Neutropenia: diagnosis and management
Jing Zhang·Xiao-Yan Wu·Run-Ming Jin
Neutropenia is defined as an absolute neutrophil count(ANC) of less than 1500/μL.According to the number of circulating neutrophils,neutropenia is divided into mild (1000/μL ≤ ANC < 1500/μL),moderate (500/μL ≤ ANC < 1000/μL),and severe (ANC < 500/μL) [1].The etiology of neutropenia is shown in Table 1.The most common causes of neutropenia are pathogen infections.Other causes include immune disorders,malignant diseases and other hematolohic diseases,malnutrition,and the use of drugs.Congenital neutropenia is relatively rare,with a prevalence of 3–8.5 cases per million [2].Excluding these causes,neutropenia lasting for more than three months is known as chronic idiopathic neutropenia (CIN) [3].
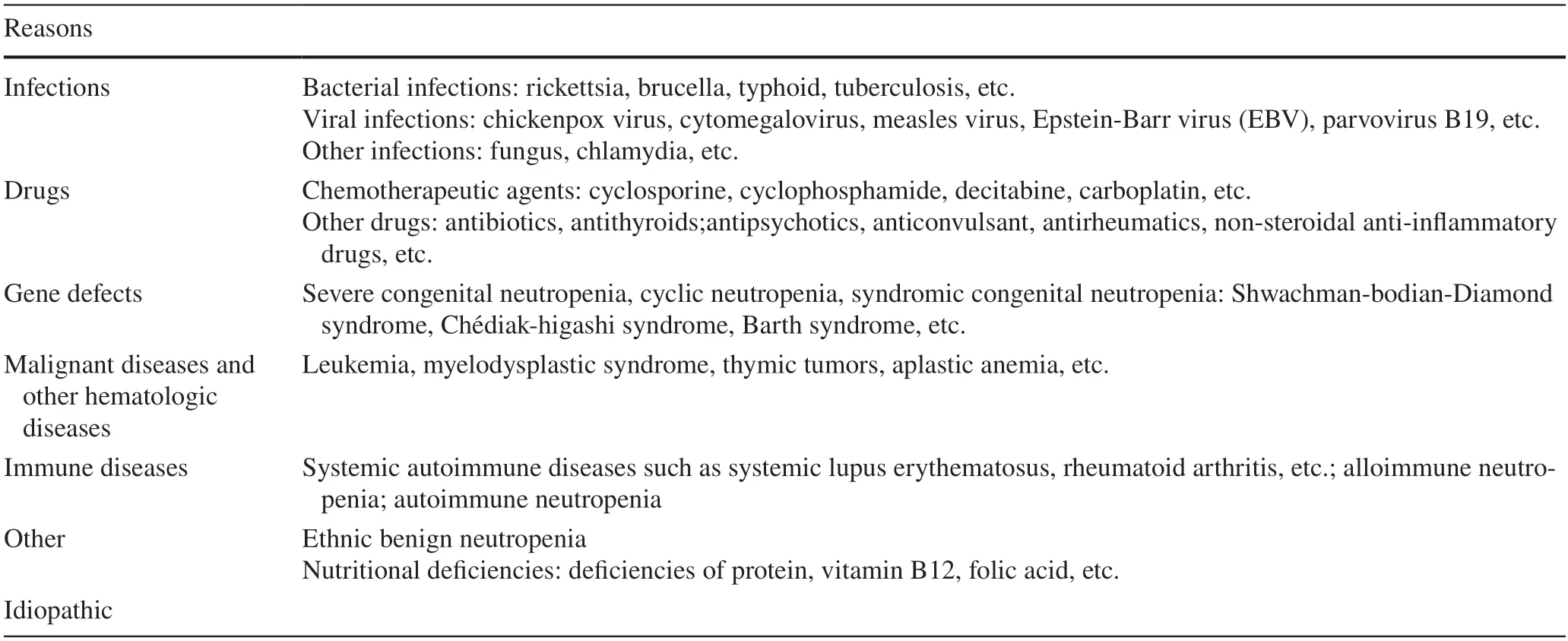
Table 1 Etiology of neutropenia
When neutropenia is monitored in children,the patient's medical history needs to be considered initially.Family history is of great value for the diagnosis of the same or similar diseases in the same family,and the history of infection and medication can help to determine the cause of neutropenia.Degree,duration,and accompanying symptoms of neutropenia provide clues to diagnosis.Pathogen infection and drug use often lead to transient neutropenia,and when neutropenia is accompanied by other cytopenias and characteristic clinical manifestations,the etiology may be related to genetic defects or hematological malignancies.
After a detailed clinical history and thorough physical examination,the etiology of neutropenia can be confirmed by appropriate clinical laboratory tests.Initial laboratory tests should include complete blood count (CBC),reticulocyte count,and peripheral blood smear.Repeated CBC more than three times helps to determine whether the neutropenia is transient.Pathogen infection testing,nutrient testing(B12,folic acid,or copper),and autoantibody testing can be considered.Bone marrow testing is selected depending on the severity of neutropenia or if multiple blood cell lines are abnormal.Genetic testing is mandatory when children are suspected of having congenital neutropenia.In patients with mild neutropenia and exclusion of other hematologic problems and abnormalities,regular CBC and differential white blood cell counts are sufficient.
Neutropenia due to pathogen infection or drug use is usually transient.Pathogen infection can diminish neutrophil production and can accelerate neutrophil destruction by directly suppressing bone marrow or by increasing the production of anti-neutrophil antibodies.Almost all viruses cause a transient reduction in neutrophils,whereas some viruses,such as Epstein-Barr virus (EBV) and parvovirus B19 infection,cause secondary myelosuppression,resulting in persistent ANC suppression [4,5].Various bacterial,fungal,and chlamydial infections can also lead to decreased production or increased destruction of neutrophils.Chemotherapeutic medicines,nonsteroidal anti-inflammatory drugs(NSAIDs),and anti-thyroid medications can all cause direct suppression of myeloid cells in a dose-dependent manner [6].In addition,there are drug-related activation metabolites that mediate the immune response,such as antibiotics,hypoglycemic agents,and antihistamines [7].Some medications,such as butazone and chlorpropamide,enter the body as semiantigens that attach to leukocyte proteins to generate complete antigens,inducing the body to produce auto-antibodies and destroying neutrophils [8].Furthermore,specific genetic susceptibility is also involved in the development of the disease,with some human leukocyte antigen (HLA) types,such as theHLA-B38gene,which increase the risk of developing clozapine-induced neutropenia [9].Antibiotics and antiviral drugs are usually considered cautiously because bacterial and viral infections are also common causes of neutropenia.Nonchemotherapy drug-induced neutropenia can be mild,well-tolerated neutropenia (ANC > 500/μL) or life-threatening granulocytopenia (ANC < 200/μL) [10].Neutropenia can occur at any point during drug use,and ANC monitoring should be taken into account when using drugs that are likely to cause neutropenia.Neutropenia from both causes usually recovers gradually because the cause disappears.Non-severe neutropenia generally does not require specific treatment.
Absolute neutrophil values consistently below 1500/μL are seen in certain races,including Africans,Yemenite Jews,Ethiopian Jews,Arabs,Caribbeans,and people of West Indian descent,and are referred to as ethnic benign neutropenia (EBN) [11].EBN has been shown to be associated with a single nucleotide polymorphism (SNP) of theDARC/ACKR1gene on chromosome 1,additional genetic variation inCXCL2on chromosome 4 andCSF3on chromosome 17 [12,13].Most EBN patients have ANC between 1000 and 1500/μL,no increased risk of infection,no secondary neutropenia,no other cytopenias,and no splenomegaly or lymph node enlargement;therefore,no special treatment is required [11].
Neutropenia can occur secondary to malignant diseases and other hematologic diseases,such as thymic tumors,T-cell large granular lymphocytic leukemia,hairy cell leukemia and lymphoma.Neutropenia is commonly occurring in up to 80% of patients with T-cell large granular lymphocytic leukemia;and patients are often accompanied by lymphocytosis and moderate splenomegaly [14].Patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) usually present with neutropenia along with changes in other blood cell count classifications.
Severe malnutrition can affect bone marrow development.Neutropenia caused by deficiency of nutrients,such as vitamin B12,folic acid and copper,is usually complicated by megaloblastic anemia and thrombocytopenia.One study has shown that vitamin D can promote the differentiation of neutrophil progenitor cells by expressing an antimicrobial peptide: pre-LL-37 [15].
Congenital neutropenia caused by inherited genetic mutations is a primary immunodeficiency disorder in which patients not only present with recurrent respiratory,cutaneous and oral mucosal infections,as well as clinical signs of associated syndromes (Table 2),but are also at risk for progression to myelodysplastic syndromes and leukemia.ELANEis a common genetic defect in congenital neutropenia,seen in over half of the severe congenital neutropenia(SCN,ANC < 500/μL) and almost all cyclic neutropenia(CyN).Mutations inELANEresult in the synthesis of nonfunctional and misfolded neutrophil elastase (NE),causing the unfolded protein response (UPR),and sustained endoplasmic reticulum stress ultimately leading to apoptosis [16,17].In addition,genetic defects inGFI1,HAX1,VPS45,JAGN1,WASandCSF3Rcan also lead to severe neutropenia[18].CyN,which also hasELANEmutations,has a different phenotype from SCN,characterized by periodic neutrophil count oscillations with reciprocal monocytosis,and infection occurs during periods of low neutrophil count [19].Studies have suggested that the mild course of CyN is related to the diversity ofELANEmutations and UPR inhibiting factors[19,20].
The family history of inheritance is very important for the diagnosis of congenital neutropenia,especially for patients with the same or similar manifestations in the family.Some gene mutations affect both hematopoietic and non-hematopoietic tissues,and patients may have congenital malformations and abnormal functions of other organs.For example,Chédiak-higashi syndrome (CHS) and Griscelli syndrome type II (GSII) with partial oculocutaneous albinism,Shwachman-Bodian-Diamond syndrome (SBDS) and Pearson marrow-pancreas syndrome (PMPS) with pancreatic insufficiency,and glucose-6-phosphatase catalytic subunit 3 (G6PC3) deficiency and Barth syndrome (BTHS) with cardiac dysfunction [19].Therefore,clinicians need to take a detailed family history in addition to a careful physical examination.Bone marrow testing is necessary for children suspected of severe neutropenia.Bone marrow biopsy and aspiration help to determine if the patient has a defect in bone marrow production and also help to rule out neutropenia due to diseases such as leukemia,MDS or aplastic anemia.With the widespread use of gene sequencing,genetic mutations related to neutrophil production and function have been identified,and some gene defects are accompanied by structural and functional abnormalities in other tissues and organs (Table 2).Genetic diagnosis helps to diagnose the type of congenital neutropenia and to estimate the prognosis of malignant transformation to MDS/AML in patients.
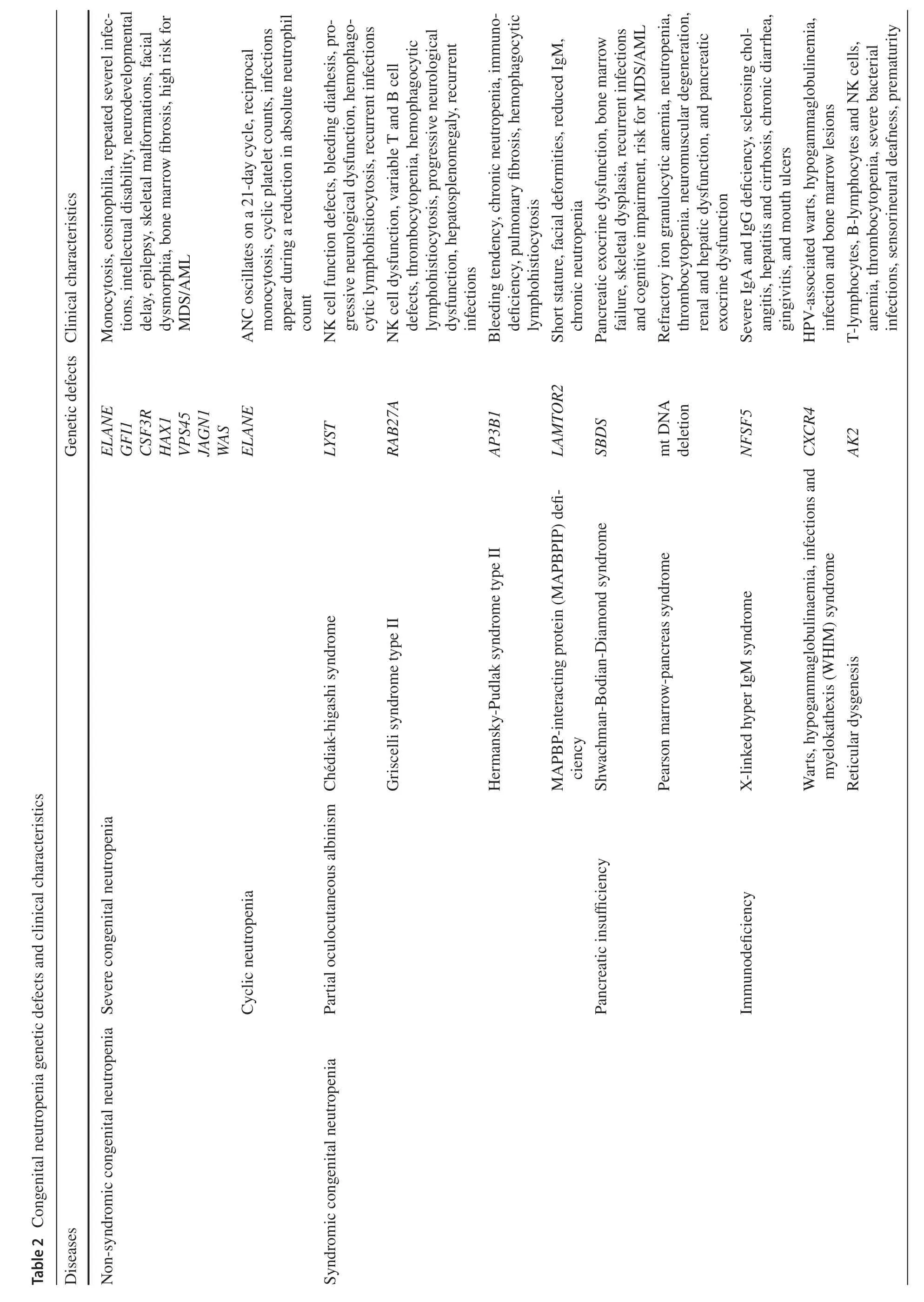
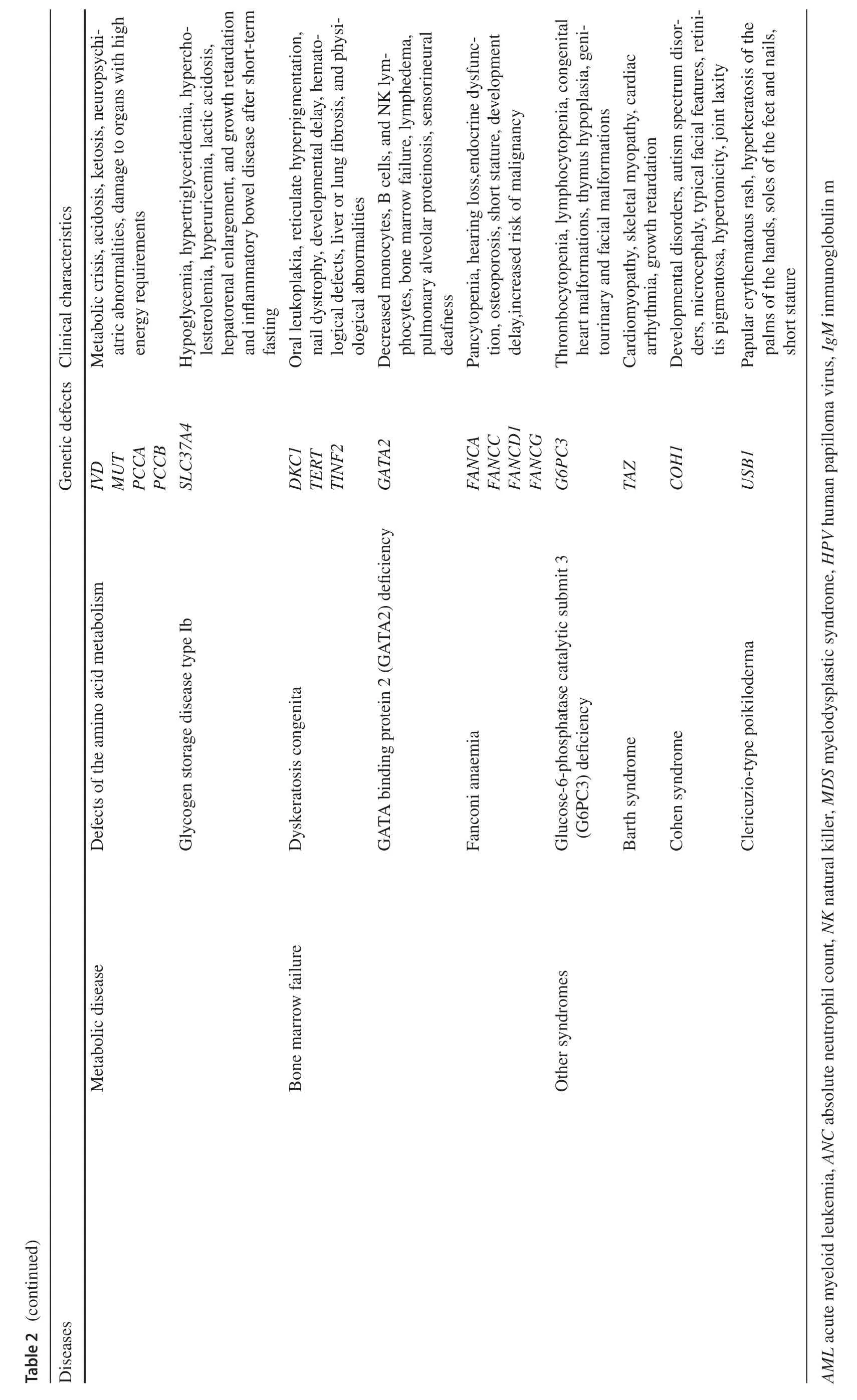
Patients with congenital neutropenia have a cumulative leukemia incidence of more than 20% after 20 years,mainly AML,but also acute lymphoblastic leukemia (ALL) and chronic granulocytic-monocytic leukemia (CMML) [21,22].Granulocyte colony-stimulating factor (G-CSF),an important treatment for SCN,has been shown to increase the risk of MDS/AML.A report of SCN patients treated with long-term G-CSF shows that the annual risk of MDS/AML within 10 years is about 2.3% per year,and the mortality rate of MDS/AML was about 22% after 15 years [23].Heterozygous mutations in the G-CSF receptorCSF3Rare found in approximately 80% of SCN-MDS/AML patients[24].However,theCSF3Rmutation is usually not sufficient to cause malignant transformation.A mutation in the geneRUNX1,which is closely related to cell differentiation,is also required.RUNX1gene mutation is the most common somatic secondary mutation in SCN-MDS/AML and contributes to the development of AML in concert withCSF3R[25].We suggest that children with ANC < 500/μL and repeated infections can take low-dose G-CSF (3 μg/kg/day)every day to avoid side effects such as bone pain caused by high dose,and adjust the dose flexibly according to the target(ANC > 1000/μL).Hematopoietic stem cell transplantation(HSCT) can be considered for children who cannot achieve the target with G-CSF > 50 μg/kg/day,and a report revealed that patients with high-dose G-CSF (> 15 μg/kg/day) may also benefit from it [26].In addition,routine analysis ofCSFR3andRUNX1mutations is necessary in SCN patients with long-term G-CSF use.Congenital neutropenia patients can be screened for somatic mutations or abnormal karyotypes by annual targeted genetic sequencing to estimate the risk of malignant transformation [27].
Immune neutropenia is caused by antibody-mediated destruction and includes both alloimmune neutropenia and autoimmune neutropenia (AIN),which often overlap because both usually involve the same neutrophil antigen(HNA) [28].Alloimmune neutropenia is attributed to the transfer of maternally produced antineutrophil antibodies through the placenta to paternal antigens expressed on the surface of neonatal neutrophils.Patients are usually found shortly after birth and present with delayed cord separation,umbiliculitis,skin infections,fever and (rarely) more serious conditions,such as meningitis or pneumonia [29].Pre-eclampsia or pregnancy-induced hypertension,hemolysis,elevated liver enzymes and low platelet count (HELLP)syndrome,preterm birth;intrauterine growth retardation,premature rupture of membranes and chorioamnionitis increase the risk of neonatal neutropenia,which is associated with a reduced supply of neutrophils from the bone marrow and depletion of neutrophils by infection [29–31].When the mother has CIN or AIN,the infant may present with transient severe neutropenia [32,33].Therefore,several blood counts should be done after birth in newborns with suspicious clinical manifestations and with high-risk factors during pregnancy and delivery.The incidence of autoimmune neutropenia is approximately 1 in 100,000,and the median age at diagnosis is eight months (5–15 months) [34].The antibodies disappear with the natural resolution of the disease within five years in most cases.Idiopathic neutropenia is usually diagnosed when there are no detectable neutrophil antibodies in the patient's body.Anti-neutrophil antibody detection methods include the granulocyte immunofluorescence test (GIFT),the granulocyte agglutination test (GAT)and the monoclonal antibody immobilization of granulocyte antigen (MAIGA) test;however,all these tests have a certain false-negative rate and false-positive rate,and repeated antibody detections may be helpful for diagnosis [35,36].
Diagnosis of CIN and AIN is difficult,but both diseases have a good prognosis.Most patients with AIN and CIN have only mild to moderate neutropenia,manifested by mild upper respiratory tract infections,occasional gingivitis,and very rarely severe or invasive infections [37].Most patients recover within 20 months after diagnosis,with a median time of 13 months (5–26 months),with no significant risk of recurrence.Patients with mild AIN and CIN rarely progress in severity,so bone marrow assessment and gene sequencing are advised.Daily care of the child,such as maintaining excellent oral hygiene,good nutritional status and healthy living patterns,can help to reduce the risk of infections.
Currently,G-CSF is commonly used as a drug for neutropenia.In most neutropenic children,a low dose of G-CSF(< 3 μg/kg/day) is sufficient to raise the ANC to a level that prevents infection.As for whether to use antibacterial agents preventively,doctors can decide based on the neutrophil count and the patient's clinical symptoms.There have been many studies on the efficacy of G-CSF.A follow-up study found that long-term use of G-CSF therapy in patients with cyclic neutropenia reduces the risk of sepsis,and no other blood complications or AML cases have been found [38].However,there are currently no randomized clinical trials or large-scale observational treatment studies on the use of G-CSF in children with severe immune neutropenia.
In conclusion,an in-depth understanding of the causes of neutrophils can help to improve and optimize the current diagnosis and treatment methods,thereby improving the prognosis of patients.Clinicians can help in the diagnosis of neutropenia based on history,physical examination,and laboratory tests.For children with congenital neutropenia,the focus of treatment should be on prevention and treatment of infection,protection of related organ function,regular bone marrow examination,and genetic monitoring to prevent malignant transformation.As more HNA antibodies are discovered and as the technology for neutrophil antibody testing improves,the efficiency of AIN detection will increase.
AcknowledgementsThe authors of this paper would like to thank all the people in the Department of Pediatrics of Wuhan Union Hospital for their continuous support.
Author contributionsJZ contributed to writing the original draft.XW and RJ helped in writing–reviewing and editing.All authors read and approved the final manuscript.
FundingNone.
Data availabilityNot applicable for commentary.
Declarations
Ethical approvalNot needed for a commentary.
Conflict of interestAuthor Run-Ming Jin is the editorial board member of World Journal of Pediatrics.The paper was handled by the other Editor and has undergone rigrous peer review process.Author Run-Ming Jin was not involved in the journal's review of,or decisions related to,this manuscript.
 World Journal of Pediatrics2022年11期
World Journal of Pediatrics2022年11期
- World Journal of Pediatrics的其它文章
- Instructions for Authors
- Incident respiratory disease among youths using combustible tobacco,electronic nicotine products,or both: a longitudinal analysis
- Third-line therapies in patients with Kawasaki disease refractory to first-and second-line intravenous immunoglobulin therapy
- A novel HNF4A mutation identified in a child with maturity onset diabetes of the young
- Integration of multiscale entropy and BASED scale of electroencephalography after adrenocorticotropic hormone therapy predict relapse of infantile spasms
- Effect of early atopic sensitization in children aged 0–2 years on the development of asthma symptoms at 9–11 years of age
