Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
Mengchi Sun,Weiyue Bn,Ho Ling,Xing Yu,Zhonggui He,Qikun Jing,*,Jin Sun,*
a Wuya College of Innovation,Shenyang Pharmaceutical University,Shenyang 110016,China
b Huzhou Central Hospital,Affiliated Huzhou Hospital,Zhejiang University School of Medicine,Affiliated Central Hospital Huzhou University,Huzhou 110016,China
Keywords:Inflammatory bowel disease (IBD)Nanotechnology-based nanomedicines(NMs)Prodrug strategy Pathophysiological microenvirnoment Passive targeted and active targeted NMs
ABSTRACT Inflammatory bowel disease (IBD) is a chronic and recurrent disease of the gastrointestinal tract,mainly including Crohn’s disease (CD) and ulcerative colitis (UC).However,current approaches against IBD do not precisely deliver drugs to the inflammatory site,which leads to life-long medication and serious side effects that can adversely impact patients’adherence.It is necessary to construct optimal drug delivery systems (DDSs) that can target drugs to the region of inflammation,thereby improve therapeutic effi-cacy and reduce side effects.With the burgeoning development of nanotechnology-based nanomedicines(NMs) and prodrug strategy,remarkable progresses in the treatment of IBD have been made in recent years.Herein,the latest advances are outlined at the intersection of IBD treatment and nanotherapeutics as well as prodrug therapy.First,the pathophysiological microenvironment of inflammatory sites of IBD is introduced in order to rationally design potential NMs and prodrugs.Second,the necessity of NMs for the IBD therapy is elaborated,and the representative nanotherapeutics via passive targeted and active targeted NMs developed to treat the IBD are overviewed.Furthermore,the emerging prodrug-based therapeutics are summarized,including 5-aminosalicylic acid-,amino acid-,and carbohydrate-conjugated prodrugs.Finally,the design considerations and perspectives of these NMs and prodrugs-driven IBD therapeutics in the clinical translation are spotlighted.
1.Introduction
Inflammatory bowel disease (IBD),mainly includes Crohn’s disease (CD) and ulcerative colitis (UC),affects a great number of patients worldwide.It is a multifactorial,chronic recurrent,and heterogeneous disease [1,2].Its pathogenesis has not been completely understood yet.It occurs intensively in Europe and is uncommon in Asia,suggesting that it is directly connected with industrialization and changes in lifestyle [3].For example,the insufficient intake of vitamin A and an increase in the intake of fat,sugar,and food additives might be closely associated with the development of IBD [4].As a hot topic for discussion,the symptoms of IBD are explored by biologists and pharmacists,mainly including the damage of mucus layer in the gastrointestinal tract,functional deficiency of intestinal barrier,and dysbiosis of gut microbiota [5].The current therapies can maintain remission with aminosalicylates (mild to moderate IBD),corticosteroids and biological drugs (moderate to severe IBD).However,these convention drugs in clinical practice generally have the following problems,including life-long medication,poor efficacy,high recurrence rate and serious side effects,which is attributed to the loss of targeting at the site of inflammation in the colon.
Inflammation-targeted drug delivery in the colon gains much attention all the time to deliver therapeutics (drugs,proteins,peptides) locally in treatment of gastrointestinal tract (GIT) diseases[6,7].The physiological microenvironment of GIT allows the design of miscellaneous dosage to control oral colon-specific drug release,such as pH of the gastric fluids,gut microbiota,intestinal epithelial permeability,intestinal bacterial enzymes,inflammatory factors,difference in absorption and release kinetics.According to pathophysiological characteristics,the rationally designed nanotechnology-based nanomedicines (NMs) hold great potential to open a new avenue for IBD therapy.The intestinal tissue permeability is increased in the endothelium and epithelium of inflamed colon,where the tight junctions (TJs) between cells are enlarged due to the loss of cellular integrity upon the activation of proinflammatory cytokines [8].Thus,NMs with small sizes can passively accumulate at the sites of inflammation due to the “leaky”intestine (the increased intestinal permeability and enlarged tight junctions),which is likely accompanied by enhancing NMs uptake by the infiltrating immune cells and controlling drug release to sustain drug concentration at the site of inflammation [9,10].Moreover,in the treatment of colitis,some specific ligands or antibodies could recognize and bind to certain overexpressed receptors,adhesion molecules,proteins and other features on the surface of colonic epithelial cells and immune cells in the inflammation.The ligands/antibodies-modified NMs would actively target inflammatory region,improve the therapeutic efficacy,and reduce the adverse reactions to normal tissues [11].NMs can also improve the physicochemical properties of drugs themselves,including solubility,stability and immunogenicity.In addition,the prodrugs therapy has been recognized as another effective strategy on colon-targeted drug delivery against IBD in the last decades.The basic mechanism of prodrug strategy is that a series of covalentconjugated compounds undergo enzymatic degradation in the intestine and are largely metabolized by colonic bacteria,which further triggers the release of active ingredients at colon regions[12,13].
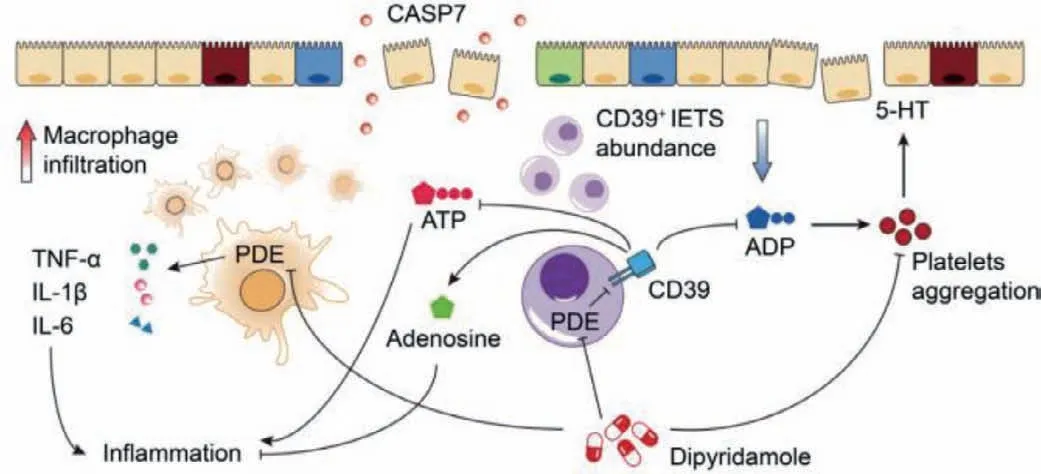
Fig.1.Common pathogenics and therapeutic pathways of IBD.Reproduced with permission [14].Copyright 2019,Elsevier Ltd.
In the past years,many efforts have been made to develop numerous NMs and prodrugs for the IBD treatment.Given the significant progress,we provide a review to timely summarize the latest advances in these fields and shed light on future directions for IBD treatment.First,this review provides a comprehensive overview of the pathological features of IBD,including pH,transit time,intestinal permeability,intestinal bacteria,and inflammatory factors.Second,we outline recent trends in the applications of NMs and prodrugs in IBD therapy based on the emphasis on emergent pathological characteristics.Moreover,the research directions and prospects of IBD treatment are summarized,which provides the ideas for the clinical transformation of inflammation-targeted drug delivery systems against IBD.
2.The pathological characteristics of IBD
The pathological process of IBD has changed the physiological function of colon.It affects the colon-related physiological characteristics of IBD patients,including colon pH,transit time,intestinal pressure and permeability,gut microbiota and inflammatory factors [14,15].These characteristics involved in the IBD pathogenesis are represented as below (Figs.1 and 2).
2.1.Colon pH
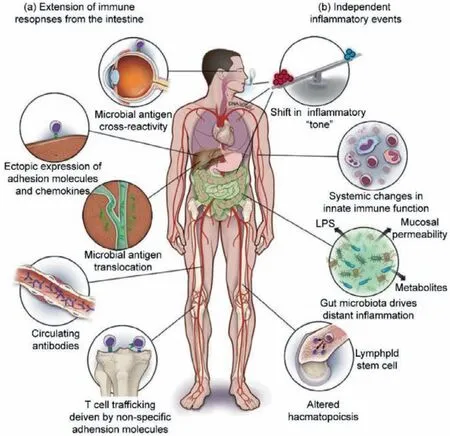
Fig.2.Other potential mechanisms of IBD.Reproduced with permission [15].Copyright 2018,Oxford University Press.
Under healthy conditions,the pH values of the small intestine and colon are within the range of 5.5-7.5 [16].However,the intestinal pH value is affected by many factors,including the production of mucosal bicarbonate and lactate,bacterial fermentation of carbohydrates,absorption of short-chain fatty acids by mucosa,and intestinal transport.The occurrence and development of IBD affect all these factors and cause abnormal colonic pH value [17].A couple of studies have reported that the colon pH in IBD patients with mucosal inflammation and epithelial wounds was significantly acidic (pH 2.3-5.5) [18,19].However,some studies have reported differences in the average colonic pH value of CD and UC patients (~5.3 and ~3.5,respectively) [17,18].The changes in colonic pH of IBD patients mainly depend on the disease status[17,20].
2.2.Transit time
Under normal circumstances,the transit time of the gastrointestinal tract depends upon the diet,activity and disease status.Among them,the discrepancy of transit time in the healthy people and IBD patients lies on many factors,including intestinal fluid peristalsis,the composition of gut microbiota,and intestinal length[21].Normal gastric emptying occurs within 2 h and arrives at the colon in 5 h [22].In IBD patients,both the UC and CD subtypes have been reported to have the phenomenon of delayed transit times,except for some patients with malnutrition,having faster transit times [23].Since diarrhea is common in IBD,which generally decreases the colonic transit time due to the increased lumen fluid content.Meanwhile,the decrease in the diversity of intestinal microbiota might also influence the transit time [24].In addition,many CD patients undergo blind ileum resection,which results in a shorter transit time through the junction of the ileum,thereby reducing the transit time through the small intestine.
2.3.Intestinal pressure and permeability
In IBD patients,the reduction in the contractile force of the colon reduces the colonic pressure [25].In the normal gastrointestinal tract,the epithelial cells are carefully connected by tight junctions (TJs) where they can seal the gap between two nearby adjacent epithelial cells and maintain the gastrointestinal epithelium in intact form.In IBD patients,damage of TJs would result in the absorption of antigen through the endocytosis pathway and the subsequent release of pro-inflammatory cytokines (including INFγ,TNF-α),inducing intestinal inflammation.
2.4.Gut microbes
In the gastrointestinal tract,there are more than 500 bacterial species,constituting the inherent microbiota with a certain ecological location,which plays an important role in maintaining the physiological functionsin vivoand digestion of indigestible foods,preventing invasive pathogens [8,26].The aerobic microbiota (bacteria,Bifidobacterium,and true bacteria) in the colon can degrade the polysaccharide/Carbohydr.Polym.,which are left undigested in the stomach and small intestine,into smaller monosaccharides that can be used as energy sources [9].Generally,a decrease in microbial diversity and population stability can be observed in IBD patients [27].It was found that the IBD patients had 25% fewer bacterial genes in their metagenome than that of normal persons [28].The bacteria-derived enzymes in the colon,including various reductases and hydrolases,such asβglucosidase,β-xylosidase,β-arabinosidase,β-galactosidase,nitroreductase,azoreductase,deaminase,and urea hydroxylase,are also important,which can further degrade the undigested polysaccharides and dietary fibers in the small intestine [14].In a nutshell,the pathological changes in the gut microbiota of IBD patients affect the composition and diversity of gut microbiota and also the secretion of these enzymes.
2.5.Key factors in the pathogenesis of IBD
2.5.1.Role of ROS
Accumulating evidences suggest that the high levels of ROS are produced at the site of acute arthritis and the mucosal ROS concentrations are elevated only at the inflammation site,demonstrating the association of ROS with disease progression [29,30,31].The excess of ROS can damage the components of the extracellular matrix and cause tissue damage [32].Activated neutrophils are a known potential source of free radicals in patients with UC,while monocytes/macrophages could produce free radicals in patients with CD.ROS-induced damage causes recruited and resident cells to lose their ability to conduct electrons through the mitochondrial electron transport chain in a controlled manner and the uncoupling of the electron transport chain is accepted by molecular oxygen,resulting in focal deficiency in oxidative stress oxygen and ROS are produced in necrotic tissue.
2.5.2.Role of MAPK activated protein kinase 2 (MK2)
MK2,a pro-inflammatory factor,acts a key role in IBD.A series of experimental results on MK2-deficient mice suggested that compared with the wild type mice,the colonic epithelium injury of MK2-deficient mice was relatively mild [33].After seven days,inflammatory cytokines IL-6 and TNF-αlevels of MK2-deficient mice was lower than that of the wild type mice,and histological score of MK2-deficient mice was distinctly higher than that of the wild type mice.In addition,the positive ROS signals in colon sites of the wild type dextran sulfate sodium (DSS)-induced mice were more obvious than that of MK2-deficient mice.Besides,the activation efficiency of Akt,p38mitogen-activated protein kinase (p38MAPK)and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in MK2-deficient mice would decrease,leading to the reduction of ROS production,thus the reduction of IBD incidence and severity.Recent researches suggested that MK2 could be a therapeutic target for IBD.It had been reported that MK2 inhibitor MMI-0100 can temper the risk of DSS-induced colitis in mice [34].
2.5.3.Role of CD177+neutrophils
Neutrophils are not only indispensable in the pathogenesis of IBD,but also act an active role in the recovery of intestinal structure and the IBD treatment [35].Neutrophils,as one type of transient effector cells,belong to the innate immune system.They are the most abundant type of white blood cell population in the blood,which could accumulate quickly at the site of inflammation.The aggregation of neutrophils in the intestine is one of the IBD symptoms.The bacteria-derived lipolypolysaccharide (LPS),DNA or inflammatory mediators under the inflammatory environment induce neutrophils to migrate into the lamella propria and across the epithelial barrier.Neutrophils in contact with invading microorganisms would exert multiple antibacterial effects which contained phagocytosis,the ROS release and degranulation to eliminate gut harmful microbes [36].
Neutrophils play a positive effect on the IBD treatment,which are closely connected with the expression of CD177.CD177,an approximately 58-64 kDa glycoprotein,is expressed in neutrophils and neutrophilic myelocytes [37].It is expressed about 50% in peripheral blood neutrophils of healthy donors,which expression level would boost in inflammatory sites [38].It has been reported that CD177 in neutrophils could bind platelet endothelial cell adhesion molecule-1 (or CD31),mediate the neutrophil-endothelial cells interactions and promote neutrophil transmigration [37].The role of CD177 in the nosogenesis of IBD is analyzed as follows[39].First,deficiency of CD177 makes DSS-induced colitis more serious in mice,illustrating a protective action of CD177+neutrophils in the IBD therapy.Second,compared with CD177-neutrophils,CD177+neutrophils could facilitate the formation of neutrophil extracellular traps (NET).Previous researches have verified that the release of NET is a pathway that highly activates neutrophil to remove harmful microorganisms and inhibits pathogen transmission[40].Moreover,CD177+neutrophils could produce less interferon(IFN)-γ,IL-17A,IL-6 and higher levels of IL-22 and transforming growth factor (TGF)-βwhen stimulated by LPS IL-22,on the one hand,directly acts on epithelial cells and promotes the recovery of intestinal epithelial barrier by motivating the reconstitution of mucus and the proliferation of epithelial cells and increase the expression of antibacterial proteins Reg3βand Reg3γ[41],further resulting in bacterial clearance [42,43].In addition,CD177+neutrophils could also elevate the expression proper level of ROS,which is helpful to enhance the bactericidal activity and protect the intestine.
2.5.4.Other factors
The tumor necrosis factor-alpha (TNF-α),interleukin (IL),and interferon-gamma (IFN-γ)-related molecular mediators are also key factors for mucosal wounds,apoptosis,and enhanced permeability at the wound site [44,45].CD is characterized by the overexpression of TNF-α,IFN-γ,and IL-17 [46],while the UC has an increased production of pro-inflammatory cytokines,including IL-5,IL-10,and IL-133 [47-49].In addition,the overall expression level of trans-membrane tight junction proteins and junction adhesion molecules in the IBD patients is reduced,resulting in the barrier function impairment,intestinal permeability enhancement of microbial endotoxins and ligands,and occurrence of systemic inflammatory reactions [50].
2.6.Current clinical reality of IBD therapies
Classic anti-inflammatory drugs like 5-ASA are important antiinflammatory drugs and often used in the anti-inflammatory treatment of patients with ulcerative colitis.However,5-ASA has limited efficacy in alleviating clinical symptoms and tissue inflammation in patients with Crohn’s disease [51].In addition,corticosteroids have been used for remission induction of ulcerative colitis,but they are not suitable for maintaining remission of IBD [52].Current immunosuppressive drugs used in the treatment of IBD include azathioprine,mercaptopurine,methotrexate,cyclosporine A and tacrolimus,which could act the patients with ulcerative colitis.Anti-TNF drugs have been also introduced into the clinical treatment of IBD,such as infliximab,adalimumab,golimumab and certolizumab pegol [53,54].At present,anti-TNF drugs like infliximab has been proved to be effective in the induction and maintenance of remission of ulcerative colitis and Crohn’s fistula.Furthermore,anti-TNF drug (infliximab) combined with immunosuppressant (azathioprine) has shown to have better effectiveness than azathioprine alone [55].Recently,various new therapeutic drugs,such as cytokine inhibitors (IL-6-I-6R blockers),transcription factors inhibitors (GATA3),cytokine signalling modulators (JAK inhibitors or SMAD7 blocker) and new anti-T-cell-activation,and migration strategies,are currently being evaluated in controlled clinical trials [56].The typical examples of traditional medicines in IBD therapy are shown in Table S1 (Supporting information).
3.The applications of nanomedicines (NMs) in IBD therapy
3.1.The necessity of NMs against IBD
IBD is a chronic inflammatory disorder of the gastrointestinal tract.More seriously,patients develop into colon cancer if inflammation-associated patients get a lot worse.Traditional medicines,including 5-aminosalicylic acid (5-ASA),methylprednisolone (MPS),and dexamethasone (DEX),have been applied for the treatment of IBD,as listed in Table S1.These medicines mainly concentrate on alleviating the symptoms of IBD through suppressing the immune responses.However,the clinical outcomes of the existent medications are far from satisfactory as expected due to their low therapeutic efficiencies and serious adverse effects [57].Most traditional small-molecule-based therapies for IBD still exist off-targeting challenge,resulting in acute complications,such as opportunistic infections,malignancies,and even hepatotoxicities.Besides,the epithelial cells in healthy colon are covered with a thick layer of mucus that allows the symbiotic and beneficial bacteria to colonize and provide a barrier against the pathogenic bacteria but the loss of the thin mucus layer and crypt is observed in the mice with colonic inflammation.This is due to the massive production of ROS and pro-inflammatory cytokines at the inflammation site.A small amount of ROS released from colon cells can play a protective role for epithelial cells and perform the intracellular signal transduction in the normal intestinal tissues.However,while the ROS contents was increased dramatically in intestine during inflammation,the excess of ROS could damage the intestinal mucosa and might develop microbial infection,further exacerbating the inflammation [58,59].Thurs,leveraging the target feature of therapeutic agents could precisely eliminate the excess of ROS contents in the diseased tissue and maintain a certain ROS contents level for normal function.
In addition,the occurrence and development of intestinal inflammation are closely associated with the balance of intestinal microbiota [60].Interestingly,the most diverse and abundant sites of the intestinal microbiota are the terminal ileum and colon,which are also the regions of high IBD incidence.This confirms an inseparable relationship between the intestinal microbiota and IBD.Considering the daily ingestion of multiple foods and chemicals,the physiological environment in gastrointestinal tract is more hostile than the other body parts.It is easy to damage the delicate balance of beneficial bacteria in the gut.Therefore,this might be another criterion for the ideal IBD medicines to maintain or mend the balance of intestinal microbiota.
The last decades have witnessed the enormous progress in using nanotechnology in the biomedical field.The emerging nanotechnology-based NMs have shown a great potential to facilitate the efficient and precise delivery of IBD therapeutic agents.Compared with traditional medicines,the superiorities of NMs are as follows.First,the NMs has higher specificity due to the enhanced permeability and retention (EPR) or ligand-receptor binding effect,which enables the NMs to be accurately delivered to the inflammation site (Fig.3B),thereby reducing the systemic side effects.Second,wrapped by carriers,the NMs can maintain stability of therapeutic agents and not be easily destroyed even under the hostile physiological conditions,thereby preventing the pharmacological inactivation.Meanwhile,owing to on-target activities of NMs,the therapeutic efficiencies of NMs against IBD are higher than that of traditional medicines.Third,relevant studies have proved that some of the NMs can promote community resilience at the inflammation site and normalize them.This ability is not available in the traditional IBD therapeutic agents [61].In this section,we discuss the applications of NMs in many aspects of IBD treatments and show that the nanotechnology has great potential in the field of IBD treatment.
3.2.The typical applications of NMs in IBD therapy
According to the specific modes,the NMs are divided into the active and passive targeted modalities.The passive targeted modality is mainly based on the physical and chemical properties of NMs,while the active targeted modality targets the related proteins,which could regulate metabolism,thereby achieving the purpose of IBD treatment.This review outlines the use of emerging nanotechnology-based NMs for IBD therapy.The common applications of NMs in IBD therapy are listed in Table S2 (Supporting information).
3.2.1.Applications of passive targeted NMs
The passive targeted NMs are common and have attracted much attention of researchers for the IBD treatment.These NMs with specific shape and size can affect thein vivofate of drugs and enhance the inflammatory tissue distribution based on the epithelial enhanced permeability and retention (EPR) effect [62,63].The advantages of passive targeted NMs are as follows.Compared with traditional IBD medicines,NMs make drugs delivery precisely and effectively.Meanwhile,their preparation process is simple and has wide applications.
The passive targeted NMs (RNPO),containing a redox polymer(Fig.3A),were first introduced.Vonget al.synthesized a redox amphiphilic block copolymer methoxy-poly(ethylene glycol)-bpoly[p-4-(2,2,6,6-tetramethylpiperidine-1-oxyl)oxymethylstyrene](MeOPEG-b-PMOT).The stable nitroxide radicals were served as a hydrophobic side chain of this copolymer [61].The anti-oxidative nitroxide radicals and biocompatible PEG shells constructed the core-shell micelles (RNPO) which effectively removed a large amount of ROS produced in the inflammatory site,reducing the production of inflammatory factors.Besides,the RNPOcould also regulate gut microflora.An increase in the abundance ofE.coliandStaphylococcuswas positively correlated with the severity of inflammation.The abundance ofE.coliandStaphylococcusunder the RNPOtreatment in the DSS-induced UC mouse models would gradually reach the normal value.More importantly,when the RNPOwas directly applied to the healthy mice,there were no apparent changes in the abundance of intestinal bacteria.Moreover,the RNPOcould selectively accumulate bacteria at the inflammatory sites [64].The intercellular space at the inflammatory site was larger than that in the normal tissue.The NMs with nano-size can infiltrate and accumulate at the inflammatory siteviaEPR effect.The nitroxide radicals-contained RNPOcould further enhance the EPR effect at the inflammatory site.The study also found a dose-response relationship between the RNPOand DSS-induced UC mice [65].The DSS-induced UC mice did not change in response to the injection at a low dose RNPO(50 mg/kg)but the medium (100 mg/kg) and high (300 mg/kg) doses of the RNPOinjection could produce significant changes.
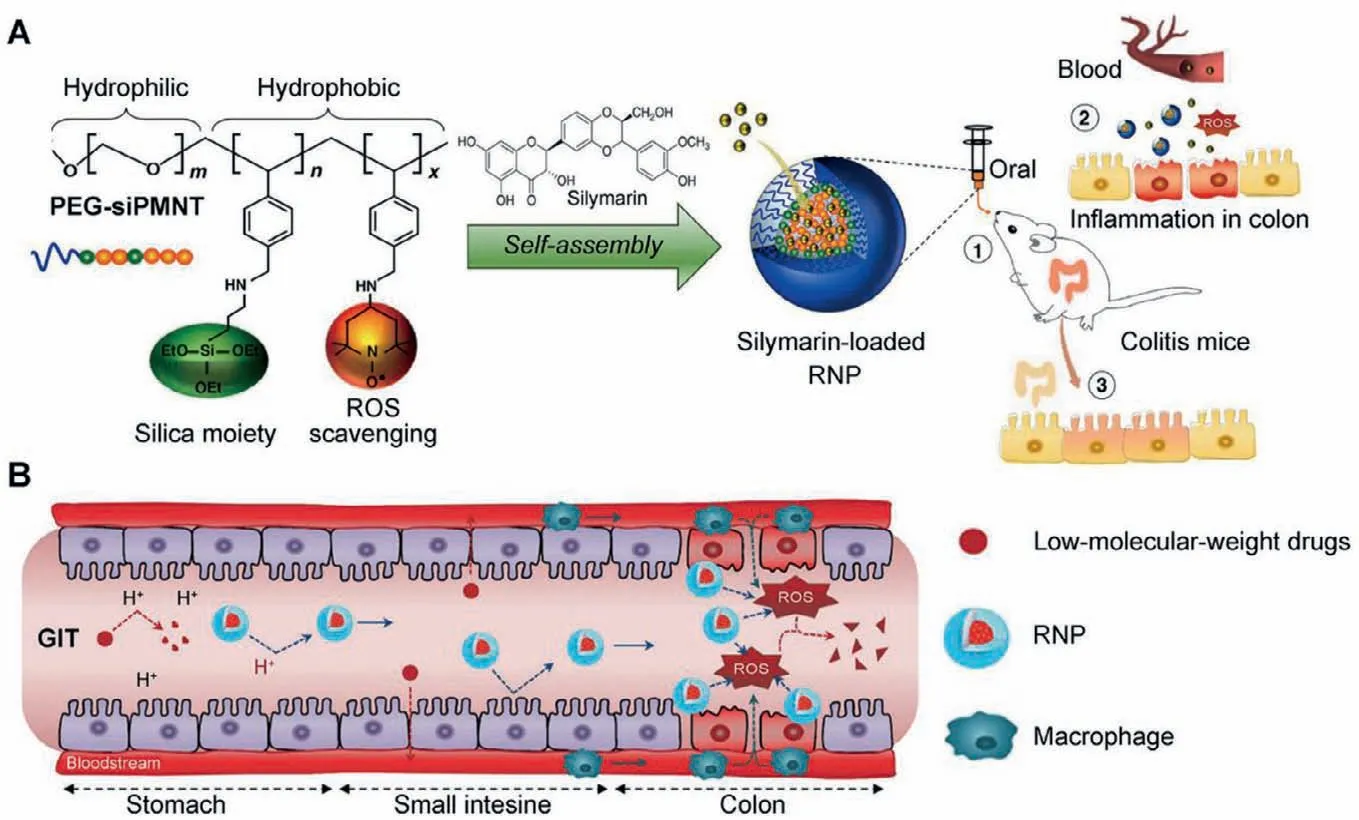
Fig.3.(A) Design of redox nanoparticle (RNP) and the illustration of the anti-inflammatory effect of RNP in colitis model mice.Reproduced with permission [59].Copyright 2021,Elsevier Ltd.(B) RNP is stable and withstands the harsh conditions of the gastrointestinal tract (GIT) and reaches the colon to scavenge ROS,especially at sites of inflammation.Reproduced with permission [85].Copyright 2018,Elsevier Ltd.
At present,a great progress has been made in the development of anti-cancer NMs for enhancing the therapeutic effects of “old”drug molecules.This strategy of developing new cancer NMs derived from repurposing the “old” drugs has been described in detail by Yang and Shi [66].As some clinically limited uses of drugs for the treatment of IBD have been discussed previously,the traditional "old drugs" can be transformed into "new NMs" using nanotechnology.
Given its excellent ROS scavenging and pro-inflammatory cytokine inhibiting capability,5-ASA has become one of the clinically important drugs for the treatment of mild IBD [67].Unfortunately,it is rapidly absorbed in the small intestine and only reaches the colon in a small amount,leading to its failure in IBD treatment as colon is the site for high IBD incidence.To address the above problems,researchers have developed several 5-ASA-containing NMs.Varshosazet al.designed and prepared a type of NMs,containing chitosan as the carrier material and 5-ASA as the active drug [68].In addition,another type of NMs,where the 5-ASA was loaded by the biodegradable polymer PCL,was designed by Pertuitet al.[69].Moulari’s team loaded the 5-ASA into silica materials,assembling a novel 5-ASA-silica NM.Compared with free 5-ASA,these NMs with different carriers and preparation processes have excellent characteristics for the IBD treatment,including precise targeting,great pharmacological activity,and few side effects.
DEX is a type of glucocorticoid and the main drug for the clinical treatment of severe IBD.Similar to other traditional IBD medicines,DEX has low bioavailabilityin vivoand lacks the precise targeting characteristic,causing different degrees of side effects in patients,including nausea and vomiting,hypertension,hyperglycemia,and urinary retention [70,71].Addressing the above challenges,Leeet al.reported that the DEX-loaded spherical polymeric nano-constructs (DEX-SPNs),having a size of about 162 nm,were prepared using phacoemulsification technology [72].The major carrier material was poly (lactic-co-glycolic acid) (PLGA),which was wrapped with a single layer of lipid shell and modified with PEG and fluorophore Cy-5 on its surface.The fluorescence images ofin vitroRAW 264.7 cell experiments showed that the DEX-SPNs were quickly swallowed by macrophages and then decomposed to release DEX,inhibiting the production of pro-inflammatory cytokines,such as TNF-α,IL-1βand IL-6.Notably,the capability of DEX-SPNs to inhibit the pro-inflammatory cytokines was comparable to that of the free DEX.After the injection of Cy5-SPNs into DSS-induced enteritis mice,the near infrared fluorescent results showed that the Cy5-SPNs specifically accumulated at the intestinal inflammation site,avoiding the side effects caused by the lack of specificity.
Except for the glucocorticoid drugs,immunosuppressive agents also have a wide range of applications in the clinical treatment of IBD.They are generally used for the patients with severe IBD,who cannot use glucocorticoid drugs.However,the traditional immunosuppressants have toxic side effects.Therefore,it is necessary to develop them into NMs.A typical immunosuppressive agent tacrolimus (FK506),an inhibitor of calcineurin,could modulate the multiple pro-inflammatory cytokines for the effective treatment of IBD [73].However,the FK506-treated patients developed the symptoms of nausea and vomiting and the severe patients were even at the risk of renal damage due to the lack of specificity.Lamprechtet al.encapsulated the FK506 into polymer PLGA and developed NMs (FK506-NPs).The results showed that compared with the free FK506,the FK506-NPs could effectively reduce the nephrotoxicity and increase the accumulation of drugs at the intestinal inflammation site [74].In addition,azathioprine (AZA) and methotrexate (MTX) are the commonly-used immunosuppressive drugs for the clinical treatment of IBD.Similar to FK506,the AZA and MTX also cause side effects,which cannot be ignored when developing powerful therapeutic strategies [75,76].Therefore,the AZA and MTX have also developed into NMs: AZA-chitosan NM and MTX-PAMAM dendrimer NM.Related trials have revealed that both the NMs could reduce side effects and had better therapeutic effects,which proved the feasibility of nanotechnology-based NMs for the IBD treatment again [77].All in all,the 5-ASA-NMs,DEXSPNs,FK506-NPs,AZA-chitosan NMs,and MTX-PAMAM dendrimer NPs are the typical representatives of developing the "old drugs"into "new NMs",making the application of nanotechnology a significant strategy for the "renewing" of drugs.
In addition to the above NMs developed from traditional medicines,the natural product-based NMs have also been focused recently,such as curcumin (CUR),thymoquinone (TQ),resveratrol (RES),silymarin,andOphiopogon japonicuspolysaccharides(OJPs) [78].The IBD would cause patients a lot of pain,such as blood in the stool.The IBD might further deteriorate and develop into colon cancer,which is a life-threatening condition for the patients.Therefore,the combination of the conventional antiinflammatory and anti-cancer drugs used for the treatment of IBD would not only relief symptoms but also reduce the incidence of colon cancer.For example,the combined application of chitosancurcumin self-assembly nanosystem (nCUR) and chitosan-7-ethyl-10-hydroxycamptothecin self-assembly nanosystem (nSN38) followed this idea.CUR is a natural medicine with many pharmacological activities,such as excellent ROS scavenging capability.SN38,a highly effective DNA topoisomerase I inhibitor,can effectively induce the apoptosis of various cancer cells.However,both CUR and SN38 have a common feature: very low solubility in water.The oral administration of both free drugs is difficult to obtain satisfactorily therapeutic effects.Chitosan,with many excellent properties,has been widely used as a carrier for the preparation of NMs.In a study,CUR and SN38 were reasonably combined into the chitosan side chain,successfully solving the problem of insolubility in water.Both nCUR and nSN38 had better synergetic capabilities for the treatment of IBD and colon cancer.The nCUR has been proved to have similar anti-inflammatory effects to that of free CUR,effectively inhibiting the expression of inflammatory cytokinesin vitro,such as IL-1β,IL-6,IFN-β,TNF-α,iNOS and MCP-1.This inhibitory effect was positively correlated with the dose.In addition,nSN38 produced a large number of inflammatory factors during its anticancer activity,which was inhibited by nCUR,thereby better exerting anti-cancer efficacy of nSN38 [79].Moreover,compared with the free drugs,the nCUR could be precisely deposited at the inflammatory site in the intestines and showed beneficial therapeutic effects,including the restoration of body weight,relieving diarrhea and blood in the stool,and reduction of mouse mortality.On the other hand,the nSN38 could specifically target and treat the lesions of colon cancer,effectively preventing the further deterioration of IBD.As compared to their individual administration,the combined use of nCUR and nSN38 (nCUR/nSN38) could protect the intestines and inhibit tumor proliferation.To sum,designing the combined application of nCUR and nSN38 solved their problem of insolubility in water and specificity,obtaining a satisfactory synergistic effect for the treatment of IBD and the inhibition of colon cancer (Fig.4).
The biocompatible chitosan-based resveratrol nanoparticles(RES-CTS-NPs),a novel type of NMs for the IBD treatment,were designed by Iglesiaset al.The active substance in this NM is resveratrol (RES),which is a multifunctional natural compound found in red wine.Many studies have demonstrated its antioxidant,anti-tumor,and anti-inflammatory activities [80-83].Its antiinflammatory activity is important for the IBD treatment.Similar to the anti-inflammatory agents,RES could decrease the level of pro-inflammatory cytokines,such as TNF-α,IL-1β,IL-6 and IL-8.The reason for combining RES into NPs is the low water solubility and chemical instability of free RES.The study proved that the RES-CTS-NPs exhibited a good prospect in the field of IBD therapy[84].Besides,Lozano-perez and colleagues encapsulated RES in silk fibroin-based NPs (RES-SFNPs) and further investigated its nanotherapeutic potential against IBD.The RES-SFNPs exhibited immunoregulatory characteristics after incubation with macrophages under basic conditions and inhibited their activation after induction with LPS.In addition,in vivoexperiments have clearly shown that the RES-SFNPs demonstrated better therapeutic effects than the individual administration of drug-free SFNPs and free RES by the down-regulation of inflammatory response and recovery of damaged mucosa.
Selenium (Se) is an indispensable element for human growth.It inhibits the formation of ROS in mitochondria by modifying protein mercaptan groups,achieving anti-inflammatory and antioxidant effects.However,the high toxicity and low bioavailability of Se have hindered its clinical application.Se-based NPs (SeNPs)were designed,which had been testified that SeNPs indeed have better characteristics than free selenium.Silymarin is a natural product with strong antioxidant activity,which could not only repair damaged epithelial cells,but also enhance the activity of antioxidant enzymes and inhibit the production of pro-inflammatory cytokines [85].Miroliaeeet al.demonstrated that SeNPs combined with silymarin have synergetic antioxidant effects and strong pro-inflammatory cytokine inhibition capabilities compared to silymarin or SeNPs alone on the model of trinitrobenzene sulfonic acid(TNBS)-induced colitis mice [86].
The OJPs,extracted from a Chinese traditional medicine namedOphiopogon japonicus,have a variety of pharmacological activities,such as liver protection and anti-myocardial ischemia effects[87,88].Furthermore,their activities,including anti-inflammatory activity and protecting the integrity of intestinal epithelial barrier,have been also discovered recently.The OJPs have attracted more attention from the researchers for IBD treatment.However,owing to its short half-life,the OJPs are quickly clearedvialiver metabolism after oral administration.To address the problem,Lin designed one new NM,named OJPs-loaded chitosan-whey proteins nanoparticles (OJPs-CS-WP NPs).First,the chitosan has positively charges and whey proteins are negatively charged,thereby ensuring successful assembly of OJPs-CS-WP NPs through electrostatic interactions.Second,the chitosan enhances the adhesion of drugs in intestines and facilitates their absorption,making it suitable for oral administration [89-91].Third,both the chitosan and whey protein have immunomodulatory activities,in which the OJPs-CSWP NPs have a significant therapeutic advantage over the free drugs [92,93].
3.2.2.Applications of active targeted NMs
The EPR effect-based drug delivery seems to be highly uncertain in a variety of patients,due to the severity and heterogeneity of inflammation [62].The active targeted NMs refer to the use of modified carriers as "missiles" to accurately and specifically deliver the drugs to their target site and exert their desired effects[94].The most commonly used modified molecules are the ligands of receptors and monoclonal antibodies [95,96].The therapeutic agent can be directed into the targeted area through the ligand-receptor interactions,which reduce nonspecific recognition(Figs.5A and C),thereby avoiding the drug delivery to the offtarget tissues [97].However,owing to high technical complexity,the current developments are still in the pre-clinical stage.In this section,we introduce the active targeted NMs for IBD therapy.
Notably,the silk fibroin (SF)-based nanoparticles (SFNPs) exhibit innate anti-inflammatory activity,healing capacity,and lysosomal environmental responsiveness to drug release.The antiinflammatory activity of SFNPs is comparable to that of the immunophilin derivative,PEP-1-FK506BP24.Recently,the on-target delivery capacity of SFNPs can be greatly improved by the precise modification of SF molecular sequencesviatransgene techniques or chemical reactions.As an important glycoprotein in the treatment of IBD,the integrin is a specific receptor for arginine-glycineaspartic acid (RGD),which is highly expressed in the gastrointestinal tract of the IBD patients [98].One targeted strategy is to conjugate the RGD onto the surface of SFNPs.In the animal models of IBD,the rectal administration of RGD-SFNPs significantly reduced the damage of the superior colonic cortex,infiltration of immune cells,and oxidation state of the colon (Fig.5B) [99,100].The histological appearance of colon tissue and mRNA expression level of the pro-inflammatory cytokines also showed much stronger anti-IBD activities of RGD-SFNPs than that of the non-functional SFNPs.
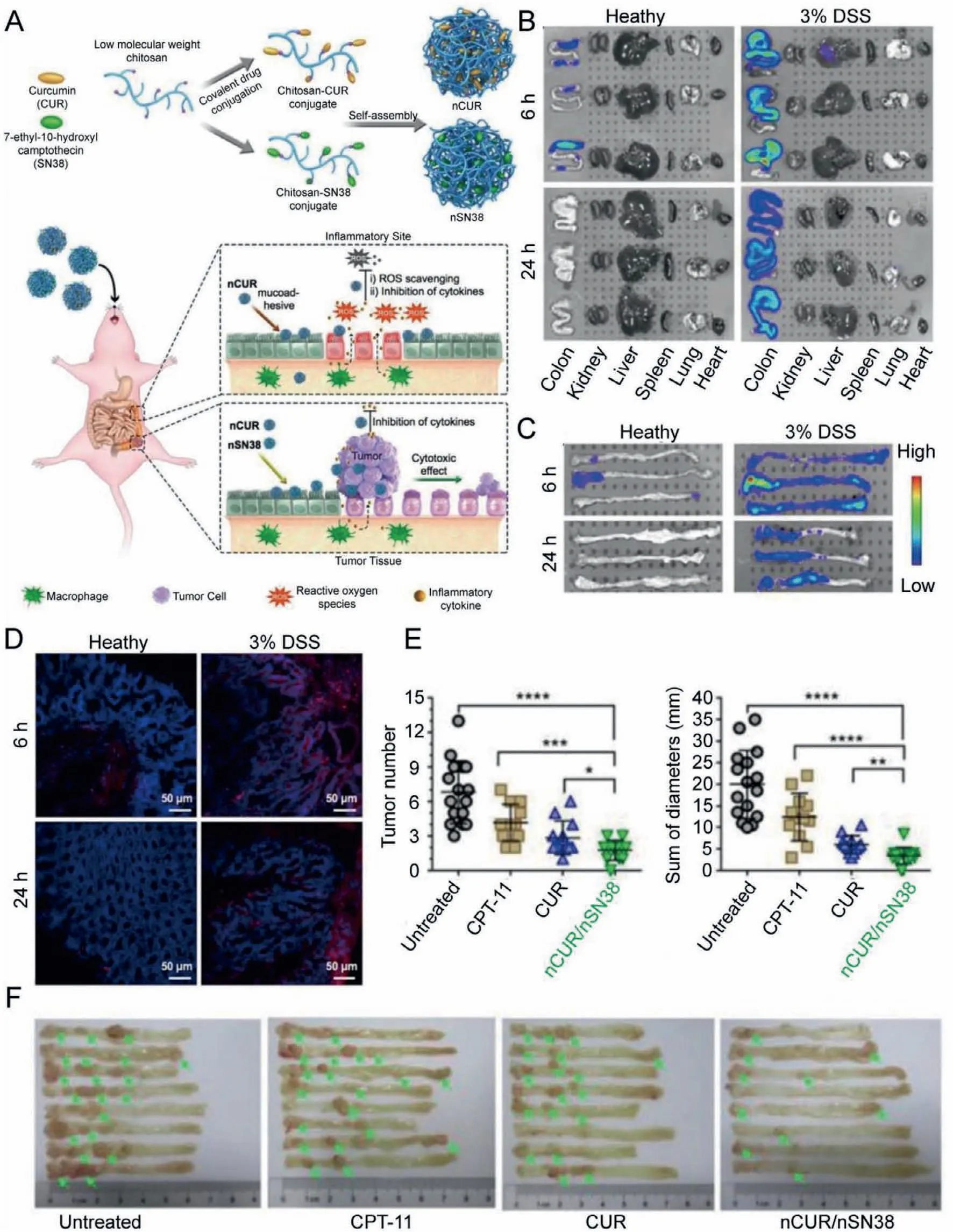
Fig.4.(A) Schematic of the self-assembly of chitosan-drug conjugates to form nanotherapeutics (i.e.,nCUR and nSN38) and oral administration of the nanotherapeutics for CAC treatment.(B) Representative in vivo fluorescence images for the evaluation of nanoparticle distribution.(C) Quantification of fluorescence intensities.(D) Fluorescence intensities.(E) Tumor diameters and tumor size distribution.(F) Representative images of murine colons.Reproduced with permission [79].Copyright 2019,Ivyspring International Publisher.
Another active targeted NM for IBD is hyaluronic acid-bilirubin NM.Hyaluronic acid (HA) is a glycosaminoglycan biopolymer,which is found in synovial fluid and extracellular matrix and has immunomodulatory properties,including the regulation of macrophages,antimicrobial peptides,and CD4+T (Treg) cells [101].Owing to the efficacy of HA,the free HA and HA-based NMs have been studied for the treatment of IBD,but their therapeutic efficiencies are obstructed due to the exist of hyaluronidase[102,103].In addition,bilirubin (BR) is a hydrophobic by-product of the decomposition of hemoglobin found in bile and exhibits the strong ROS scavenging,antioxidant,and cell protection activities [104,105].The covalent compound of HA and BR assembled as NMs could address the drawbacks of the individual drug in the treatment of IBD.In this NMs,the HA shell solved the problem of BR insolubility in water and allowed the oral administration of NMs to target the inflammatory intestinal epithelial cells through HA-CD44 interactions.Meanwhile,the BR core could enforce the NMs hyaluronidase resistance and strong ROS clearance.According to Leeet al.,the HA-BR NMs could treat the IBD from many aspects [5]: First,compared with free HA,the HA-cholesterol conjugate and its oxidization form,HA-BR NMs,have strong ROS clearing capability,thereby protecting the colonic epithelium cells from ROS damage.Second,the HA-BR NMs can normalize the expression pattern and mRNA levels of ZO-1 and occludin-1,which play a key role in the intestinal homeostasis.In addition,when tested in DSS-induced UC mouse models,the NMs could prevent their systemic exposure to fluorescein isothiocyanate (FITC)-dextran after the oral administration,demonstrating that the NMs could promote the restoration of intestinal barrier function.Finally,the NMs therapy significantly reduced the local levels of pro-inflammatory cytokines,such as IL-1,TNF-αand IL-6,and increased that of the anti-inflammatory cytokines,such as IL-10 and transforming growth factor-beta (TGF-β).It is important to note that the NMsmediated induction of IL-10 and TGF-βis transient and returns to the baseline levels 10 days after the cessation of NMs therapy.
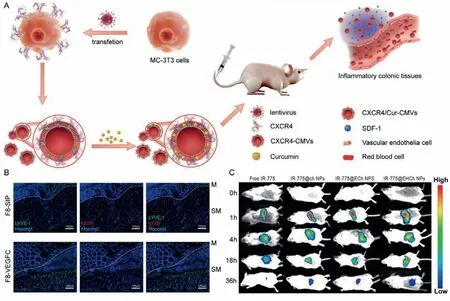
Fig.5.(A) The schematic illustration of active targeted nanoparticle CXCR4-CMVs fabrication,inflammation targeting,and modulation in vivo.Reproduced with permission[94].Copyright 2021,Wiley-VCH GmbH.(B) Representative pictures of immunofluorescence labeling of the inflamed colon after F8-SIP (upper panels) or F8-VEGFC (lower panels) treatment for LYVE-1 (green),CD31 (red),and Hoechst (blue).Dotted white lines mark the separation between the mucosa (M) and the submucosa (SM).Reproduced with permission [96].Copyright 2019,American Chemical Society.(C) In vivo fluorescence images of mice treated with free IR-775 and active targeted nanoparticles.Reproduced with permission [99].Copyright 2021,Elsevier Ltd.
3.3.New attempt: gene-based nano-therapeutic strategy
Gene therapies,regulating intracellular gene expression,could serve as a useful tool for targeting “undruggable” targets to treat the IBD [106].Benefiting from the progress of molecular biology technology and bioengineering in the past decade,the usage of exogenous nuclear acids (NAs) such as DNA,mRNA,antisense oligonucleotides (ASOs),small interfering RNA (siRNA) and geneediting systems (like CRISPR-Cas) can precisely edit gene and minimize the amount of harmful genes Yinet al.[107-109].Many great works have been done to summarize the mechanism of gene therapy.In this study,we focus on the oral nanoparticle-mediated gene therapies for the treatment of IBD.
Oral delivery of NAs has a variety of advantages over parenteral delivery,such as non-einvasiveness,sustained or controlled release,selective targeting and easy for use [110-112].However,multiple barriers of gastrointestinal tract (GIT) bring the following challenges for gene therapies.For example,gastric acid can destroy glycosidic bonds and denature the NAs’structures.The transformation of pH in small intestine and large intestine requires the stability of NAs in a wide range of pH.The presence of proteolytic enzymes,such as pepsin,can degrade NAs [113].In addition,the peristaltic activity of the digestive tract obstructs the contact between the foreign particles and epithelial cells,reducing the possibility of cellular uptake.Mucus lining,as an additional barrier,can capture and remove foreign particles through electrostatic and hydrophobic interactions.Hence,NAs-encapsulated carriers need to be designed to protect NAs against inactivation and bypass the above obstacles,further promoting cellular uptake of NAs [111].
IBD occurred along with the the overexpression of ROS.The ROS-sensitive delivery carriers could be degraded,releasing the therapeutical NAs to the target site [114,115].Wilsonet al.designed the ROS-sensitive thioketal nanoparticles (TKNs) through step-growth polymerization [116].They encapsulated siRNA engineered by DOTAP-derived liposomes in TKNs.The biodistribution model showed that TKNs accumulated at the inflammatory site in DSS-induced colitis mice and decreased the expression of proinflammatory cytokines.Peppaset al.designed the mannosylated bioreducible cationic polymer (PPM) which could assemble NPs assisted by sodium triphosphate (TPP).These NPs remained stable in gastric pH and were degraded by trypsin,releasing siRNA embedded NPs.In vitrostudies confirmed that the NPs are easily absorbed by macrophages,and the encapsulated siRNA can downregulate the expression of TNF-αin macrophages [116].
Chitosan can easily form chitosan-derived NPs with NAs through electrostatic interaction between amine and phosphate groups [117].However,chitosan is insoluble in neutral and alkaline pH,which reduces its therapeutic effect on intestinal inflammatory sites.Chemical modification strategy is used to improve the therapeutic efficacy of chitosan-derived NPs [118].For example,galactose-modified chitosan NPs could effectively bind to overexpress macrophage galactose/N-acetyl galactosamine-specific lectin in the surface of activated macrophages during inflammation,contributing to receptor-mediated uptake of these NPs.A hard trimethylammonium charge was introduced into galactosylated chitosan,which can maintain its ability to target macrophages[119].In addition,thiol group is further introduced to galactosylated chitosan,assisting in adhering to mucoprotein [120].
4.Applications of prodrugs systems in gut inflammation therapy
Currently,commercial drugs in clinical practices generally have some disadvantages,such as low solubility and stability,and undesirable side effects.In response to these obstacles,the prodrugs,inactive conjugates metabolizedin vivoto release the parent bioactive components,have been also widely utilized.Prodrugs refer to the covalent combination of a pharmacologically active drug and a carrier.After enzymatic conversion,the active drug is releasedin vivo.This conversion depends on the type of bond present because the conjugated high-activity compounds can be specifically bioactivated in the presence of the high-activity microbial enzymes(azoreductase-galactosidase xy-xylosidase,nitroreductase glucosidase deaminase,etc.) [121].The prodrugs can produce their desired effects at a lower dose due to being released at a specific site under the action of an enzyme.In addition,they have many advantages.For example,for the certain active drugs,the modification of prodrugs reduces their side effects and increases their stability and bioavailability.In addition,the prodrug approach is easier than the other types of colon-specific drug delivery systems.
For their synthesis,the bonds between the carriers and drugs are usually selected by the functional group of the drug molecules.The commonly used prodrugs for the clinical treatment of IBD are divided into 5-ASA-conjugated prodrugs,amino acid-conjugated prodrugs,and carbohydrate-conjugated prodrugs based on the types of conjugates as listed in Table S3 (Supporting information).Chemical structures of various prodrugs are shown in Table S4(Supporting information).
4.1.5-ASA-conjugated prodrugs
The 5-ASA is a commonly used drug for the treatment of IBD but its application is affected by excessive absorption in the proximal intestine.Among the prodrugs that have been reported,the azo-conjugates of 5-ASA have been widely accepted.The azoconjugates are metabolized by the intracellular enzymes and extracellular reduction [122].The azo-conjugated prodrug is stable in the upper part of the gastrointestinal tract and is cleaved in colon by the azo reductase produced by the intestinal microbiota to achieve the on-target effects.The azo-5-ASA prodrug has many advantages in the IBD treatment.As the molecular size and hydrophilicity of prodrug molecules increase,the early absorption of the active agent in the small intestine is minimized.Sulfasalazine(SASP) is a commercially available 5-ASA prodrug,which is prepared by linking the 5-ASA to sulfapyridineviaan azo bond.Khanet al.showed that the superior therapeutic effect of SASP in the UC treatment was due to the decomposition of the azo bond in the colon by bacteria into sulfapyridine (SP) and 5-ASA,and almost all the doses reached the colon intact [123].The two drug molecules would be released in the colon by the decomposition of azo bonds,exerting the antibacterial and anti-inflammatory effects at the same time.More studies have shown that the inert or pharmacologically-active molecules as carriers and 5-ASA,which are bound through azo bonds to form prodrugs,such as olsalazine,ipsalazine,balsalazine,and APAZATM,can also effectively release the active drugs in the colon.The further optimization of the carrier can enhance 5-ASA’s efficacy,improve tolerance,and reduce the side effects [124,125].
4.2.Amino acid-conjugated prodrugs
Amino acids as carriers can reduce the toxicity of drugs as well as improve their bioavailability,effectiveness,and transport to the large intestine in IBD and other colorectal diseases [121].Two hydrophilic polar groups,a carboxyl group and an amino group,could increase drug’s water solubility and reduce their membrane permeability,thereby reducing the partial absorption of drugs in GIT.In addition,it was pointed out that the certain amino acid prodrugs exhibited different hydrolytic activities due to the different microbial populations and parts of the intestine,with on-demand release and accumulation at the target site [126].The researchers used salicylic acid (SA) as a model drug to study the effects of the type and configuration of amino acids on the absorption of the prodrugs.Nakamuraet al.prepared a series of prodrugs by combining a series of non-essential amino acids,such as glycine,tyrosine,methionine,L-alanine,and glutamic acid with SA [127].Among them,studies have indicated that the SA-glycine prodrug was absorbed in GIT and was not suitable to be delivered into the colon.The SA-glutamic acid prodrug could increase the hydrophilicity and length of the carrier amino acid and reduce the membrane permeability of the conjugate.This prodrug could not be absorbed and degraded in the upper GIT but hydrolyzed into SA by the colonic enzymes.Additionally,it has been found that the intestinal enzymes are enantioselective.When administered orally,the SA-L-alanine was metabolized to SA by the rabbit intestinal microbiota;however,the conversion rate of SA-D-alanine to SA was negligible [128].
4.3.Carbohydrate-conjugated prodrugs
4.3.1.Glycoside conjugates/glycoside prodrugs
Some drugs can provide aglycones and form glycosides with sugars.They are large and hydrophilic and do not pass easily through biofilms after ingestion.The glycosides are broken down only by glycosidases and the glycoside conjugates are generally considered as good colon-specific carriers because of the production of glycosidase in GIT by anaerobic microbiota in the large or small intestine [129].However,the transit time of drugs in large intestine is longer than that in the small intestine,giving an advantage to glycoside drugs to have therapeutic efficacy following the glycosidic bond hydrolysis takes time.Studies have suggested that the formation of glycoside conjugated with anti-inflammatory steroids can reduce their systemic side effects without affecting their efficacy [130].Researchers used steroids as model drugs to examine the effects of different active drugs,glycosyl groups,and other factors on targeting the colon site.Friend and colleagues developed DEX-21-β-glucoside and prednisolone-21-β-glucoside for the delivery of steroids to colon [131].When taken orally,less than 1% of the active drugs in the two free steroid forms could reach cecum,while nearly 60% of the DEX-21-βglucoside prodrug reached cecum within 4-5 h and underwent hydrolysis.However,the effects of prednisolone prodrug were not obvious.To compare the effects of different sugar groups as carriers,Friend and Chang also prepared several anti-inflammatory steroids,including DEX,prednisolone,hydrocortisone and flucortisone glycosides,galactosides,and cellobiose [132].In vitrostudies found that the galactosyl prodrugs were hydrolyzed faster than the corresponding glycosides and cellobiose.In addition to altering the transmission by aglycone,the stereochemistry of the glycosidic bond can also be conducted to change the release rate and site.The diet can induce intestinal bacteria to produce certain enzymes,and bran diet can increase the production ofα-glucosidase.The control of glycosidase activity through diet might be very useful to normalize and increase the glycosidase activity in IBD patients,reducing enzyme levels [131].
4.3.2.Glucuronide conjugates/glucuronide prodrugs
Glucuronide conjugates can also be used to deliver the IBD drugs to colon site.The binding of glucuronic acid-binding is the most common biphasic metabolic reaction,which increases the polarity of the drug and promotes excretion.However,bacteria in the lower part of the gastrointestinal tract would secreteβglucuronidase,release the glucuronide in the intestine,then be reabsorbed.For the entire gastrointestinal tract,the gradient of glucosidase activity between the distal small intestine and cecum is much lower,indicating that the glucuronide prodrugs are less prone to early hydrolysis in the small intestine and can be delivered more specifically to colon.Haeberlinet al.prepared a prodrug of glucuronic acid in combination with DEX,named DEX-β-D-glucuronic acid [133].The study found that in normal mice,the intramedullaryβ-D-glucuronidase activity in the distal small intestine increased by 30 folds as compared to cecum and the DEX was effectively released in the colon.By comparing the conventional,UC,and germ-free rats,the largest hydrolytic activity was observed in the intestinal contents of conventional rats,followed by UC rats.The germ-free rats displayed very low lumenβ-Dglucuronidase activity.A similar study conducted in the normal and UC rats showed that the glucosinolate prodrugs using colventconjugating of DEX and budesonide could deliver the high levels of drugs to the colon region [134].
4.3.3.Dextran conjugates/dextran prodrugs
Dextran is a homopolysaccharide composed of glucose as monosaccharide units,which are connectedviaglycosidic bonds.These bonds can be hydrolyzed by glucanase.Dextranase shows high enzymatic activity in the presence of anaerobic Gramnegative bacteria,especiallyBacteroides[135].These bacteria are almost absent in the upper part of gastrointestinal tract but their concentration in the colon is as high as 1011/g.The glycosidic bonds in the dextran conjugate can be hydrolyzed by the glucanase in the colon to produce a prodrug oligomer,which is then further broken down by the colonic esterase to release the free active drug in colon,thereby achieving the target-specific delivery [136].Studies on glucocorticoid-dextran conjugates have demonstrated that the dextran can block drug absorption/hydrolysis in small intestine.In cecum and colon,the glucocorticoids were released and used to treat experimental colitis in rats,causing almost no adrenal suppression [137].
4.3.4.Cyclodextrin conjugates/cyclodextrin prodrugs
Cyclodextrin is covalently combined with active drugs to form prodrugs with an advantage in delivering the drugs to colon.Due to its high biocompatibility,safety,and hydrophilic and bulky nature,it cannot be absorbed in the gastrointestinal tract [138-140].It is resistant to gastric acid,saliva,and pancreatic amylase.The colonic bacteria (mainlyBacteroides) can stimulate the cyclodextrin enzyme activity,which would degrade the carbon sources and ferment them into small sugars,thereby releasing the active drugs[141-143].In addition,the formation of short-chain fatty acids by cyclodextrin fermentation is beneficial for maintaining the health and integrity of the colonic epithelium.The schematic diagram of cyclodextrin coupled release was shown in work of Zou’s team[144].They synthesized highly active 5-ASA conjugated withα-CyD,β-CyD andγ-CyD.The study found that these prodrugs had similar therapeutic effects after oral administration in rats and can effectively be delivered drugs to the colon part.
5.Conclusions and perspectives
In conclusion,this review summarizes the application of NMs and prodrugs strategy for the treatment of inflammatory enteritis.First,we systematically sorted out the pathological characteristics of IBD that play a key role in its pathogenesis,such as colon pH,transit time,intestinal pressure and permeability,gut microbes,and inflammatory factors.Second,by comparing with the traditional IBD drugs and analyzing the properties of NMs,we discussed the direct and indirect need of NMs to treat IBD.In addition,the existing prodrugs for the IBD therapy were discussed and the previous reported examples were enumerated for further demonstration and the summary of anti-IBD nanomedicines and prodrugs under clinical trials are shown in Table S5 (Supporting information).
Nowadays,although the clinical applications of novel drug delivery systems (DDSs) for the treatment of IBD are still in their infancy,some clinical reports have shown outstanding advantages of the NMs and prodrug approach for the IBD treatment.In recent years,researchers have conducted clinical trials to study the targeting potential of different DDSs to target IBD.In order to fully clarify the impact of key factors,the test formulations in clinical trials by Lautenschlägeret al.were prepared according to the GMP requirements [144].The test preparations contained hydrophilic PEGfunctionalized PLGA micro-and nano-particles,positively-charged chitosan-functionalized PLGA micro-and nano-particles,and nonfunctionalized PLGA micro-and nano-particles.By analyzing the experimental data of 101 patients with IBD and a control group of healthy volunteers,the following findings were suggested.First,as compared to the micro-particles,the nano-particles showed more displacement and deposition in both the healthy and inflamed mucosa.Second,as compared to the healthy control group,the deposition rate of micro-and nano-particles at the inflammatory site was higher in the IBD patients.In addition,the PEG-functionalized nano-particles had the highest translocation through the healthy(2.31%) and inflammatory mucosa (5.27%).Furthermore,the transfer (3.33%) and deposition rates (10.8%) of the PEG-functionalized nano-particles through the inflamed mucosa were significantly higher than those of the healthy mucosa (0.55%,P=0.045: 4.1%,P=0.041,respectively).In a nutshell,in the IBD patients,the NMs had better targeting specificity and promising prospects in the treatment of IBD and the PEG-functionalized nano-particles can be used as good delivery vehicles.Oral administration is the most common and preferred route of administration for the treatment of IBD,although some administration obstacles existed in the digestive tract,such as pH profiles and intestinal peristalsis [145].Prodrugs can overcome the physiological obstacles due to degradation of prodrugs through colonic microflora microorganisms and colonic enzymes.In addition,IBD patients need long term treatment [121].The use of anti-IBD prodrugs with targeting specific colon tissue can reduce the drug dose.Meanwhile,the incidence of systemic side effects in these patients can also be reduced by oral prodrugs.Thus,prodrugs are considered to be one of the most practical methods for targeted treatment of IBD patients [146].
However,the development of novel DDSs (e.g.,NMs and prodrug strategy) for the IBD treatment still faces many challenges.First,the targeting of new formulations has been improved as compared to the traditional medicines but there is still room,needing to be explored.Second,there are few reports on the utilization of new DDSs for the clinical treatment of IBD,which also reflects the immaturity of the development of formulation for the treatment of IBD.Finally,the preparation of formulation is difficult and the quality evaluation requirements are strict.The improvement of preparation process is also a big problem,which needs further investigation.Of course,it’s crucial to note the safety and structural stability of drugs in the conversion of clinical technology.Therefore,continued in-depth exploration of the pathogenic mechanism of IBD andin vivosafety and stability evaluation will help the development of new drug delivery systems for IBD.
Declaration of competing interest
The authors ensure that there are no conflicts to declare.
Acknowledgments
This work was supported by National Key R&D Program of China (No.2021YFA0909900),National Natural Science Foundation of China (No.81773656),Liaoning Revitalization Talents Program (No.XLYC1808017) and Shenyang Youth Science and Technology Innovation Talents Program (No.RC190454),China Postdoctoral Science Foundation (No.2020M680986) and General Project of Liaoning Provincial Department of Education (Nos.LJKZ0927 and LJKQZ2021034).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.061.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
- A review on treatment of disinfection byproduct precursors by biological activated carbon process
