted on by the experts as overall consensus (strong recommendation),approaching consensus (weak recommendation) and divergent consensus (not recommended).Results In the 1st Delphi round,a questionnaire of 414 items was prepared based on the experts’ responses to ten open-ended questions.In the 2nd Delphi round,59.2% of these items were accepted as pre-recommendation.In the 3rd Delphi round,62.6% of 246 items were accepted for inclusion in the proposals.The final version recommendations consisted of 154 items.Conclusions This study includes comprehensive and detailed answers for every problem that could be posed in clinical practice for the management of PD,and recommendations are for all pediatric patients with both oropharyngeal and esophageal dysphagia.
Keywords Diagnosis·Dysphagia·Pediatric·Recommendation·Rehabilitation
lntroduction
Swallowing difficulty,known as dysphagia,is a term that encompasses all difficulties that occur in the safe,effective,and adequate delivery of food to the stomach [1,2].Although this term is sufficient to describe the disorder for adults,dysphagia in the pediatric age is much more than that.Swallowing,one of the most basic physiological functions of humans begins in the early gestational period and continues to develop and vary after birth [3].Since swallowing is a variable and continuous process,a wide range of complicated symptoms and signs are seen in pediatric dysphagia (PD) as in adults [1–3].
The prevalence of PD is reported in a wide range,ranging from 0.9% to 94%,depending on the definition of dysphagia,the population studied,and the methods used [1,2,4–8].Although a large portion of this rate is made up of children with disorders,approximately 25% are children with normal development [9–11].This means that PD can occur not only in children with disorders,but also in children with normal development.Thus,PD is a bigger problem than we thought.
The complex nature of PD necessitates algorithms for its management,including identification,diagnosis,treatment and follow-up.There are several guidelines,reviews and meta-analyses for the management of PD in the literature[1,2,10,12–18].However,some of these articles focus on a single phase of swallowing,such as the oropharyngeal phase;some involve a limited part of management such as diagnosis or rehabilitation;and some address a specific etiology such as cerebral palsy (CP).Currently,there is no comprehensive and multidisciplinary recommendation study that covers all aspects of PD.
This study sought recommendations with methods that can be used in clinical practice to fill this gap in our country and in the world,from the perspective of experienced multidisciplinary experts.
Methods
This study was carried out by a multidisciplinary expert group between May 2021 and September 2021 by a threeround modified Delphi survey via e-mail.
Aim and scope of recommendations
Dysphagia is defined in this study as any difficulty in bolus passage from mouth to stomach [1].In other words,oropharyngoesophageal dysphagia was included in the study.The recommendations in this study were planned for all pediatric ages and subgroups according to age-related development intervals that were defined as newborn (1st month of life),infant (ages between 2nd month and 2nd year),young child(preschooler: ages between 3rd and 6th year),older child(school-age: ages between 7th and 12th year) and adolescent (ages between 13th and 17th year) [19].The recommendations,including the subtitles management,diagnosis,rehabilitation,and follow-up,were established.This study does not include eating-feeding disorders without dysphagia,and these recommendations cannot be applied to adults ≥ 18 years of age.
Methodology of recommendations
Formation of multidisciplinary expert team
A multidisciplinary group of experts developed these recommendations.At the beginning,an executive team was formed consisting of four members of the Turkish Society of Physical Medicine and Rehabilitation (PMR)-Dysphagia Working Group who have been involved in the child’s age for at least ten years.Consultant experts were then selected by this executive team.The selection of consultant experts required that they treat pediatric patients (with or without dysphagia) and/or actively follow up or treat these patients for at least five years [20,21].Moreover,experts from almost all regions of the country,such as the north,south,east and west,were invited so that the study was not limited to only one region of the country.Hence,20 physiatrists,25 different pediatric specialists (pediatric neurology,neonatology,nephrology,pulmonology,gastroenterology,psychiatry,and developmental pediatrics),five otolaryngologists,two plastic surgeons,five dentists,five speech-language pathologists(SLP),five physiotherapists and five dietitians were invited to this study via e-mail.
An expert group was formed with 41 of the 72 experts who had received an invitation letter agreeing to share their opinions and knowledge from their own perspective as experienced professionals in pediatrics and/or PD.Although the study began with 41 experts,it was completed with 28 consultant experts [12 physiatrists,eight pediatricians (one neurologist,two pulmonologists,two gastroenterologists,one psychiatrist and two developmental pediatricians)],three otolaryngologists,one plastic surgeon,one dentist,one SLP,one physiotherapist and one dietitian in 27 centers and 17 cities.
Recommendation generation process
The recommendations were created through a seven-step process.In the 1st step,the main bibliographic databases(PubMed,EMBASE,Cochrane Library,etc.) were searched to identify appropriate question patterns by the executive team,which used a number of key words such as “child”,“children”,“pediatric” and “dysphagia”.This team conducted four online interviews,each lasting approximately one hour,and drafted ten open-ended questions that provided unlimited comments [22].
In the 2nd step,this form of 10-question prepared by the executive team was sent via e-mail to 41 members of the consultant expert group who agreed to participate in the study.The aim of this 1st Delphi round was to allow experts to express their opinions and suggestions on pediatric dysphagia management,diagnosis,rehabilitation,and follow up issues without restrictions.The experts were asked to provide their detailed and unrestricted opinions within four weeks.
In the 3rd step,the opinions and comments of 38 experts who filled out the questionnaire were collected and evaluated by the executive team,and a 414-item questionnaire was created through online interviews.In the 4th step,this 414-item questionnaire was sent to the experts as the 2nd Delphi round.Experts were asked to score each item and return the questionnaire within six weeks to determine the strength of the recommendation.
All items were scored on a 10-point scale (0–10),where 0 point represented “I totally disagree”,and 10 points represented “I totally agree”,and the strength of the recommendation was determined for each item and classified as strong recommendation (overall consensus,OC),weak recommendation (approaching consensus,AC),and no recommendation (overall divergence,OD).Although many different methods were used in the literature to evaluate the strength of the recommendations,three measures [percent(%),median value,and interquartile range (IQR)] were used to increase the strength of recommendations for each of the items in this article [23–25].According to this method,OC was determined if the agreement rate for 8–10 points was ≥ 80% and the median value was between 9 and 10,and the IQR was ≤ 2;AC (meaning that there was no OC but meaningful support) was determined if 8–10 points,agreement rate for 8–10 points was 65%-79% and the median value was between 8 and 10,and the IQR ≤ 3;and OD(meaning that there was significant disagreement within the group) was considered if the agreement rate for 8–10 points was < 65% or the median value was < 8 or the IQR was > 3.
In the 5th step,the questionnaire was revised according to the experts’ scores for each item and the items (pre-recommendations) defined as OD were removed.The OC and AC items were revised with comments and/or details,and all suggested explanations were added to both the items and the recommendation list.In the end,246-item recommendations were created.
In the 6th step,the final version of the recommendations was sent to the experts in the 3rd Delphi round and given six weeks to score the recommendations.In the 7th step,the strength of the recommendations was evaluated for the last time by the executive team based on the final scores of the experts and the final version of the recommendations(154 items) was created (Figs. 1,2,3).
Statistical analysis
Descriptive statistics from SPSS version 22.0 (SPSS Inc.,Chicago,IL,USA) software were used for statistical analysis.The strength of agreement was calculated for each item using percentages (%) (responses of 8–10 points),median values and IQR with Kappa method [26,27].
Results
In the 1st Delphi round,a questionnaire of 414 items was prepared in line with the experts’ responses to ten open-ended questions.In the 2nd Delphi round,59.2%of these items (OC and AC) were accepted as pre-recommendation,168 items were removed.In the 3rd Delphi round,62.6% of 246 items were accepted for inclusion in the proposals.The distributions of the strength of the recommendations in the 3rd Delphi round were given in Supplementary Tables 1,2,3,4.
The final version recommendations were 154 items,including 99 strong (OC),39 weak (AC) and 16 not recommended (OD).As management subsection: four items(n=4,100% OC);diagnosis subsection: 69 items (n=39,56.5% OC;n=24,34.8% AC;andn=6,8.7% OD);rehabilitation subsection: 64 items (n=41,64.1% OC;n=14,21.9% AC;andn=9,14% OD);and follow-up subsection:17 items (n=15,88.2% OC;n=1,5.9% AC;andn=1,5.9% OD).
Discussion
This study includes a presentation of the opinions and suggestions of multidisciplinary experts from all over Turkey about PD.The experts’ opinions were analyzed according to the three-round Delphi method to determine the management of dysphagia in pediatric patients of all ages.Finally,the recommendations were prepared in as much detail as possible,shedding light on almost all questions and problems that may be encountered in clinical practice.
General principles of rehabilitation
When a baby is born,it is expected that the baby can breathe independently and swallow safely.In a healthy infant born at term,this is possible with optimal healthy functioning of systems (such as neurological,gastrointestinal,respiratory,cardiovascular,and musculoskeletal systems) and an optimal mother-infant relationship,and it has been rarely reported that chronic dysphagia is observed in healthy infants and children who meet these conditions [12,28,29].However,an infant is not a shrunken adult,and unlike adults,the development of PD is associated with multiple factors or already exists at birth.This is because all body systems,especially the head and neck structures that play a role in swallowing function,are different from adults,especially in newborns,infants,and young children [12].Swallowing changes and develops from birth.
Although all phases of swallowing in a newborn infant are involuntary and reflexive,local (such as angulation of the nasopharynx,downward displacement of the larynx,elongation of the pharynx,and multidirectional tongue movements)and general (such as disappearance of primitive reflexes,improvement of organization and coordination of the cortical,sensory,and motor peripheral nervous system,and postural control) anatomical,physiological and functional changes occur with growth.Eventually,adult swallowing function develops with voluntary control of the oral phase[30,31].As a result of this constantly changing and evolving process,swallowing can be affected in the presence of many conditions that cause growth and developmental disorders.Thus,very different and heterogeneous symptoms and signs arise from the classic dysphagia symptoms and signs seen in adults.[11,32].
Risk factors
With the developments in medicine in recent years,most preterm infants are kept alive and the prevalence of dysphagia is increasing (40%) [33–37].Prematurity has been reported as one of the most important risk factors for developing dysphagia [30,35,36].The risk of dysphagia in preterm infants increases to 95%-100% due to the combination of many factors such as immaturity of neurological,cardiac,pulmonary and gastrointestinal systems,the presence of structural and genetic abnormalities of body systems,birth trauma,the presence of asphyxia and the methods used for asphyxia [28,32,38–44].Additional medical and surgical conditions such as infection,tracheostomy,intubation,and drug effects also contribute to the development of dysphagia.As for children and adolescents,neurological diseases such as CP,cognitive impairment from any cause,neuromuscular disorders,congenital and acquired anatomical and structural changes (such as craniofacial malformation,tracheoesophageal fistula,and tracheostomy),cardiopulmonary and gastrointestinal system diseases [such as gastroesophageal reflux (GER) and eosinophilic esophagitis] are important risk factors for dysphagia [2,7,12,14,17,42,45–47].
In this study,we stated the first recommendation as a strong one: “Dysphagia should be considered in all newborns,infants and children with any risk factors for PD and/or dysphagia symptoms and signs”.In addition to this recommendation,we described the risk factors,symptoms and signs that may cause oropharyngoesophageal dysphagia for easy implementation in practice (Fig. 1).In this study,the risk factors were divided into child-related and mother/caregiver-related risk factors.This is because nutrition is mother/caregiver/family dependent,especially in the newborn and infant period.In fact,all pediatric ages with disability have this dependency [48,49].The caregiver's feeding behavior that is not appropriate for the child's development,wrong beliefs,and poor positioning can cause dysphagia[28,50].In addition,it has been reported in the literature that there is a dynamic process that is closely related between mother and child in terms of eating and feeding.It has been reported that the feeding behavior adopted by the mother may be associated with conditions such as nutritional deficiency,physical,mental,and metabolic diseases [45,50,51].For these reasons,"the mother/caregiver attempting to feed with nutrition and consistency inappropriate for the child's developmental level and having an eating-feeding relationship disorder" were defined as risk factors that may be associated with the mother.In addition,it was predicted that feeding in a poor position and posture may also cause dysphagia,and this was accepted as a weak recommendation by our experts,as they considered it more of a secondary cause.Apart from that,prenatal and natal factors and low education and socioeconomic levels of the mother were rejected as factors that can be associated with dysphagia due to secondary reasons.

Fig.1 Summary of the recommendations I. OG orogastric,NG nasogastric. a Weak recommendation
Symptoms and signs
In most papers in the literature,the diagnosis is based on the presence of symptoms and signs [3,30,32–38,47,52].These symptoms and signs are in a broad spectrum and can complicate the diagnosis.Therefore,dysphagiarelated symptoms and signs were also detailed in our study (Fig. 1).In the newborn and infant period,symptoms such as weak sucking,difficulty sucking,inability to grasp the breast,sucking-swallowing synchronization disorder,restlessness,crying,sweating,body contraction,arch form and apnea attack during feeding;in all pediatric age,accumulation of food in the mouth,choking,fatigue,coughing,gagging and vomiting during feeding,spilling of food from the mouth,drooling unrelated to the teeth,difficulty chewing and inability to chew,wheezing and wet voice and hoarseness during/after swallowing,difficulty swallowing,multiple swallowing and tilting of the head,painful swallowing,feeling stuck during/after feeding,persistent chronic cough and vomiting,GER symptoms,history of recurrent pneumonia,failure to gain weight,failure to grow,growth and development arrest in the last 2–3 months,prolonged meals (over 30 minutes) were considered strong recommendations.
This study also voted to suggest that swallowing function should be considered in all pediatric age regardless of the presence of risk factors and/or symptoms/signs,but this was rejected because the purpose of this study was to propose recommendations for use in clinical practice and avoid unnecessary applications/workload.
Diagnosis
A recommendation list of 69 items was developed for the diagnosis of PD under the subheadings of management,clinical evaluation,and instrumental evaluation.Of these,39 were strong recommendations,24 were weak recommendation,and six were not recommended (see the Supplementary diagnosis for details on diagnosis section).
Rehabilitation
In recent years,PD has been defined as a pediatric feeding disorder associated with age-inappropriate reduced oral intake and medical,nutritional,feeding skill and/or psychosocial dysfunctions [11].According to this definition,medical conditions include risk factors such as neurological,gastrointestinal,and cardiopulmonary disorders,nutritional factors such as malnutrition and dehydration,nutritional skill disorders such as disorders of oral/pharyngeal sensorimotor functions,and learned aversion,requiring the use of alternative feeding methods with texture and postural modification,psychosocial dysfunction including child,caregiver and feeding environment such as stress,disruptive behavior and use of compensatory strategies by the caregiver can cause dysphagia in children [2,11,29,32].Two or more of these factors often coexist in the pediatric age group.Therefore,as with diagnosis,treatment is complex,multifactorial and multidisciplinary [11].Accordingly,the goal should be to correct the existing dysfunction(s) from a multidisciplinary perspective.
Rehabilitation management principles
In this study,13 strong recommendations were made as rehabilitation management principles.First,the goal of rehabilitation and the person(s) who should serve this goal were defined and these suggestions received the highest (100%)score from all experts.These recommendations were: “the aim of PD rehabilitation should be to ensure adequate nutrition and fluid intake in the safest and least restrictive way,to prevent the development of dysphagia complications,to prevent pulmonary complications and aspiration,to make feeding skills as functional as possible and to support growth”,“dysphagia treatment of infants/children should be managed by a multidisciplinary team (multidisciplinary team identified at diagnosis).Active participation of the family/caregiver should be ensured in this team”.
Also added to these recommendations were “treatment should be individual,etiology and pathology specific”.Because ensuring the sustainability of dysphagia treatment is possible by determining the cause of dysphagia and its treatment.Therefore,the strong recommendations “in patients with PD,determining the underlying cause and treating the cause is the first-line treatment method.This treatment can be medical (such as drugs for gastroesophageal reflux and eosinophilic esophagitis),surgical methods (structural and anatomical disorders,such as tracheoesophageal fistula,stenosis,tracheal web),an apparatus (such as orodental apparatus,ISMAR,Castillo morales) or botulinum injection (such as sialorrhea) and can be the first-line method” were made.
The difference between this study and other similar studies is that patients who do not have dysphagia but have more than one risk factor should be included in a rehabilitation program that includes compensatory methods such as caregiver training,positioning and environmental modification.Because in the presence of a neurological developmental disorder in an infant or child,dysphagia may go undetected for the time being,but it is not impossible to think that dysphagia may develop in the future with complications and malformations,such as cognitive dysfunction or postural impairment that may develop as a result of the current disease [53].
Rehabilitation modalities
While the evidence for dysphagia rehabilitation in adults in the literature is more adequate and clearer,there is not enough evidence and a comprehensive rehabilitation algorithm in PD.In this study,with the clinical experience of our experts,they evaluated in detail almost all modalities that have been and can be applied in dysphagia rehabilitation,and created a rehabilitation algorithm.
This study identified 51 recommendations for rehabilitation modalities for both oropharyngeal dysphagia (OPD)and esophageal dysphagia (ED).The modalities defined as OC were first-line,and the modalities defined as AC were included in the treatment algorithm as second-line (Fig. 2)(see the Supplementary rehabilitation modalities for details on rehabilitation modalities subsection).
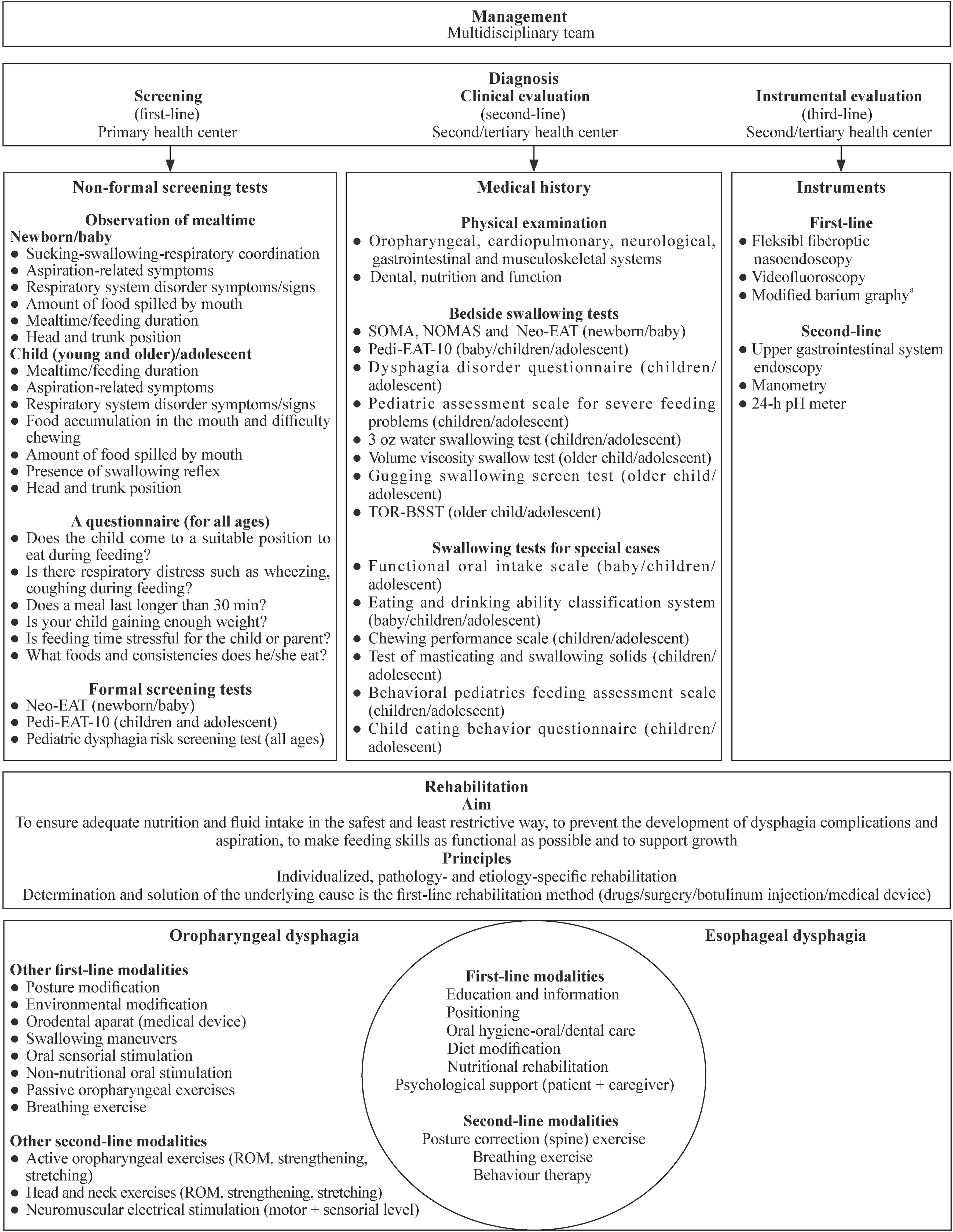
Fig.2 Summary of the recommendations II. Neo-EAT neonatal eating assessment tool,Pedi-EAT-10 pediatric eating assessment tool-10,SOMA schedule for oral motor rating scale,NOMAS neonatal oral motor rating scale,TOR-BSST Toronto bedside swallowing screening test,ROM range of movement. a Weak recommendation
Follow-up
In this study,17 follow-up recommendations were created,of which 15 were OC (strong recommendation),one AC(weak recommendation),and one OD (no recommendation).Little is known in the literature about the follow-up management of patients with PD,and this management is often tailored to the case,etiology,and treatment [13,17,32,54].Although a personalized follow-up interval was recommended in our study,patients were defined as those at "low and high risk of complications" in order to facilitate application in practice (Fig. 3),and accordingly,a recommendation was made to perform applications at wide(3 months–9 months) or frequent intervals (1 per month).
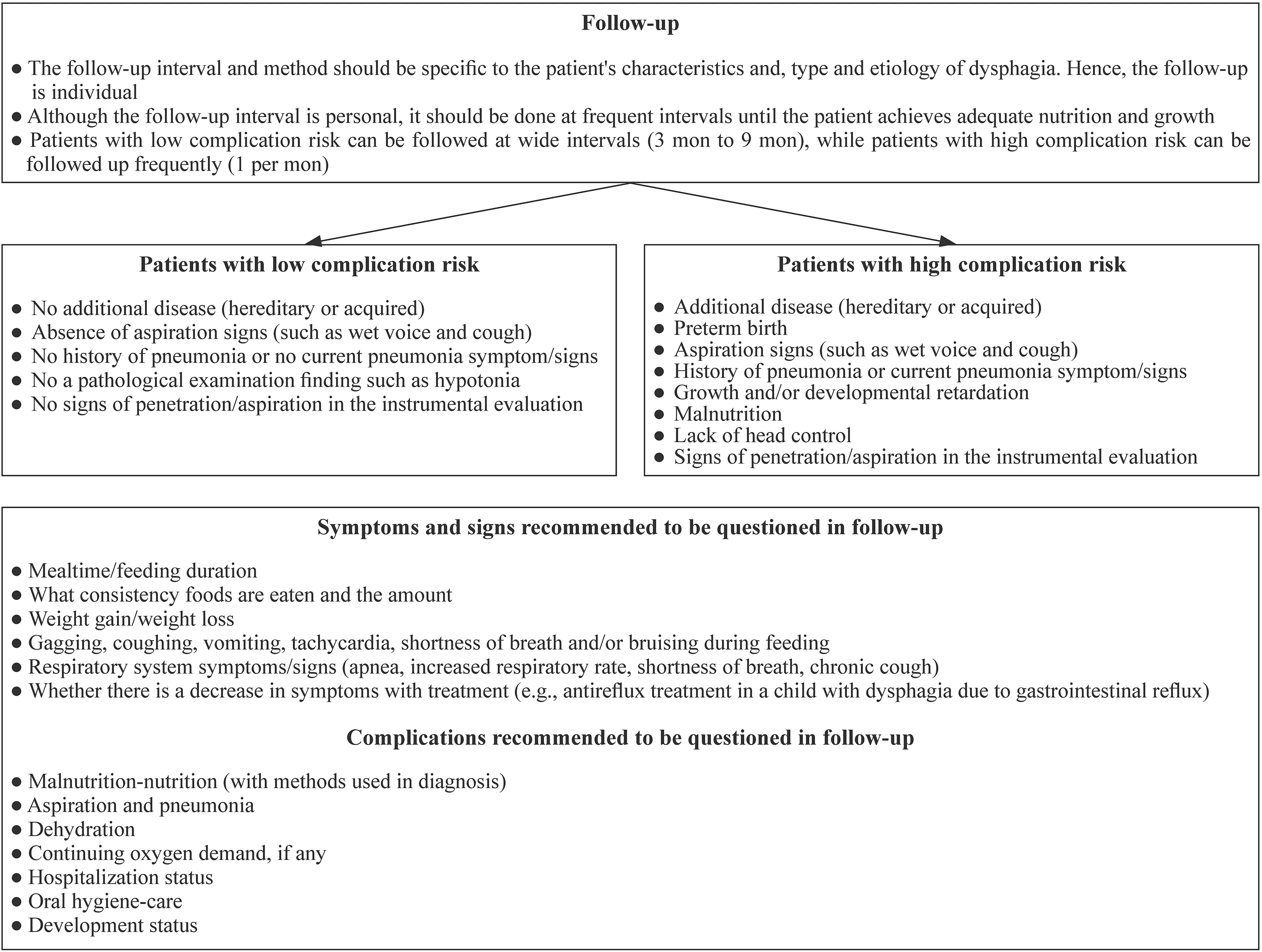
Fig.3 Summary of the recommendations III
Patients at low risk of complication should be included:“no additional disease (hereditary or acquired),absence of aspiration signs (such as wet voice with feeding,voice change,and cough),no history of pneumonia (at least three times a year) or no current pneumonia symptom/signs,and no pathological examination finding such as hypotonia”.If an instrumental evaluation was performed,it was defined as “no signs of penetration/aspiration in the instrumental evaluation” in addition to the above parameters.The high complication risk patient was defined as the presence of at least one of the conditions: “existence of additional disease(hereditary or acquired),history of pneumonia or current pneumonia signs,preterm birth,no head control,presence of aspiration signs,growth and/or developmental retardation,malnutrition,and severe penetration-aspiration signs in instrumental evaluation".In addition,what should be included in a follow-up form in this study were defined as “symptoms and signs of dysphagia,possible complications,continuity of rehabilitation methods applied,and nutritional assessment” (Fig. 3,Supplementary Table 4).These query titles were created for ease of use in practice.Depending on the characteristics of the center to be implemented,more comprehensive forms containing these titles can be created.
In conclusion,the management of PD is still a topic in need of improvement in evidence-based medicine,unlike dysphagia in adults,which has made significant strides and is supported by evidence.This study attempted to create comprehensive and detailed answers for every problem that might be posed in clinical practice for the management of PD,and created recommendations for all pediatric age groups,both for OPD and ED.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519-022-00584-8.
Author contributionsUE,ES,GE,and SEK contributed to conceptualization,methodology,and software.UE,ES,GE,SEK,KB,KND,YD,DAM,TE,TC,GE,UZ,CP,AEU,MG,BS,AS,UN,YN,UC,UMC,BF,TE,AMS,SIA,CD,AZ,AR,and NF contributed to data curation and original draft preparation.ES contributed to supervision.UE contributed to visualization,investigation,software,validation,reviewing and editing.All the authors approved the final version of the manuscript.
FundingThere is no funding source.
Data availabilityThe datasets are available from the corresponding author on reasonable request.
Declarations
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (No.2021/110-08) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interestNo financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article and contributors' statement.The authors have no conflict of interest to declare.
 World Journal of Pediatrics2022年11期
World Journal of Pediatrics2022年11期
- World Journal of Pediatrics的其它文章
- Instructions for Authors
- Incident respiratory disease among youths using combustible tobacco,electronic nicotine products,or both: a longitudinal analysis
- Third-line therapies in patients with Kawasaki disease refractory to first-and second-line intravenous immunoglobulin therapy
- A novel HNF4A mutation identified in a child with maturity onset diabetes of the young
- Neutropenia: diagnosis and management
- Integration of multiscale entropy and BASED scale of electroencephalography after adrenocorticotropic hormone therapy predict relapse of infantile spasms
