The photocatalytic ·OH production activity of g-C3N4 improved by the introduction of NO
Jun Jing,Ki Qi,Guohui Dong,Mengmeng Wng,Wingkei Ho
a School of Environmental Science and Engineering,Shaanxi University of Science and Technology,Xi’an 710021,China
b Department of Science and Environmental Studies and Centre for Education in Environmental Sustainability,The Education University of Hong Kong,Hong Kong,China
Keywords:Active oxygen species Nitrogen oxide Photocatalysis Organic pollution g-C3N4
ABSTRACT The efficiency of photocatalytic pollutant removal largely depends on the ability of the photocatalytic system to produce hydroxyl radicals (·OH).However,the capability of photocatalyst to produce ·OH is not strong at present.Advancing the capacity of photocatalytic system to produce ·OH has always been a tough problem and challenge in the field of environmental science.In this research,it was found that introducing nitric oxide (NO) into the graphitic carbon nitride (g-C3N4) photocatalytic system could memorably enhance the ability of producing ·OH group.This study provides a new idea for improving the capacity of photocatalytic ·OH production.
Active oxygen species (AOS) are effective green oxygenants for environmental-pollution control.AOS have many types,including superoxide radical (·O2-),hydrogen peroxide (H2O2),and hydroxyl radical (·OH) [1-8].To remove environmental pollution,many methods such as electrochemistry,Fenton reaction,and photocatalysis have been developed to produce·OH.Electrochemistry method requires substantial electric energy,and Fenton reaction requires extra H2O2[9-11].Moreover,these two methods may harm the environment.For example,electrochemistry method may release metal ions and harmful gas,and Fenton reaction may produce iron sludge [12,13].Photocatalytic technology has suffi-cient energy source (sunlight) and is environmentally friendly [14].Meanwhile,photocatalytic technology could produce·OH under mild conditions.Thus,photocatalytic·OH production has the potential for practical applications [15].However,the·OH production efficiencies of photocatalysts remain at a low level because the recombination rate of photogenerated electron and hole is relatively high.Many methods have been developed to solve this problem.For example,the photocatalyst can be modified by various modification methods (e.g.,doping,heterojunction formation,and noble-metal deposition) to suppress the recombination of photogenerated electron and hole,or a sacrificial agent may be added to improve the redox half-reaction [12,16-18].However,photocatalyst modification is a complicated process [19-21],and adding a sacrificial agent may cause environmental pollution.For example,Xiao’s team attempted to improve the photocatalyst with ozone (O3) as a sacrificial agent [5,7,8,15].O3is known to be harmful to human health and the environment because of the lung damage it inflicts.Thus,developing a new method of improving the photocatalytic production of·OH is very important.
Nitrogen oxide (NOx),a major pollutant in the atmosphere,originates from the combustion of fossil fuel in boilers and motor vehicles (exhaust gas) [22].The N atom of NO has an unpaired electron,so NO possesses strong reducing property (Fig.S1 in Supporting information) [23,24].Therefore,we believe that NO can be used as a sacrificial agent for photogenerated hole,meaning that photocatalytic AOS production can be improved by NO bubbling.Meanwhile,NO can be oxidized to NO2by using a photogenerated hole,and then the generated NO2is absorbed by water (became NO3-) [25].In this case,the NO in the exhaust gas can be used and further removed.To verify the above analysis,the visible-light photocatalyst g-C3N4was used to produce·OH through NO bubbling.We found that the introduction of NO could improve the photocatalytic·OH generation activity of g-C3N4.In this process,the structure and activity of g-C3N4are very stable [22,26].The improvement mechanism was studied systematically.The details can be found as below.
We detected the generation of active oxygen species (AOS) with or without NO gas (500 ppb,diluted by air stream) bubbling.Figs.1a and b show the EPR signal of·O2-.No clear·O2-signal was observed in darkness.After the light was turned on,·O2-can be produced in g-C3N4suspension and g-C3N4/NO suspension.However,the bubbling of NO did not significantly affect the intensity of generated·O2-.Figs.1c and d display the generated H2O2under dark and light-irradiation conditions.H2O2can be produced only under light irradiation in the presence of g-C3N4sample.Similarly,with·O2-,NO bubbling did not significantly affect the intensity of generated H2O2.As for·OH,the effect of NO bubbling was highly significant.Fig.1e shows that·OH was undetected in darkness.After the light was turned on,·OH can be produced in g-C3N4suspension and g-C3N4/NO suspension (Fig.1f).Surprisingly,the concentration of generated·OH in the g-C3N4/NO system was nearly twice as high as that in the g-C3N4suspension.Therefore,NO bubbling helped g-C3N4produce more·OH under light irradiation (see Supporting information for details).
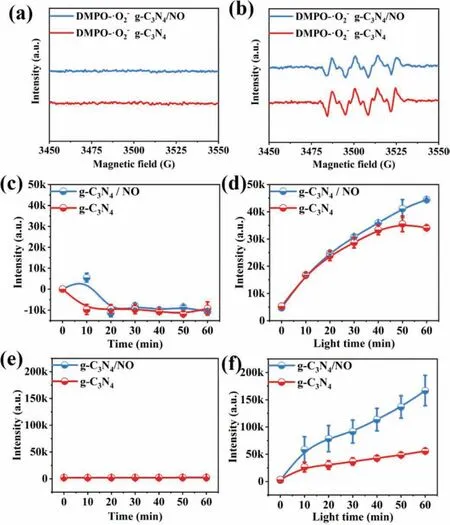
Fig.1.(a) DMPO EPR spin-trapping for ·O2- radicals in dark and (b) under visible light illumination (λ >420 nm).(c) Fluorescence test of H2O2 production in dark and (d) under light irradiation.(e) Fluorescence test of ·OH production in dark and(f) under light.
To understand the mechanism of NO in inducing g-C3N4to produce more·OH under light irradiation,we analyzed the converted products of NO during photocatalysis.In the photocatalytic reaction of NO in the gas-solid phase (Eq.1 and Fig.2a),most reaction products were NO2[3].However,the NO2signal disappeared when the hole scavenger was added,suggesting that NO can be oxidized to NO2by hole.This can be further proved by the hole capture experiment (Fig.S3a in Supporting information).Interestingly,NO2cannot be detected when the photocatalytic reaction proceeded in the liquid-solid phase (Fig.2b).This result suggested that the generated NO2decomposed (Eq.2) or absorbed (Eq.3) in water.If NO2decomposes,the generated oxygen free radicals (·O) would react with water to produce·OH [27].To verify this mechanism,NOxwas bubbled in water (without the presence of photocatalysts)under light irradiation (λ= 420 nm).The results are displayed in Fig.2c.·OH can be detected in the NO2-bubbled water but not in the NO-bubbled one.These results confirmed Eqs.2 and 4.Accordingly,NO2photolysis can contribute to the increase in·OH yield.Fig.2d shows that NO2photolysis (70 ppb) could produce only 3.33% of the·OH increment.Thus,other reasons for the·OH increment besides NO2photolysis must exist.The photolysismechanism diagram of NO2is shown in Fig.2e.Extra NO2would be absorbed by water and became NO3-.Besides,the conversion rate of NO to NO3-is 47%.(Fig.S4 in Supporting information).
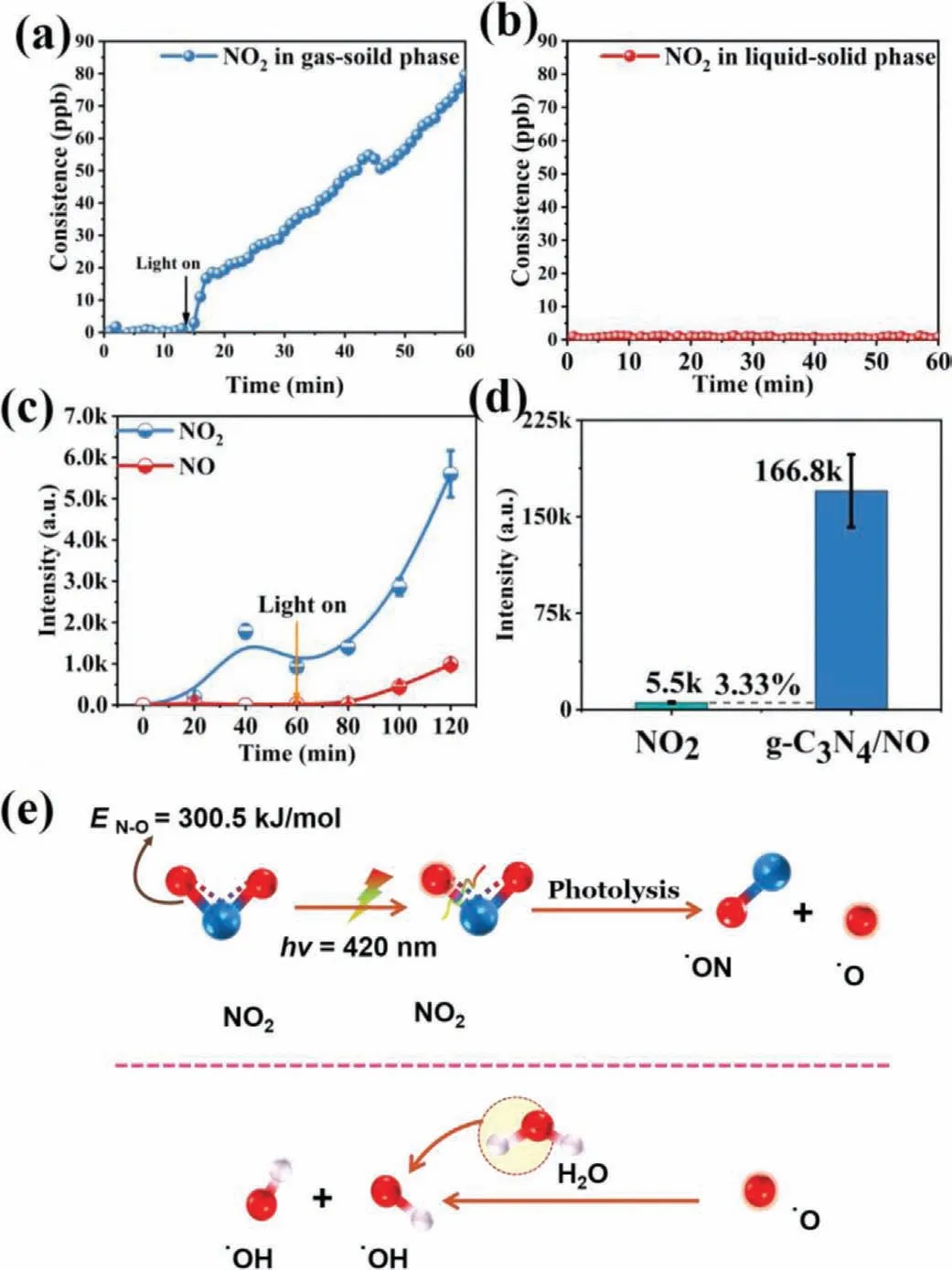
Fig.2.(a) NO2 concentration in the gas-solid phase reaction and (b) NO2 concentration in the liquid-solid phase reaction.(c) The amount of hydroxyl radicals produced over time.(d) Histogram comparison of the ·OH yield of NO2 to the total ·OH yield.(e) Schematic diagram of NO2 photolysis to produce hydroxyl.
Since NO could consume hole and inhibit the recombination of photogenerated carriers.Interestingly,NO bubbling obviously improved the photocurrent signal of g-C3N4under the same condition.And the further confirmation could be found in Fig.S3b(Supporting information).This phenomenon ensured that NO could inhibit the recombination of photogenerated carriers.Consequently,the valence band of g-C3N4can provide more excitation electrons for the higher fluorescent signal (Fig.S3c in Supporting information).For a visual expression,the reaction-mechanism diagram is shown in Fig.S3d (Supporting information).The more consumption of photogenerated hole could be created,the more adsorbed O2molecules could be reduced.
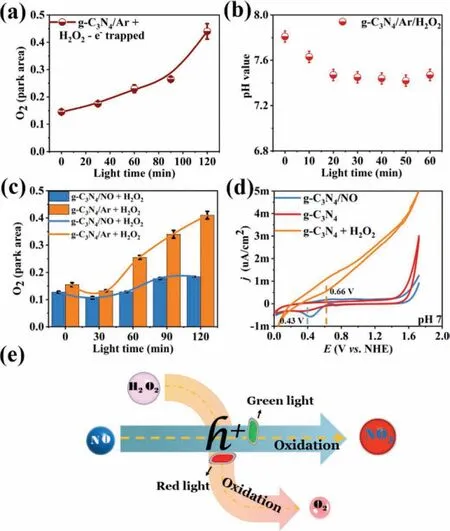
Fig.3.(a) O2 production under argon atmosphere plus hydrogen peroxide AgNO3 as capture electron condition.(b) Change of pH value of hydrogen peroxide added in argon atmosphere.(c) O2 production of CN and H2O2 under NO atmosphere (blue)and argon atmosphere (orange).(d) Cyclic voltammetry curves of the g-C3N4 in NO ambience and pure g-C3N4 and g-C3N4 with H2O2.(e) Schematic diagram of the H2O2 decomposition with the presence of NO.
Based on the redox potential of H2O2/O2(0.659 V) [28]and the valence-band potential of g-C3N4(1.4 eV) [29],H2O2may be oxidized to O2by the photogenerated holes (Eq.5).This speculation can be proven by H2O2catalytic-decomposition experiment.(Figs.3a and b and S2.3 in Supporting information).To investigate the effect of NO on H2O2catalytic decomposition,NO was introduced in the above experiments.Fig.3c shows that the amount of produced O2significantly decreased after NO introduction,indicating that NO can inhibit the oxidation reaction of H2O2caused by photogenerated holes consumed.Meanwhile,the redox potential of NO/NO2(0.428 V) was smaller than that of H2O2/O2(0.659 V),which can be proven by the cyclic voltammetry curve in Fig.3d.In this case,H2O2can be protected by NO from the oxidation of photogenerated holes,just like the drawing in Fig.3e.Therefore,more H2O2can be used to produce·OH (Eq.6).

After·OH generation,it diffused in water.However,NO is insoluble in water,so NO cannot consume the·OH generated in our system.When NO was oxidized to NO2by photogenerated holes,NO2was adsorbed by water and became NO3-and NO.However,NO3-cannot be further oxidized,so it cannot consume the generated·OH.In this case,NO2did not consume the generated·OH in our system.Based on the above experimental results and discussion,although NO was used as the hole sacrificial agent,and it did not consume·OH.For this reason,our system could accumulate more·OH for the degradation of organic pollutants [30](Fig.S5 and S2.4 in Supporting information).Herein,Fig.4 can be well matched with this theme.
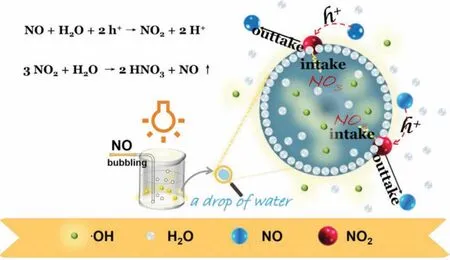
Fig.4.Conversion process of NOx in the photocatalytic reaction: Schematic diagram of the dissolution of NOx in reaction system.
Stability is an important evaluation indicator for the application value of one technology or sample [31].Accordingly,cycling experiments were conducted to investigate the stabilities of this technology and g-C3N4sample [32,33].Fig.S6a (Supporting information) shows that the·OH yield did not decrease after five cycles of use.Meanwhile,the degradation rate of tetracycline performed using our technology was very stable throughout the five cycles of repeat experiments (Fig.S6b in Supporting information).These results suggested that our technology was stable and recyclable.The stability can be further confirmed by the XRD,FT-IR,UV-DRS,and SEM characterizations.A detailed analysis can be found in Figs.S6c,S6d,S7 and S8 (Supporting information).
In conclusion,the introducing NO could significantly enhance the photocatalytic·OH production ability of g-C3N4.After a series of mechanism studies,we found that there were four reasons for this improvement: (1) The·O produced by the NO2photolysis can react with H2O to form·OH;(2) NO can be used as a hole-capture agent,which makes g-C3N4generate more photo-generated electrons to reduce O2;(3) H2O2can be protected by NO from being oxidized by holes;(4)·OH cannot be consumed by NO or NO2.Since NO could enhance the·OH production,NO was introduced in the photocatalytic tetracycline removal process.It was found that NO could enhance the mineralization rate of tetracycline from 59%to 86%.Moreover,the presence of NO in the photocatalytic process does not affect the stability of g-C3N4.
Declaration of competing interest
The authors report no declaration of competing interest.
Acknowledgments
Financial support by the National Nature Science Foundation of China (Nos.21876104 and 21603271) is gratefully appreciated.The work described in this paper was also partially supported by the General Research Fund (Nos.18300920 and 18301117) of Research Grants Council,the Department Collaborative Fund (No.04490),Dean’s Research Fund (No.FLASS/DRF 04554) of the Faculty of Liberal Arts and Social Sciences,The Education University of Hong Kong,Hong Kong Special Administrative Region,China.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.12.071.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
