Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
Ke Tin,Limin Hu,Letin Li,Qingzhu Zheng,Ynjun Xin,Gungshn Zhng,*
a College of Resource and Environment,Qingdao Engineering Research Center for Rural Environment,Qingdao Agricultural University,Qingdao 266109,China
b School of Ecology and Environment,Zhengzhou University,Zhengzhou 450001,China
Keywords:Persulfate Sulfate radical Activation Organic wastewater Advanced oxidation processes
ABSTRACT In recent years,with the emergence of new pollutants,the effective treatment of wastewater has become very important.Persulfate-based advanced oxidation processes have been successfully applied to the treatment of wastewater,such as wastewater containing antibiotics,pharmaceuticals and personal care products,dyes,endocrine-disrupting chemicals,chlorinated organic pollutants,and phenolics,for the degradation of refractory organic contaminants.This paper summarizes the production of sulfate radicals,which can be generated by the activation of persulfate via conventional and emerging approaches.The existing problems of persulfate-based advanced oxidation processes were analyzed in detail,including residual sulfates,coexisting factors (coexisting inorganic anions and natural organic matter),and energy consumption.This paper proposes corresponding possible solutions to the problems mentioned above,and this paper could provide a reference for the application of persulfate-based advanced oxidation processes in actual wastewater treatment.
1.Introduction
In recent years,with the development of industry and technology,an increasing amount of water has been polluted [1],causing many hidden dangers to human health and lives.However,with the lack of standards for water discharged by pharmaceutical plants [2]and garbage treatment plants [3]as well as water containing domestic sewage,pesticides [4]and oil [5],the categories and contents of refractory organic pollutants in water have gradually increased.In addition,refractory organic compounds have the characteristics of good stability,difficult degradation,and low biodegradability [6,7],which causes substantial problems and challenges for water supply treatment.Generally,the processes of wastewater treatment can be divided into physical methods,chemical methods,and biological methods.The physical methods include adsorption and extraction,and their operation is simple,but the disadvantage of these methods is that physical methods mainly involve the phase transfer of organic pollutants and cannot effectively decompose and remove pollutants.Due to the low biodegradability of insoluble organic pollutants,biological methods are not applicable.The most prominent process among chemical methods is the advanced oxidation process,which involves the generation of active free radicals to degrade the target contaminants.
Advanced oxidation processes (AOPs) are essential in water treatment.Due to thein situgeneration of highly active species,such as hydroxyl radicals (·OH),sulfate radicals (SO4·-),and superoxide anion radicals (O2·-),to mineralize organic pollutants into carbon dioxide (CO2),water (H2O) and small inorganic compounds,AOPs have been widely applied to mineralize refractory organic contaminants [8-10].In general,there are many advanced oxidation processes,including ozone oxidation,Fenton oxidation,Fenton-like oxidation,photochemical oxidation,and electrochemical oxidation,that are used to degrade refractory organic pollutants in wastewater by strong oxidants (ozone,hydrogen peroxide,etc.).However,these methods have some limitations,such as the poor stability of oxidants that restrict their storage and transport,in addition to the strict pH requirements of the system (2-4)[11].Therefore,a new strategy to resolve the current issue should be developed.Sulfate radicals (SO4·-),as strong single-electron oxides,can selectively and rapidly degrade many pollutants and have peaked the interest of many researchers [9].
In this article,we mainly introduce the persulfate-based advanced oxidation processes (PDS-based AOPs).Compared with·OH,SO4·-has the advantages of high redox potential (2.5-3.1 V) higher than that of·OH (1.8-2.7 V) [12],high selectivity [13],and long half-life (30-40 μs) [14].Furthermore,SO4·-can effectively react with target pollutants in a wide range of pH values (2-8) [15,16].SO4·-can degrade refractory organic pollutants in water and convert them into CO2,H2O,inorganic salt,and other small molecular substances [17,18].A large number of studies have shown that PDS-based AOPs can effectively remove refractory pollutants,such as antibiotics,pharmaceuticals and personal care products (PPCPs),dyes,endocrine-disrupting chemicals (EDCs),chlorinated organic pollutants,and phenolics [19-24].In summary,this technique is effective for the degradation of refractory organic pollutants,has received more attention from researchers and has great potential and advantages for wastewater treatment.
First,this article introduces several different activation methods and mechanisms of persulfate.In addition,the various methods of persulfate (PDS,S2O82-) activation enable its use in the treatment of a variety of contaminants.Therefore,this article describes the application of PDS-based AOP technology in different wastewater treatments in detail.Finally,the article notes the problems associated with this technology and discusses possible solutions to provide a reference for the application of PDS-based AOPs in actual wastewater.
2.Activated approaches for the generation of sulfate radicals
Sulfate radicals can be produced by the activation of peroxymonosulfate (PMS,HSO5-) and peroxydisulfate (PDS,S2O82-)[25,26].Sodium,potassium and ammonia are the normal forms of PDS.Due to its high solubility and low secondary contamination,Na2S2O8is considered the first choice for the most degradation of contaminants [27].PDS and PMS are characterized by the existence of O-O bonds [26],and it has been reported that oxidants with OO bonds can form free radicals that result in contaminant degradation [27].Compared with other oxidants,such as ozone (O3)(2.3 USD/kg),hydrogen peroxide (H2O2) (1.5 USD/kg),and PMS(2.2 USD/kg),the price of PDS is inexpensive (0.74 USD/kg) [27].However,PDS is so stable at room temperature (25 °C) that it is difficult to activate,presenting a low contribution to the degradation of pollutants [28,29].Therefore,various physical and chemical methods are used to activate PDS to generate sulfate radicals.
2.1.Traditional approaches
In the process of PDS activation,the traditional activation methods include thermal activation,alkali activation,ultraviolet(UV) activation,and transition metal ions activation.The different activation mechanisms of various systems have different activation efficiencies and main target pollutants (summarized in Table 1).Taking antibiotics as an example,some common antibiotics (cephalosporin,dichloromethane,carbon tetrachloride,ciprofloxacin,chloramphenicol,and sulfamethazine) were selected as model pollutants [20,21,30-33].Notably,alkali activation,as one of the activation methods of persulfate,has appeared several decades ago.At that time,there were few emerging pollutants like antibiotics and endocrine disruptors.With the emergence of new pollutants,the way of persulfate activation is constantly improved,and it is difficult to find a single case of alkali-activated persulfate for degradation of antibiotics and other pollutants.So it was common for alkali-activated persulfate to degrade chlorine-containing organic pollutants.Therefore,the degradation of chlorinated organics in alkali-activated PDS system is taken as an example.Several activation methods are described in the following sections.
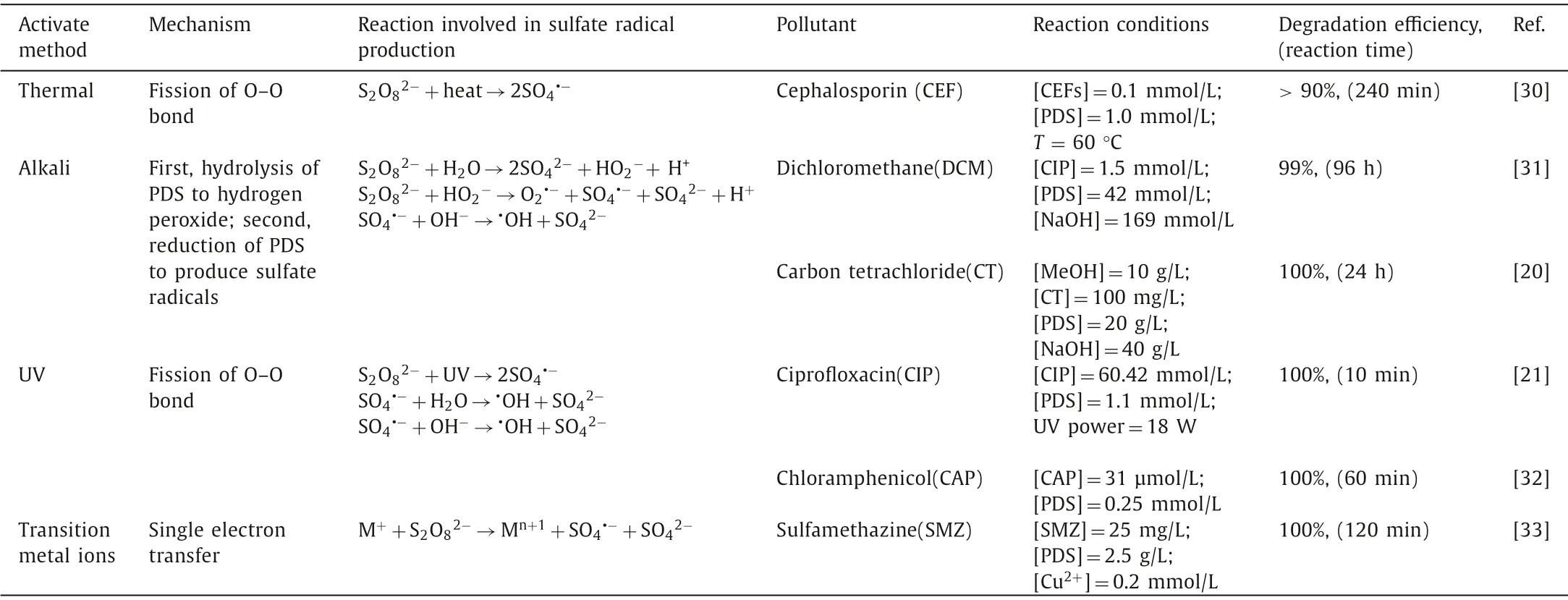
Table 1 Summary of the different PDS activation methods: mechanisms,pathways and examples.
2.1.1.Thermal activation
Thermal activation is one of the most common and simple methods [34].Thermal activation does not require the addition of any other chemicals into the reaction system and only requires that the temperature be controlled to adjust the rate of sulfate radical production [35].In addition,the activation efficiency of PDS increases significantly with increasing temperature [36].When the temperature of the system increases,the O-O bond of PDS absorbs energy and breaks,resulting in the formation of two sulfate radicals (Eq.1) [37].In addition,the reaction of sulfate radicals with water can produce·OH,but the reaction rate is very slow at room temperature (the rate constant ofk[H2O]<2 × 10-3s-1).With increasing system temperature,the amount of·OH is greatly increased (Eq.2) [38,39].In the process of the degradation of chloroxylenol (p-chloro-m-xylenol,PCMX) by thermally activated persulfate,Sunet al.found that with increasing temperature,the pollutants can be completely removed in a short time,which was attributed to the high temperature promoting PDS to produce more active species (SO4·-,·OH) (Fig.1) [40].In general,despite energy consumption,thermal activation is also one of the most efficient methods.
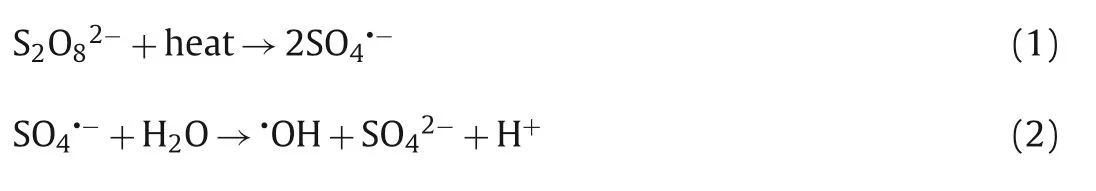
2.1.2.Alkali activation
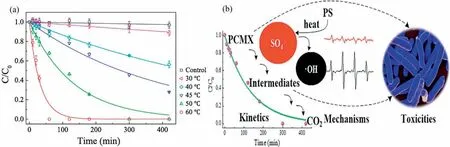
Fig.1.(a) Effect of reaction temperature on PCMX oxidative degradation by thermally activated persulfate ([PCMX]=1.579 mmol/L;[PDS]=1.5 mol/L).(b) The mechanism of thermally activated persulfate for degradation of PCMX (PS stands for persulfate in the graph).Reproduced with permission [40].Copyright 2019,Elsevier.
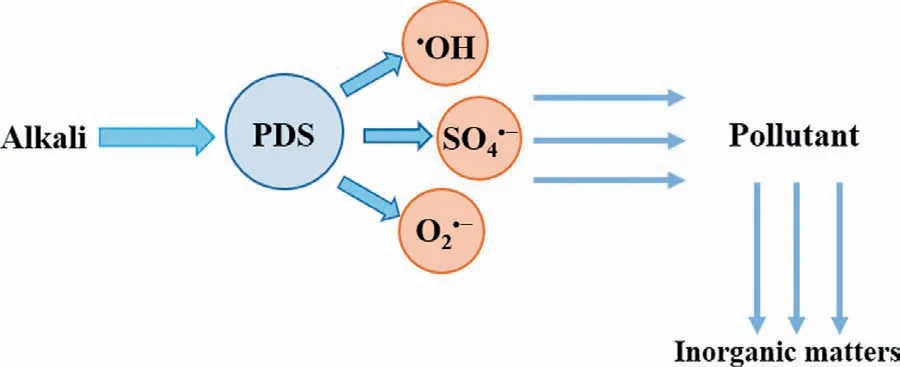
Fig.2.The mechanism of alkali-activated persulfate.
Persulfate can also be activated under alkaline conditions [41].Generally,potassium hydroxide (KOH) or sodium hydroxide (NaOH)is a strong base that can improve the pH value of the solution [27,42].First,PDS is hydrolyzed to hydrogen peroxide anion(HO2-) (Eq.3).Then,PDS is reduced by HO2-to form O2·-(Eq.4)[37,41].In addition,under alkaline conditions,the produced SO4·-in the system can also react with hydroxyl ions (OH-) to form hydroxyl radicals (·OH) to participate in the oxidation reaction (Eq.5)[37].The diversity of active species creates a wide pH range in alkali activation [20].The mechanism of alkali-activated persulfate is shown in Fig.2.For example,Dominguezet al.[31]concluded that in the use ofin situoxidation technology for the degradation of dichloromethane (DCM),the addition of NaOH can effectively activate PDS to achieve the total degradation of contaminants.In the process of degradation of carbon tetrachloride (CT) by alkali/PDS system,although methanol is used as scavenger of·OH and SO4·-[43],methanol can effectively enhance the production rate,stability and reactivity of O2·-,so that CT can be completely removed within 24 h [20].
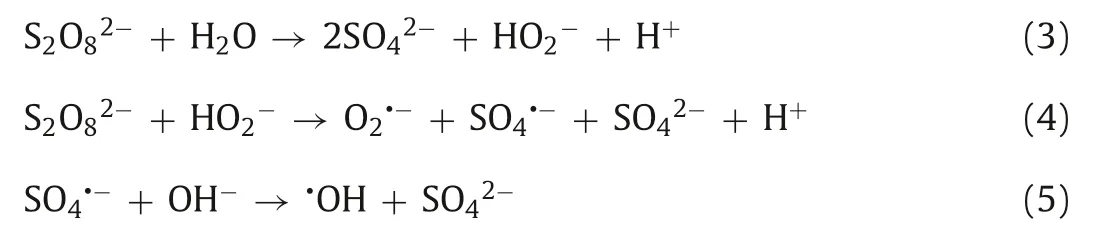
In general,alkali-activated persulfate is mostly used in chlorinecontaining organic wastewater treatment.For some emerging pollutants,such as antibiotics,dyes,and endocrine disruptors,the method of single alkali activation is not widely used.However,the alkali activation method is typically used forin situchemical remediation,and the obvious limitation is that the reaction time of alkali-activated persulfate system is very long,up to several days[31,44].
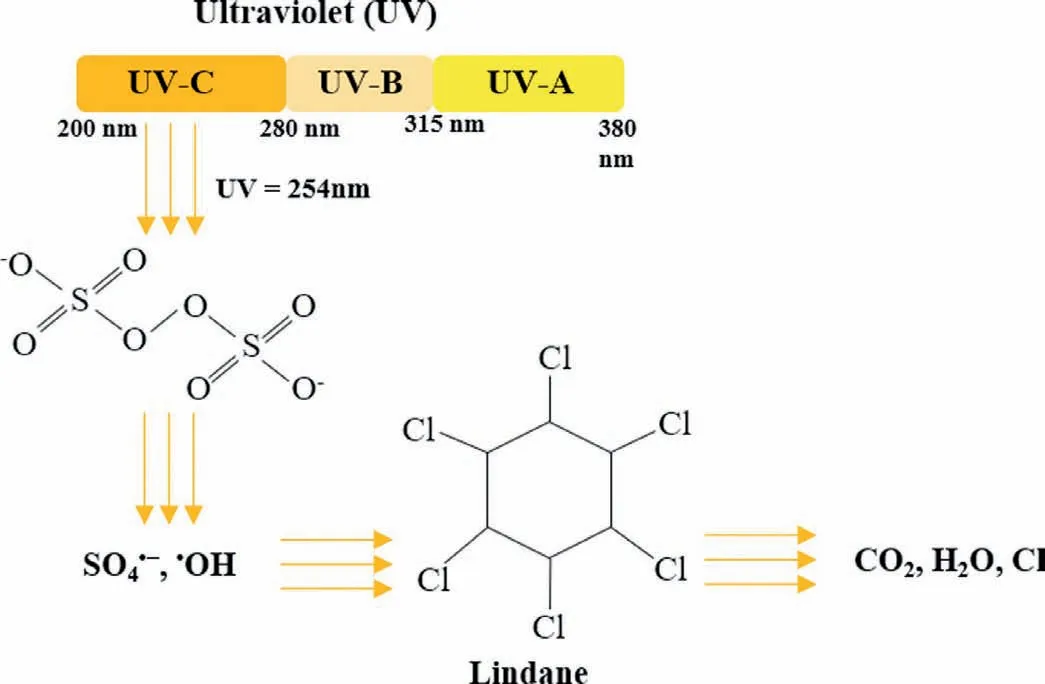
Fig.3.The degradation mechanism of lindane by UV/PDS system.
2.1.3.Ultraviolet activation
The solar spectrum is made up of three parts: 5% ultraviolet (200-380 nm),43% visible light (380-780 nm) and 52% infrared spectroscopy (IR) (780-2500 nm) [45].In addition,ultraviolet light can be carefully divided into UV-A (315-380 nm),UV-B(280-315 nm),and UV-C (200-280 nm) according to wavelength.UV-C is a nonthermal and nonchemical radiation treatment that uses physical energy and is considered safe and nontoxic [46].UVC at 254 nm is widely used in the activation of PDS because its higher energy than UV-A and UV-B,which provides a shorter reaction time for the system [32,47,48].Under UV radiation,the cleavage of the O-O bond results in the activation of PDS,and PDS can be decomposed into two SO4·-(Eq.6) [26].Furthermore,when the pH of aqueous solution increased,SO4·-could further generate·OH (Eq.5) [26,49].In a water treatment project,the degradation rate of lindane increased to 93.2% due to the activation of PDS by UV at 254 nm (Fig.3),and the degradation of lindane followed first-order kinetics [4].In the treatment of phenacetin in wastewater,the SO4·-produced by UV-catalyzed PDS played a beneficial role as the main contributor under neutral conditions [50].

In general,UV activation has the advantage of high efficiency.However,UV light accounts for a small fraction of solar light,and light sources that activate PDS is developing into visible and even solar light [51].As a traditional activation method,UV activation has good application prospects in wastewater treatment.
2.1.4.Transition metal ions activation
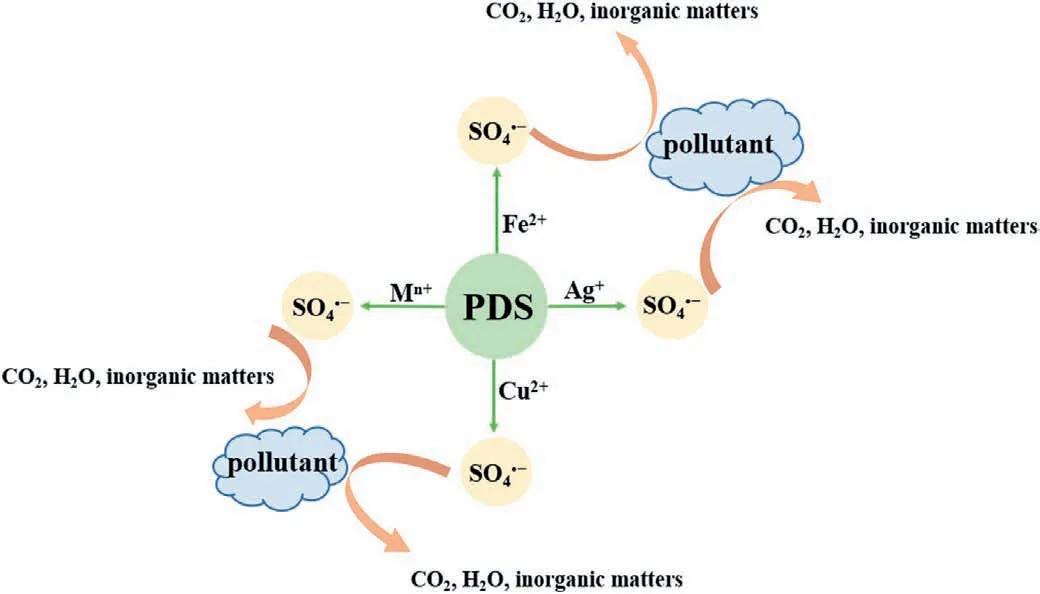
Fig.4.The degradation mechanism of transition metals activate persulfate for pollutants removal (Mn+ stands for transition metal ions in the graph).
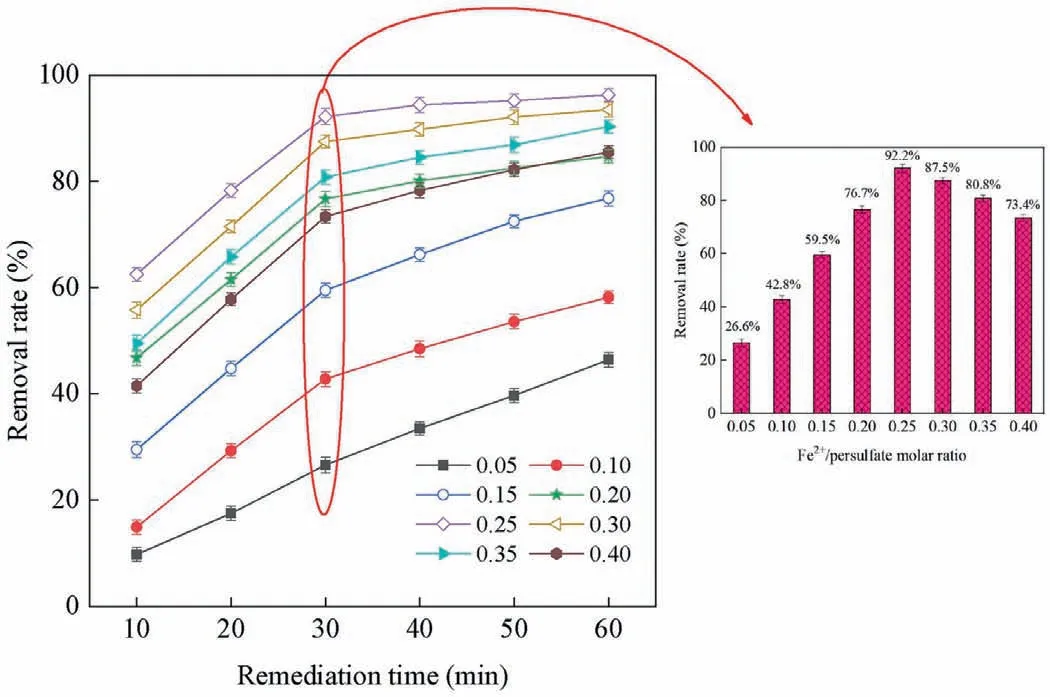
Fig.5.Pyrene degradation in polluted water at different Fe2+/PDS ratios.([pyrene]=100 mg/L;[PDS]=65 mmol/L;pH 7) Reproduced with permission [56].Copyright 2021,Elsevier.
Similar to the Fenton method (Fe2+activates H2O2to produce·OH),low valence transition metal ions (Ag+,Fe2+,Co2+,Cu2+,Mn2+,etc.) can also activate PDS to produce SO4·-.Anipsitakis and Dionysiou investigated the degradation of 2,4-dichlorophenol (2,4-DCP) by activated PDS with different metal ions.By measuring the degradation rate of 2,4-DCP,the result found that silver ion (Ag+)is the most effective catalyst in the activation of PDS,and the process of its activation of PDS can be expressed by Eq.7 [52].Fe2+is widely used in the activation of PDS because of its abundant,nontoxic,and rapid activation of PDS.Iron,as the fifth most abundant element and the second most abundant metal element in Earth’s crust,has a satisfactory price.In addition,iron’s nontoxic nature makes Fe2+/PDS technology environmentally friendly.The process of PDS activation by Fe2+is similar to that of Ag+(Eq.8) [53,54].We can conclude that due to the high reduction potential of these transition metal ions,they can catalyze PDS to produce SO4·-by single electron transfer (Eq.9,where M represents metal ions) (Fig.4) [26,55].For example,in the degradation of pyrene-polluted water,when the optimal molar ratio of ferrous ions to persulfate is 0.25,pyrene can be reduced by 92.2% in 30 min (Fig.5) [56].
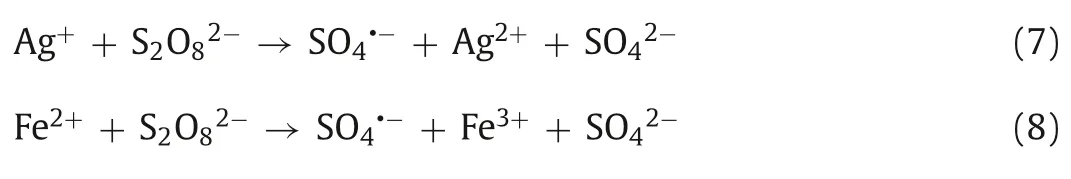
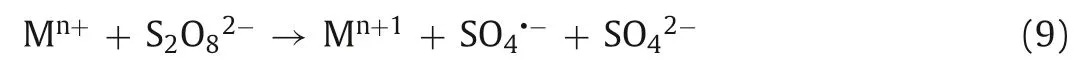
Compared with thermally activated and UV-activated PDS,metal ion-activated PDS does not require additional energy input.However,metal ions are not easy to recover,and this causes secondary pollution to the water after reaction and increases the subsequent treatment technology and difficulty [57,58].
2.2.Emerging approaches
In recent years,to overcome the limitations of traditional approaches,researchers have sought more energy-saving,resourcebased,environmentally friendly,and convenient methods.In addition,some emerging approaches are constantly being proposed and improved.
2.2.1.Microwave activation
Microwaves (MWs) are an electromagnetic spectrum with a frequency range of 300 MHz to 300 GHz [59].In general wastewater treatment,water molecules,as the main medium in the system,can effectively absorb MWs due to their polar structure [60];then,the temperature of the reaction system is instantaneously increased due to the macroscopic thermal effect caused by the rotation and friction between polar water molecules,and PDS,as an electrolyte,is effectively activated at high temperatures under MW radiation to produce free radicals that participate in the oxidation reaction (Eq.10) [43,61].It has been reported that the performance and efficiency of MW irradiation is superior to that of the traditional heating method [62,63].MW irradiation has the advantages of fast and uniform heating,low activation energy,high reaction rate and energy efficiency [64,65].More importantly,the MW/PDS system can offer mild reaction conditions that can solve the problem of equipment corrosion [66].For instance,Huet al.set the MW power at 300 W to activate PDS,and the degradation ofp-nitrophenol (PNP) reached 96.8% within 14 min.In addition,with increasing persulfate dose,the degradation efficiency also increased within a certain range [67].The short reaction time and high treatment efficiency make microwave catalytic persulfate advanced oxidation technology suitable for the treatment of refractory wastewater.

In general,the activation of persulfate by MW irradiation can effectively generate SO4·-by MW power or MW temperature controls.To measure the dominant active species in the reaction system,a quenching experiment was carried out by Zhang’s group.It is well known thattert-butanol (TBA) is used to quench·OH,and methanol (MeOH) is used to quench·OH and SO4·-according to the different reaction rates (Eqs.11-14) [43].In the quenching experiment,it was found that the degradation ability of PNP of the reaction system was slightly limited in the presence of TBA but significantly decreased in the presence of MeOH (Fig.6) [67].This completely shows the formation of·OH and SO4·-in the system,and SO4·-plays an important role in the MW/PDS system.
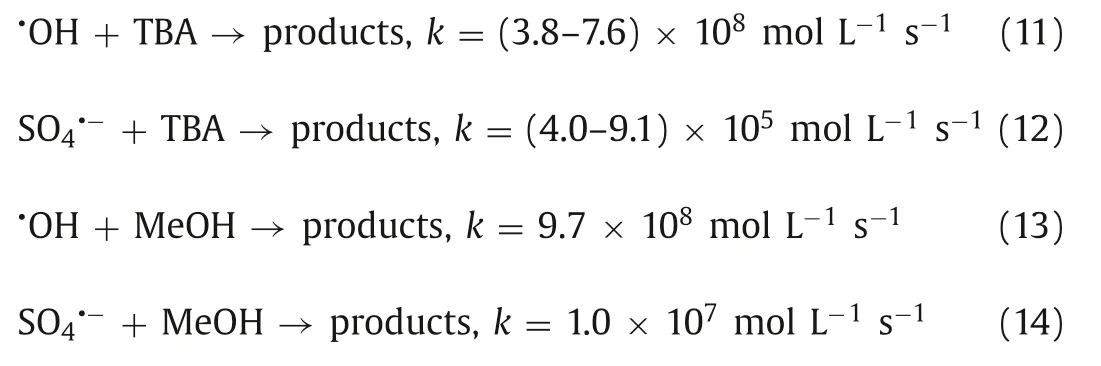
2.2.2.Metal oxide activation
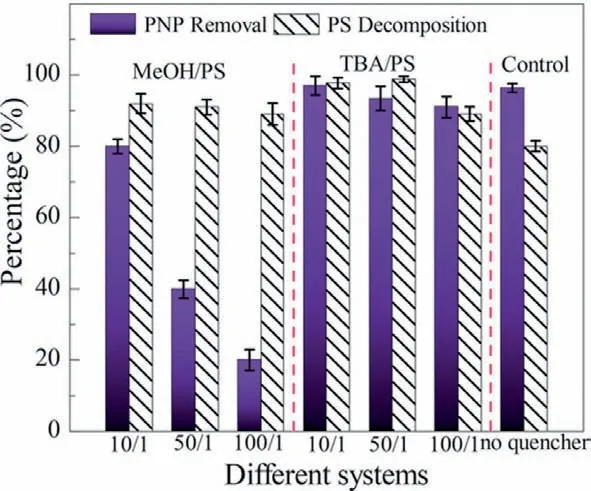
Fig.6.Quenching experiment.([PNP]=20 mg/L;[PDS/PNP]molar=15/1;MW power=300 W) (PS stands for persulfate in the graph).Reproduced with permission [67].Copyright 2019,American Chemical Society.
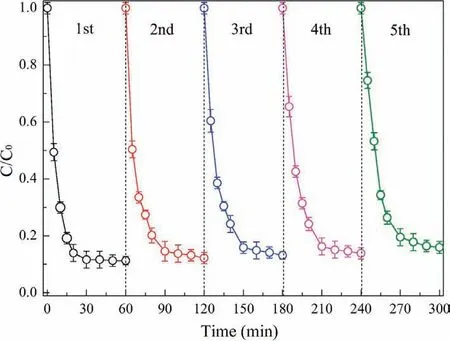
Fig.7.Operational life of CuFe2O4 nanoparticles in the CuFe2O4/PDS system.([PNP]=50 mg/L;[CuFe2O4]=30 g/L;[PDS]=8 mmol/L;pH 7) Reproduced with permission [71].Copyright 2017,Elsevier.
Compared with transition metal ions,metal oxides have been gradually applied because they are ferromagnetic and easy to recycle as heterogeneous catalysts without secondary pollution [68].As mentioned above,in transition metal ions,Fe2+is not easy to recycle,and excessive Fe2+will reduce the amount of SO4·-(Eq.15),affecting the reaction efficiency [69].As a result,the introduction of metal oxide can effectively avoid these defects.In the process of PDS activation by metal oxides,metal ions on the surface of the metal oxides activate PDS to produce free radicals.Considering Fe3O4as an example,first,in a heterogeneous system with Fe3O4as a catalyst,Fe2+is constantly generated on the surface of Fe3O4to activate persulfate to produce free radicals (Eq.8).Second,combining N2adsorption-desorption isotherms and BJH desorption pore diameter distribution measurements shows that Fe3O4has a large specific surface area,which can provide enough reaction sites for redox reactions to accelerate the removal of pollutants.Finally,Fe3O4has good reusability,and the removal rate of PNP is still higher than 90% after three Fe3O4recovery cycles [70].Similarly,in the removal of PNP from water,the CuFe2O4catalyst still maintains high catalytic activity after five recycling cycles (Fig.7) [71].
The introduction of heterogeneous systems provides a new direction for the development of water treatment methods.Currently,Fe2+is considered a good activator of persulfate since other transition metal ions are toxic [72].Therefore,iron oxides with abundant resources and favorable prices are widely valued in wastewater treatment [73].Zero valent iron (ZVI,Fe0) is widely used as a source of Fe2+because it can continuously release Fe2+into the solution and effectively recycle Fe3+on its surface [74].Wanget al.found that in the process of using ZVI to activate PDS to remove alachlor,alachlor can be degraded completely in 60 min.More importantly,these authors found that the transformation of Fe3+to Fe2+was improved (Eqs.16 and 17) [75].In particular,Nidheesh’s team pointed out the controlled release of Fe2+occurs in persulfate solutions (Eqs.18 and 19) [76].In addition,iron oxides such as hematite,goethite and ferric oxide can also provide Fe2+as iron sources [58,77].
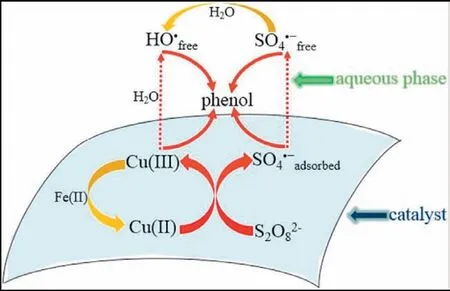
Fig.8.The mechanism for phenol degradation in the CuO/Fe3O4/PDS system.Reproduced with permission [22].Copyright 2015,American Chemical Society.
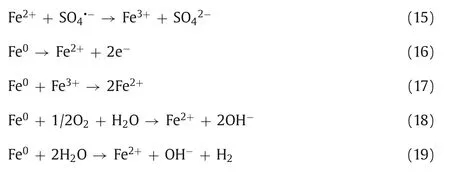
With the continuous exploration of the properties of metal oxides,based on the previous research,increasing numbers of researchers tend to synthesize new materials,such as bimetal or even polymetallic oxides,which are used in persulfate systems.For example,Leiet al.prepared magnetic CuO/Fe3O4composite materials to activate PDS to degrade phenol.Within 120 min,phenol was almost completely mineralized,and the mechanism of action is shown in Fig.8 [22].MnCuS nanocomposites continuously provide active sites to activate persulfate in order to produce sulfate radicals by redox reactions between Cu2+/Cu+and Mn3+/Mn2+and have good stability and recovery performance in this system[78].Wu’s team also found that the NixCo3-xO4system can effectively degrade tetracycline (TC) in wastewater due to the synergistic effect of Ni3+/Ni2+and Co3+/Co2+[79].Additionally,there are other metal oxides used to degrade TC in wastewater.Table 2 lists some metal oxides that degrade TC and have achieved good treatment effects [72,79-84].
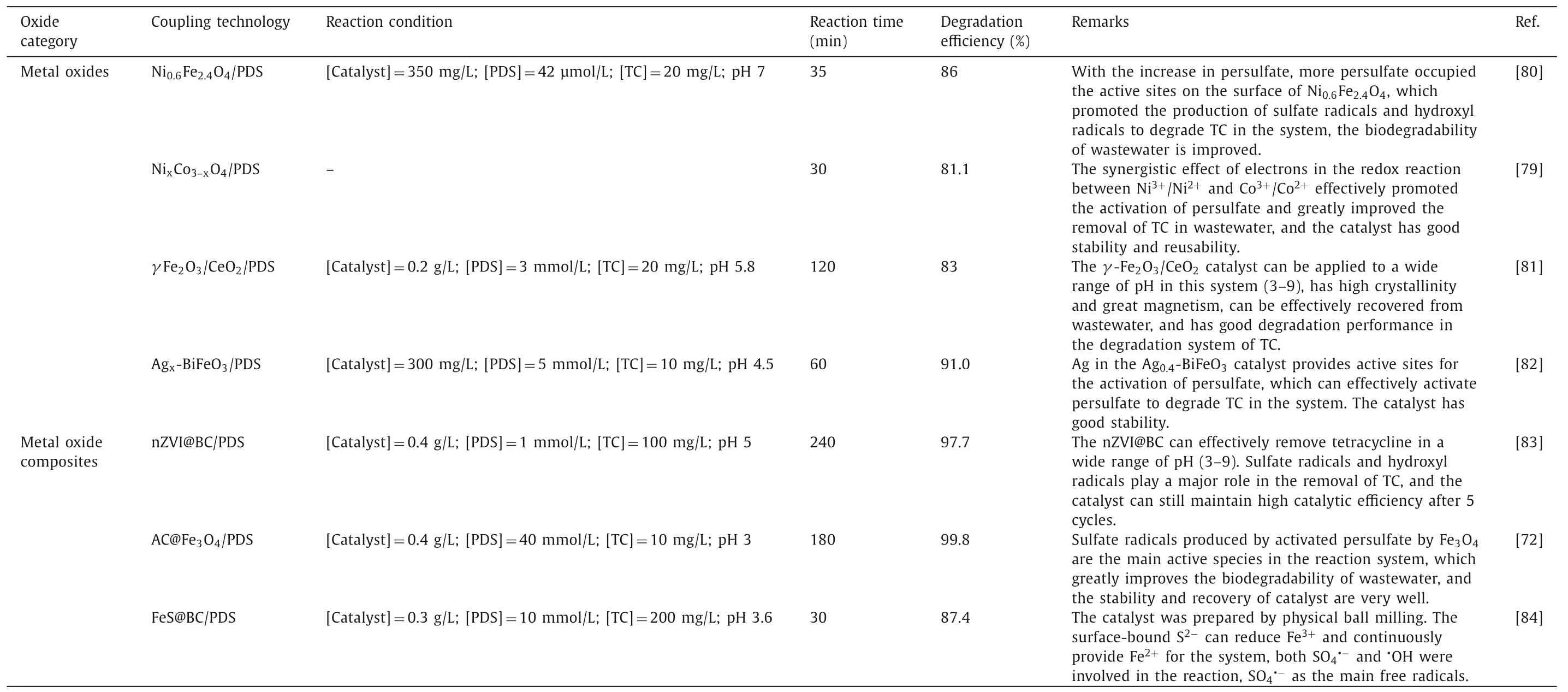
Table 2 List of some studies on the degradation of TC by PDS activation with various metal oxides as the catalyst.
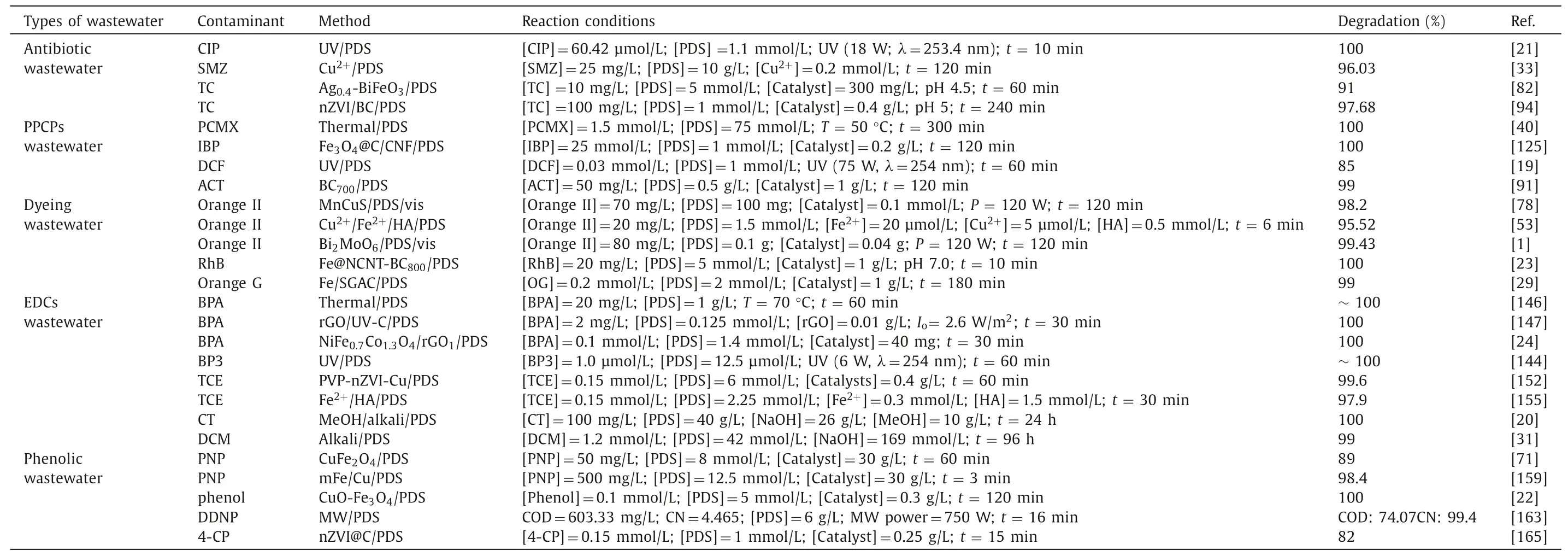
Table 3 List of different PDS-based AOPs in organic wastewater treatment.
2.2.3.Carbon activation
Carbonaceous materials have attracted substantial attention due to their nonmetallic and abundant resources [85,86].Carbon-based materials are typically used as electron donors of PDS to promote the formation of sulfate radicals (Eq.20) [26].In addition,Xia’s team also pointed out that carboxyl,carbonyl and other catalytic parts enhanced the activation of PDS by forming delocalizedπelectrons (Eq.21) and proposed an electron transfer mechanism on the oxygen functional groups on the surface of activated carbon(AC) (Eq.22) [26].
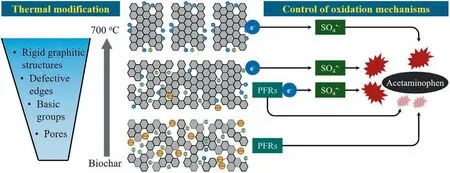
Fig.9.The degradation mechanism of BC700/PDS system to degrade ACT.Reproduced with permission [91].Copyright 2020,Elsevier.
As a kind of carbonaceous material,biochar (BC) is produced by the pyrolysis of wood,straw and other organic materials [87].Biochar has a series of functional structures,such as oxygencontaining groups and pore structures,that can provide active sites for the reaction system [88].Due to its low cost and environmental friendliness,biochar is increasingly being used as a persulfate catalyst to participate in the advanced oxidation process[89].Fanget al.found that biochar can activate PDS to produce SO4·-to degrade polychlorinated biphenyls (PCBs) in solution [90].Similarly,Kimet al.extracted biochar from apple tree pruning waste and thermally modified it.It was found that among different thermal modification temperatures,when the temperature was set to 700 °C,biochar had the most developed graphite structure and defects,which effectively activated PDS to produce SO4·-and achieved excellent acetaminophen (ACT) degradation.ACT removal was attributed predominantly to SO4·-generation accepting electrons from persistent free radicals and the graphitic structures (Fig.9) [91].In addition,it has been reported that biochar can effectively promote the removal of 2,4-DCP from wastewater by PDS due to its high specific surface area and hierarchical porous structure.·OH plays an important role in this system [92].
In addition to the preparation of biochar by pyrolysis,metal loading is considered one of the methods for improving the utilization efficiency of biochar.Biochar can be used as a catalyst carrier to prevent the aggregation of metal nanoparticles and provide more active sites [88,93].For example,nZVI particles loaded on biochar can effectively degrade TC in a wide range of pH values(3-9).Moreover,the biochar-supported catalyst still maintained high catalytic activity after five recycling cycles [94].Zhuet al.synthesized bamboo-like carbon nanotubes doped with nitrogencontaining nano-iron particles (Fe@NCNT-BC),which achieved the complete degradation of rhodamine in an Fe@NCNT-BC/PDS system within 10 min [23].
Another way to improve the utilization efficiency of biochar is the doping of heteroatoms.Due to the similar size of nitrogen and carbon atoms,nitrogen doping has attracted substantial attention.Because the electronegativity of nitrogen is higher than that of carbon,it easily forms Lewis base sites,while the difference in electronegativity contributes to the formation of defect structures.Thus,the electronic transfer capability can be accelerated [95-98].g-C3N4,a representative carbonaceous material loaded with N,with the characteristics of the low cost,biaxial structure,is the most stable carbon nitride allotrope [99].It is difficult to recycle carbonaceous materials because of their good dispersibility in solution.Therefore,many researchers focus on metal ions or metal oxides loaded on modified carbon materials as catalysts to activate persulfate and improve its recovery capacity [93,100].Wanget al.used ferrocene modified g-C3N4to activate persulfate and degrade TC in water under the assistance of visible light.On the one hand,the combination of ferrocene and g-C3N4inhibited the electron-hole pair recombination in g-C3N4and expanded the light response range.On the other hand,the iron cycle on ferrocene surface could activate persulfate and generate sulfate radicals.Finally,the catalyst can also make good use of its ferromagnetism to be recovered from the solution after the reaction,increasing its reusability [101].
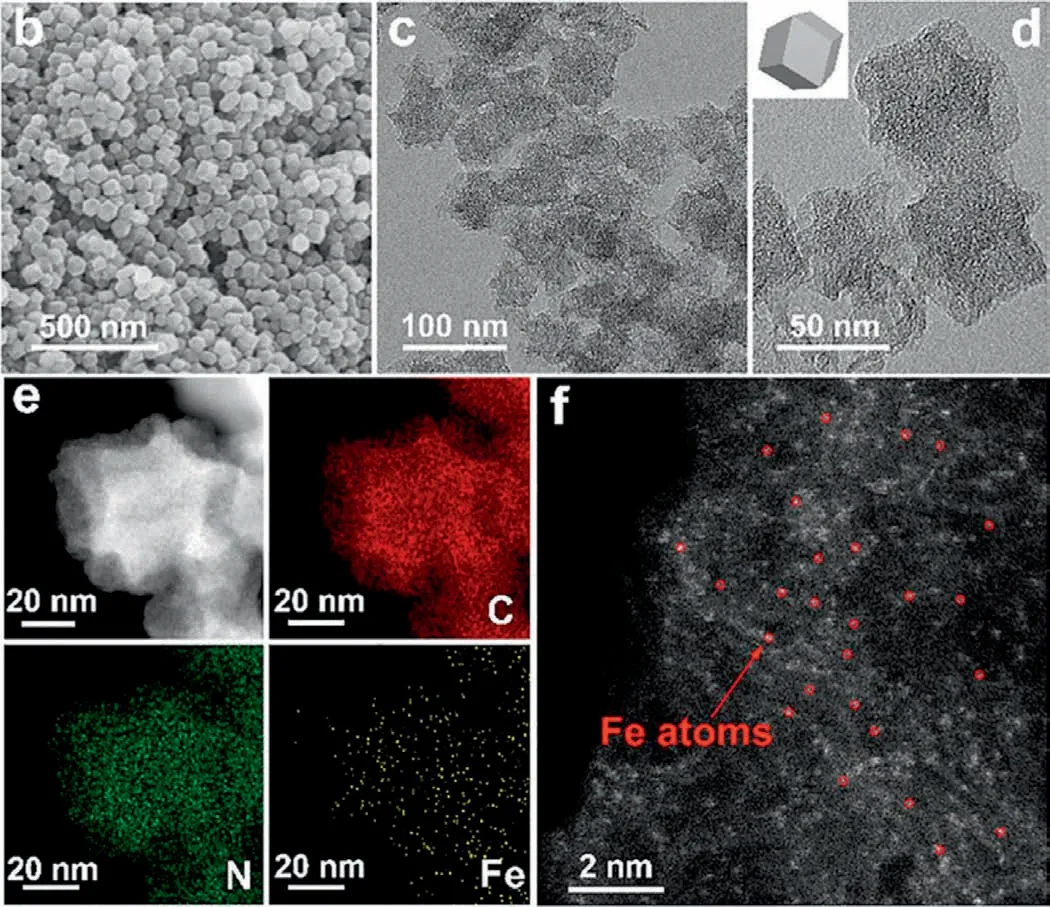
Fig.10.SEM and TEM images of SAFe-N-C.Reproduced with permission [103].Copyright 2021,Elsevier.
Moreover,other modified carbon materials are also good for persulfate activation due to their porous structure.Mao’s team synthesized a nitrogen-doped porous Co@C nanoboxes (Co@NC) embedded with cobalt nanoparticles,which activated persulfate effi-ciently and completely removedp-chloroaniline (PCA) in 2 min.It is noted that Co@NC shows very limited leaching of cobalt ions(the dissolution proportion of cobalt ions is 0.28%) [102].Duet al.synthesized single-atom iron anchored on nitrogen-doped carbon (SAFe-N-C) with iron phthalocyanine (FePc) and metal-organic framework (ZIF-8).The SEM and TEM images showed that SAFe-N-C presented a regular rhomboidal dodecahedron structure.The precipitated Fe atoms were captured by the surrounding N-C defects,and the formed Fe-Nx sites were well dispersed on the surface of N-C carrier to activate persulfate for the degradation of chloramphenicol (Fig.10) [103].Liuet al.synthesized a composite catalyst Cu/RGO that could also activate persulfate well.This synthesis method not only reduced the amount of copper (only 0.075 times of the previously reported zero-valent copper),but also the mineralization rate of 2,4-dichlorophenol can reach 69.2%within 30 min [104].On the whole,the combination and interaction of metal and non-metal with modified porous carbon have good application prospects in persulfate activation.
In addition to the radical pathway,carbon activated persulfate also exist a non-radical pathway.Renet al.proposed that PDS were adsorbed on the surface of CNT and activated to form CNT-PDS*complexes with high redox potential when used CNT/PDS system to degrade phenolic compounds (PCs) (Fig.11),and then the coadsorbed PCs were oxidized to products with low potential,which was essentially an electron transfer mechanism [105].It is worth noting that due to the potential differences in the micropollutants,the non-radical pathway of the transfer of electrons from the micropollutants to reactive complex is actually a "compulsory" electron transfer process [97].Renet al.also pointed out that the doping of compounds can improve the redox potential of CNT-PDS*,which is crucial for the decomposition of pollutants in non-radical pathways [97].
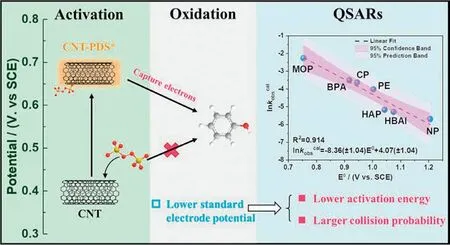
Fig.11.The non-radical pathway of CNT/PDS/PCs system.Reproduced with permission [105].Copyright 2019,American Chemical Society.
3.Application of PDS-based AOP treatment in organic wastewater
Common wastewater can be divided into antibiotic wastewater,PPCP wastewater,dye wastewater,EDC wastewater,chlorinated organic wastewater,and phenolic wastewater.Various PDS-based AOPs have been used to treat different organic wastewaters,and these PDS-based AOPs will be described in the following section.Some different wastewater treatment technologies are listed in Table 3.
3.1.Antibiotic wastewater
Antibiotics are chemical therapeutic agents used to treat infectious diseases of the humans,animals,and plants.Antibiotics can prevent the growth of and even inactivate microorganisms[106,107].Studies have found that low doses of antibiotics can be used to promote the growth of animals,so antibiotics are also widely used as feed additives.Therefore,antibiotics were increasingly being detected in wastewater,and their concentrations have increased over time.Cephalosporins (CEFs) are easy to hydrolyze and widely exist in the environment.The presence of CEFs in the environment may lead to the emergence and spread of antibioticresistant bacteria,which has attracted public attention [108].Antibiotic wastewater is usually characterized by strong antibacterial activity,low biodegradability and high chroma,which is a challenge in environmental treatment [109].
CEFs are widely used because of their good antibacterial activity,accounting for approximately 50%-70% of the total amount of antibiotics used [110,111].A study pointed out that compared with surface water and wastewater,the thermal/PDS system performed better in removing CEFs from high-salinity hospital wastewater[30].Ciprofloxacin (CIP) is one of the most pervasive antibiotics in wastewater treatment plants and is mostly used to treat infectious diseases.Dewil’s team studied the degradation of CIP by UVactivated persulfate and found that CIP antibiotics have a good removal effect and were completely removed within 10 min [21].It is worth noting that under similar conditions,the degradation of CIP by PDS occurs much more quickly than that by PMS,and the reaction time of PMS/CIP is six times longer than that of PDS/CIP,which may be attributed to the activity of radicals produced in different system is different [21,112].Sulfonamide (SMZ) antibiotics are widely used in the veterinary industry and are discharged into the environment with animal feces or urine.Zhou’s group was the first to use transition metal ions to activate PDS to degrade SMZ.In this study,Cu2+,with the best comprehensive efficiency,was selected as the activator to achieve excellent removal efficiency of SMZ within 120 min [33].In addition,the SMZ can also be completely removed by thermal activation of PDS within 90 min at 60 °C [113].
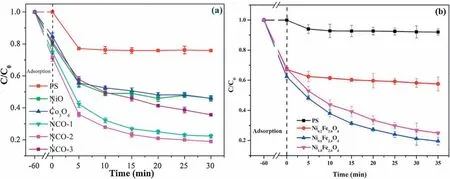
Fig.12.(a) Effects of NixCo3-xO4 on PDS activation for TC removal ([catalyst]=300 mg/L;[PDS]=105 μmol/L;[TC]=10 mg/L).Reproduced with permission [79].Copyright 2020,Elsevier.(b) Effects of Ni0.6Fe2.4O4 on PDS activation for TC removal ([catalyst]=300 mg/L;[PDS]=52.5 μmol/L;[TC]=20 mg/L) (PS stands for persulfate in the graphs).Reproduced with permission [80].Copyright 2018,Elsevier.
TC,as a broad-spectrum antibiotic,is widely used in medicine and animal husbandry [114].In recent years,researchers have used many different activated persulfate methods to degrade TC in water.Ouyanget al.used the sol-gel method to prepare Ag-doped bismuth ferrite catalyst Ag0.4-BiFeO3,which can effectively remove 91% of TC in the system at a rate constant of 0.033 min-1.The results showed that silver doping provides active sites for the activation of persulfate and promotes the degradation of TC [82].Yuan’s team and Zhang’s team synthesized the bimetallic oxides CeO2/Co3O4andγ-Fe2O3/CeO2,respectively.Both have a good removal effect on TC.In the same way,SO4·-and·OH were the active species of the reaction system,and repeatability experiments showed that the two bimetallic catalysts have good stability and reusability [81,115].Wu’s team and Wang’s team synthesized magnetic catalysts,namely,NixCo3-xO4and Ni0.6Fe2.4O4,respectively,to activate PDS to degrade TC.The catalysts showed excellent catalytic performance,and the synergistic effect of metal ions (cyclic redox reaction between metal ions) promoted the degradation reaction (Fig.12) [79,80].The synthesis of nano ZVI supported on biochar (nZVI/BC) catalyst was applied to the degradation of TC,the persulfate was effectively activated,and the degradation rate was approximately 98% [94].
In addition,many researchers have also studied the removal of chlortetracycline [116],chloramphenicol [32],sulfamethoxazole[117],sulfadiazine [118,119]and other antibiotics by activating persulfate.Herein,PDS-based AOPs have great application potential for antibiotics.
3.2.PPCPs wastewater
PPCPs are often detected in various water bodies because they are widely used in medical treatment,cosmetics,animal husbandry and aquaculture [120-122].PPCPs are stable and do not disintegrate by themselves,so they have strong persistence.In addition,PPCPs have potential adverse effects on human health and aquatic ecosystems because they are poisonous.Therefore,PPCPs are considered one of the most urgent environmental problems and have attracted great attention [123,124].
Ibuprofen (IBP),a common PPCP,is mainly used to relieve pain,inflammation,and fever due to its anti-inflammatory properties.Sun’s group constructed a Fe3O4@C/CNF/PDS system to degrade IBP in water.Carbon nanofiber (CNF) carriers can effectively avoid the agglomeration of Fe3O4,and catalysts with large specific surface areas can effectively activate PDS,which can achieve the complete degradation of IBP [125].Zhanget al.synthesized carbon dots and nanomagnets encapsulated in pomegranate-like amorphous carbon spheres and found that the catalytic materials can activate bisulfate,hydrogen peroxide,and persulfate well;among these compounds,persulfate has a good efficiency (100%) for removing IBP.The large specific surface area of nano Fe3O4can provide enough active sites for the reaction system,while the excellent electron donor and electron acceptor ability of carbon dots promote the degradation of IBP [126].Rodriguezet al.explored the effect of persulfate activated by Fe2+,Fe3+and ZVI on IBP removal.That paper pointed out that ZVI could continuously activate persulfate to produce SO4·-by controlling the slow formation of Fe2+,improve the utilization efficiency of oxidant,and achieve almost complete removal of IBP [127].Similarly,ZVI-activated persulfate can also degrade propranolol (PRO),which is a drug with ecological toxicity and bioaccumulation properties [128].
PCMX,a representative PPCP,is widely used as an antibacterial disinfectant,an active agent in hand sanitizer,and a preservative in the cosmetics and dyestuff industries owing to its excellent antibacterial properties.Zhang’s team studied the degradation of PCMX by thermally activated persulfate and its toxic changes.The study found that with increasing temperature in the range of 30 °C to 60 °C,SO4·-is continuously generated,and finally,the system realized the complete mineralization of PCMX.Additionally,toxicological experiments also showed that its ecological toxicity is greatly reduced [40].In addition,a researcher modified biochar from apple pruning waste to degrade paracetamol.The degradation mechanism is attributed to the generation of SO4·-and the electron donor and acceptor of the graphite structure in biochar [91].Diclofenac (DCF) is widely used as a sodium salt in the clinic and is also widely used in the treatment of painful inflammatory rheumatoid and non-rheumatoid diseases.Luet al.used UV at 254 nm to activate persulfate to degrade DCF.The degradation of DCF followed pseudo-first-order kinetics (1.0 × 10-3s-1).The study also pointed out that the UV/PDS system is an effective process for removing diclofenac in water [19].
In general,PDS-based AOPs can be used for the successful degradation of PPCPs.However,there are many types of PPCPs,which need to be further systematically studied.
3.3.Dyeing wastewater
Half of dyeing wastewater originates from the textile industry,followed by the printing and dyeing industry,paper and pulp industry,leather and paint industry and dye manufacturing industry,because dyes are an important component of these products [129,130].High chroma,high biochemical oxygen demand,and high dissolved solid content are the notable characteristics of dyeing wastewater;in actual production and application,to maintain the integrity of the color and structure of the fuel,the dye also has very low biodegradability,which increases the difficulty of treating dyeing wastewater [131].Among all dyes,azo dyes are the most commonly used and are easily converted into dangerous aromatic amines in oxygen-deficient environments;thus,these dyes have a potential impact on the human body and other aquatic organisms[132,133].
Acid orange 7 (AO7) dye,a representative azo dye,has a complex and stable structure and low biodegradability.Yang’s team tried to degrade AO7 with PMS,PDS and H2O2.Under the condition of heating,PDS was activated effectively,and the removal effect of AO7 was better than that of the other two (the decolorization rate was close to 100%).Under the assistance of UV light,all three oxidants were effectively activated,and the removal effi-ciency of PDS was still superior [134].Orange II is another kind of azo dye that is used for dyeing in industrial production processes and has toxic effects.Zhouet al.used a one-pot hydrothermal method to synthesize MnCuS nanocomposites to activate persulfate to degrade orange II.Under visible light,the removal efficiency of orange II was close to 100% within 120 min (Fig.13).In addition,the catalytic material has excellent reuse performance,and after three cycles of degradation,the removal efficiency can still reach 97.1% [78].Wanget al.studied the degradation of orange II by persulfate activated by a transition metal ion coupling system,that is,adding trace copper ions into an iron ion-hydroxylaminepersulfate system.The addition of trace copper ions and the synergistic effect between metal ions (cyclic redox reaction between copper ions and iron ions) broaden the pH range in which orange II can be degraded,which provides a certain basis for the actual treatment of orange II dyeing wastewater [53].Zhuet al.synthesized Bi2MoO6microspheres by a hydrothermal method to activate persulfate to degrade orange II under visible light.The excellent degradation efficiency can be attributed to the irregular nanoplates on the surface of microspheres and the active species produced during the reaction (O2·-,SO4·-,·OH) [1].Sudan dyes are commonly used in textile and food processing for coloring.Shuchiet al.first used the method of thermal activation persulfate to degrade the dye wastewater of Sudan.The total degradation of Sudan Black B was achieved in 17 min at the highest potassium persulfate dosage (750 ppm) and the optimum temperature (60 °C),which also had an excellent removal effect on the chroma.However,the dose of persulfate was relatively high,and it was easy to increase the salinity of follow-up water [135].In the MW-activated persulfate system,many other azo dyes,such as orange G (OG) [136],X-3B [137],and methyl orange [138],can also be removed well.
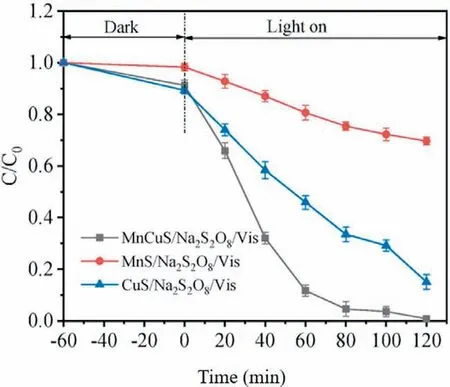
Fig.13.The degradation curves of orange II in the MnCuS/Na2S2O8/visible light system by using 0.1 mmol of MnS,CuS and MnCuS.Reproduced with permission [78].Copyright 2021,Elsevier.
In addition to azo dyes,other dyes are also degraded well by PDS-based AOPs.Zhuet al.synthesized N-doped carbon nanotubes encapsulated with Fe nanoparticles as activators of persulfate to degrade rhodamine B (RhB) dye.RhB was completely degraded in 10 min under neutral conditions [23].There are many studies on the use of the PDS-based AOPs for removing azo dyes.It is worth noting that the assistance of visible light can effectively promote the degradation of dyes and the removal of chromaticity.
3.4.EDCs wastewater
EDCs are chemicals that interfere with human hormones and physiological functions.EDCs are easy to cause metabolic disorders.EDCs usually come from industrial chemicals,personal care products,and pharmaceuticals [139-141].Benzophenone-3 (BP3) is one of the most widely used UV filters because of its excellent UV absorption properties.It has been identified as a phenolic endocrine disruptor [142,143].Lee’s study showed that the removal efficiency of BP3 by UV/PDS was better than that by UV/H2O2,and the toxicity of BP3 decreased faster in the UV/PDS system [144].In addition,Wang’s team explored the mixed removal of nonylphenol (NP)and triclosan (TCS) by thermally activated persulfate and studied the interaction of the two substances,the polymerization products produced and the reaction path in detail,which provided an important reference for the removal of mixed endocrine disruptors in wastewater [145].
Bisphenol A (BPA) is one of the most representative endocrine disruptors and is widely used.Potakiset al.used thermally activated persulfate to degrade BPA.The study found that the activation temperature was an important reason for the degradation of BPA,and the presence of chloride promoted the degradation of BPA.Finally,the toxicity test showed that the toxicity of BPA towardV.fischeridecreased during the reaction (inhibition ofV.fischeriactivity decreased from approximately 50%to approximately 14%) [146].Ozyildizet al.studied the effect of homemade reduced graphene oxide (rGO) on the oxidation of BPA in a UV/PDS system.The existence of rGO significantly promoted the degradation of BPA,and the complete removal of BPA was achieved within 30 min [147].Similarly,He’s team synthesized NiFe0.7Co1.3O4-rGO1nanocomposites to activate persulfate to degrade BPA,which has excellent catalytic activity,leading to better removal of BPA from the system with less persulfate.In addition,the study also pointed out that NiFe0.7Co1.3O4-rGO1can degrade BPA in river water and secondary effluents,which provides a feasible method for the removal of organic pollutants [24].In addition,it has been reported that metal sulfides can activate persulfate and enhance the degradation of BPA,trichlorobiphenyl and other pollutants.Surface metal active sites and reduced sulfur species accelerate the regeneration of low valence metal ions and enhance the reaction efficiency of the system [148].
3.5.Chlorinated organic wastewater
Chlorinated organic compounds are organic compounds obtained by replacing hydrogen atoms with chlorine atoms,which have high toxicity and deformity and pose a serious threat to human health [149].The toxicity of the compounds increases with increasing chlorine atoms.Most chlorinated organic compounds are manufactured by hand and are widely used as intermediates in industrial production,degreasers and solvents,such as pesticides and refrigerants [150,151].
Trichloroethylene (TCE) is a chlorinated organic substance mainly used in industrial production that widely exists in water environments due to improper disposal [152].TCE is cytotoxic and carcinogenic,and long-term exposure may damage the human central nervous system and cause harm to the human body [153,154].Idreeset al.synthesized polyvinylpyrrolidone-coated nano ZVIcopper bimetallic particles (PVP-nZVI-Cu) to activate persulfate in order to degrade TCE.PVP effectively prevented iron aggregation and condensation.The system effectively degraded TCE,and the de-chlorination rate reached 83.3% [152].Shanet al.loaded nZVInickel bimetallic materials onto biochar (nZVI-Ni@BC) to degrade TCE by activating persulfate and achieved the complete degradation of TCE.SO4·-and·OH were the main free radicals in the system [151].Wuet al.added hydroxylamine (HA) to the Fe2+/PDS system for the first time to promote the transformation of Fe3+to Fe2+,accelerate the regeneration of Fe2+,and prevent the formation of precipitation so that the system could better degrade TCE[155].
In addition,CT is a widely used chlorinated volatile organic compound with relatively high solubility (0.78 g/L) and low biodegradability,and it is listed as a priority pollutant by the US Environmental Protection Agency (EPA) [150].Dominguezet al.used alkali-activated persulfate to degrade CT and methanol as a cosolvent with less polarity than water,which led to the complete degradation of CT.In addition,the study indicated that among the free radicals produced (including O2·-,SO4·-,·OH),O2·-was the only active species that caused CT degradation [20].Lindane,a kind of hexachlorocyclohexane isomer (HCH)-γ-isomer (γ-HCH),is a common pesticide and has strong insecticidal properties [156].Santoset al.used alkali to activate persulfate to degrade lindane.It was found that when the pH was higher than 12,a rapid hydrodechlorination reaction took place,and lindane in the system was adequately removed [44].DCM is a colorless liquid chlorinated organic pollutant that is volatile and is usually used as a degreasing agent for citrus fruits,metal degreasing,and paint removal solvents.[31].DCM was listed as a possible human carcinogen by EPA.Dominguezet al.used alkali-activated persulfate to degrade DCM,and the system almost achieved complete degradation of DCM [31].Furthermore,surfactant-enhanced alkali-activated persulfate was used to remove chlorinated organic compounds [157].Alkali-activated persulfate technology has been proven to be an effective method for the treatment of chlorinated organic pollutants.
3.6.Phenolic wastewater
Phenols are commonly used in industrial production and daily life.Phenols are aromatic pollutants with high biological toxicity and low biodegradability.Long-term exposure to phenols damages the human nervous system,so their use needs to be effectively controlled [92].
PNP,as an important organic chemical raw material and intermediate,is widely used in manufacturing pesticides,dyes,rubber chemicals,pesticides,and plasticizers.[158,159].Liet al.synthesized magnetic CuFe2O4nanoparticles by a sol-gel-combustion method as a catalyst to activate persulfate in order to degrade PNP,and a good PNP removal effect was achieved (89%).The study also discussed the degradation pathway and mechanism of action in depth,which had reference significance [71].In addition,Jiet al.used microscale Fe-Cu bimetallic nanoparticles (mFe-Cu)to activate persulfate in order to degrade PNP.The synergistic effect between PDS and mFe-Cu effectively removed 98.4% of PNP in the system (Fig.14a).In addition,the reaction had a good reaction rate constant (1.91 min-1) and a rapid reaction time (3 min)(Fig.14b) [159].Additionally,Zhanget al.used the mFe/Cu-air-PDS system to degrade PNP,and the synergistic effect of copper ions and iron ions (cyclic redox reaction between copper ions and iron ions) not only overcomes the ease with which iron reactivity is affected by surface passivation but also effectively promotes the degradation of PNP.After 60 min,the removal rates of chemical oxygen demand (COD) and TOC were 71.0% and 65.8%,respectively [160].Leiet al.synthesized a CuO-Fe3O4bimetallic material through a hydrothermal method to activate persulfate.The presence of HCO3-is helpful to the degradation of phenol,and the system can completely remove phenol in 120 min [22].Sunet al.doped Mg on the bimetallic oxide CuO-Fe2O3as the activator of persulfate to degrade phenol.The doping of Mg provided more oxygen vacancies,resulting in the production of more active substances in the reaction process,which effectively promoted the degradation of phenol.This study also proposed the degradation pathway of phenol [161].Qiuet al.synthesized an N-doped mesoporous carbon catalyst (N-OMC) to activate PDS to degrade phenol,and the degradation of phenol conformed to pseudo-first-order kinetics (5.58 min-1).It is worth noting that PDS was mainly activated by the nonradical process of electron transfer and singlet oxygen formation,which provided a new reference for the activation process of persulfate [162].
Dinitrodiazophenol (DDNP),a kind of phenolic compound,is an excellent initiating explosive device that is widely used in the detonator production industry.DDNP is a dangerous chemical with explosive characteristics,and it is very important to remove DDNP from water [163,164].Wanget al.used microwave-activated persulfate to degrade DDNP;within 16 min,the chemical oxygen demand (COD) decreased by 74.04%,and the chroma was almost completely removed.It should be noted that the system was not affected by coexisting anions in the reaction process [163].In general,the MW/PDS process is an effective method for removing DDNP from water.Liet al.synthesized carbon-coated ZVI nanoparticles (nZVI@C) by hydrothermal carbonization and carbothermal reduction.The unique structure of the catalyst and carbon coatingactivated persulfate can effectively degrade 4-chlorophenol (4-CP),which proves the feasibility of large-scale application of nZVI in the environment [165].Zhou’s team used degreased swine bone as the precursor to prepare biochar and achieved the efficient degradation of 2,4-DCP in a biochar-activated persulfate system.SO4·-,·OH,O2·-and O21participate in the degradation of 2,4-DCP [92].m-Cresol is an important chemical intermediate with certain biological toxicity [166].Zou used UV/O3to activate persulfate to degradem-cresol.The degradation rate ofm-cresol was nearly 100%in 30 min by electrophilic addition,ring opening and mineralization.
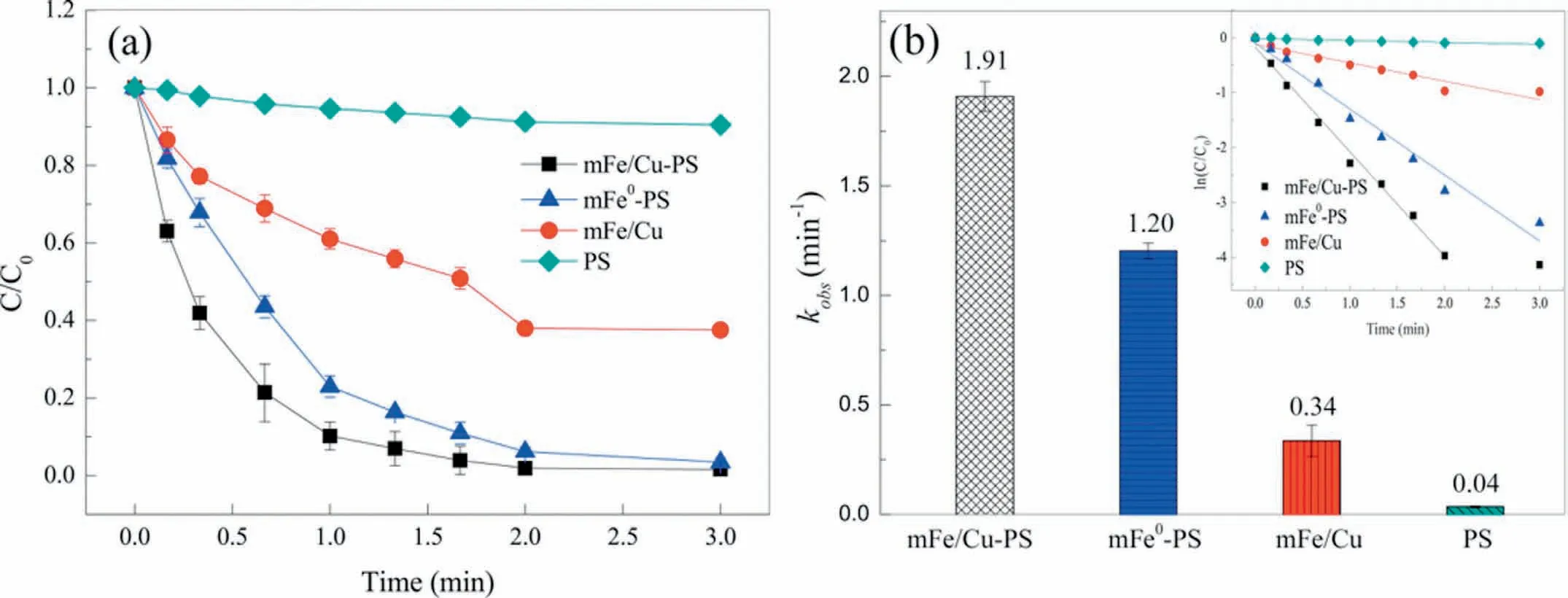
Fig.14.(a) Degradation of PNP by different systems (PS stands for persulfate in the graph).(b) Degradation kinetics for PNP removal ([PNP]=500 mg/L;[catalyst]=30 g/L;[PDS]=12.5 mmol/L;pH 3.6).Reproduced with permission [159].Copyright 2017,Elsevier.
3.7.Other wastewater
In addition to the above six pollutants,PDS-based AOPs can also be used to degrade other pollutants in water.For example,furfural [5,167],naphthenic acid [6],landfill leachate [3,168],aniline [169,170],artificial sweeteners [171],and nitriding disinfection byproducts [13].The above mentioned results show that PDS-based AOPs have great potential in wastewater treatment.
Here,we summarized a total of six different types of wastewaters.It is not difficult to find that thermal activation is one of the most traditional technologies,and thermal-activated persulfate system can be applied to most wastewater treatment.Due to the stable structure of pollutant molecules in phenolic wastewater,thermal activation may consume more energy.Therefore,many metal nanoparticles,metal oxides activation and carbon activation are used in the treatment of phenol wastewater.In addition,metal nanoparticles,metal oxides activation and carbon activation can be used in the treatment of various types of wastewaters due to the advantages of rich sources and no energy consumption,and all of which have achieved excellent removal effect.Both UV activation and MW activation have a high potential for pollutant removal,but their application is lower than that of non-energy consuming activation methods due to energy consumption issues (discussed in the next section).In general,PDS-based AOPs can be well applied to different wastewater systems.
It is worth noting that most current studies were focused on the laboratory level.Considering some restrictive factors that exist in the process of persulfate oxidation-mediated pollutant degradation,such as high salinity of water body after reaction and high energy consumption,PDS-based AOPs have not been applied to the actual wastewater treatment,so it is necessary to work to overcome the existing restrictive factors.
4.Existing problems and possible solutions in PDS-based AOP systems
As described in Section 3,PDS-based AOP technology has good application prospects in the degradation of refractory organic pollutants.However,there are several existing problems,such as the residual sulfates in the solution,energy consumption,and coexisting ions.Therefore,we aim to determine the problems and develop practical methods to solve the corresponding problems.As a result,PDS-based AOPs can be better applied to actual wastewater treatment,which will play a positive role in large-scale wastewater treatment plants.
4.1.Residual sulfate
Sulfate (SO42-) is a common contaminant in wastewater and is considered toxic to humans,plants and animals at high concentrations [172].It has been reported that drinking water containing a high concentration of sulfate ions can cause human diarrhea and other adverse reactions [173].Therefore,to protect the health of aquatic organisms,the concentration of sulfate ions should be controlled below 500 mg/L [174].In the process of degrading pollutants in wastewater,more persulfates were added to the wastewater to produce more sulfate radicals.For instance,when Hua’s team degraded DBP in wastewater,the dose of sodium persulfate reached 200 mmol/L [175];Zhou’s team added 100 mmol/L persulfate to the wastewater to degrade orange II [78].In the process of removing TPH by alkali-activated persulfate,the dose of persulfate reached 100 g/L [176].Excessive input of persulfates leads to the self-decomposition of sulfate radicals and reduces the pollutant removal efficiency;on the other hand,it will increase the concentration of SO42-in the water,which will cause secondary pollution of the water,pose a serious threat to aquatic organisms,and affect the operation of subsequent biological treatment [26,177].It is necessary to further eliminate SO42-in the effluent to meet the requirements of water discharge quality.In general,there are three methods to solve the problem of residual sulfate: double oxidant process,eosinophilic organism,and strong basic ion exchange resin.Removal rate of SO42-with different methods are listed in Table 4.
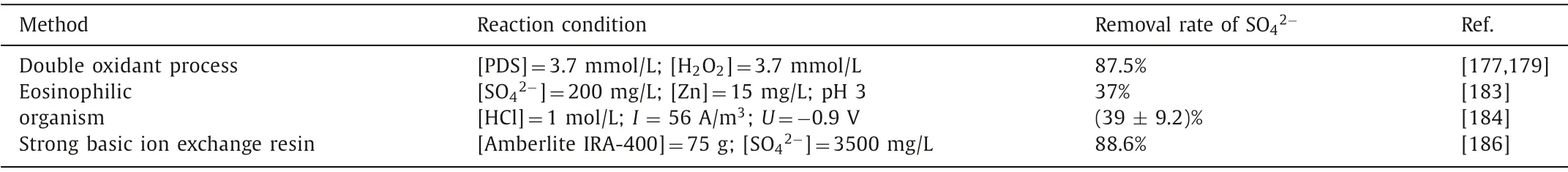
Table 4 List of removal rate of SO42- with different methods.
(1) Double oxidant process.To reduce the content of SO42-in water,studies have shown that other oxidizing substances(H2O2,O3,etc.) can be added to co-oxidize the pollutants in the system to reduce the use of persulfate [178].Additionally,due to the variety of free radicals,the double oxidant system can also have a wider range of applications.Chenet al.used microwave-induced persulfatehydrogen peroxide binary oxidant to degrade dinitrodiazophenol industrial wastewater.when then(PS)/n(H2O2)ratio was 3.7/3.7 (the dosages of PDS and H2O2were 3.7 mmol/L and 3.7 mmol/L,respectively),the removal rates of COD and CN in wastewater reached 73.55% and 98.1%,respectively[177].In the previous literature,when the dosage of persulfate was 29.59 mmol/L,the removal rates of COD and CN in MW/Fe2+/PDS system were 70% and 100%,respectively[179],indicating that the dual oxidant system can effectively reduce the dosage of persulfate,thus reducing the concentration of sulfate ion after reaction.Lai’s group developed a ZVI/H2O2/PDS system to remove PNP,and the degradation rate of PNP reached 99.9% after 6 min of treatment(Fig.15);this effect was attributed to the strong synergistic effect among ZVI,H2O2and PDS [180].In addition,Lai’s team also explored the ZVI/O3/PDS system to degrade the shale gas drilling backflow.Under the optimal conditions,the BOD5/COD value of the system reached 0.49,which effectively improved its biodegradability [181].In conclusion,the use of double oxidants can not only improve the reaction efficiency but also reduce the use of persulfate to reduce the concentration of SO42-in the effluent,which is a very promising technology.
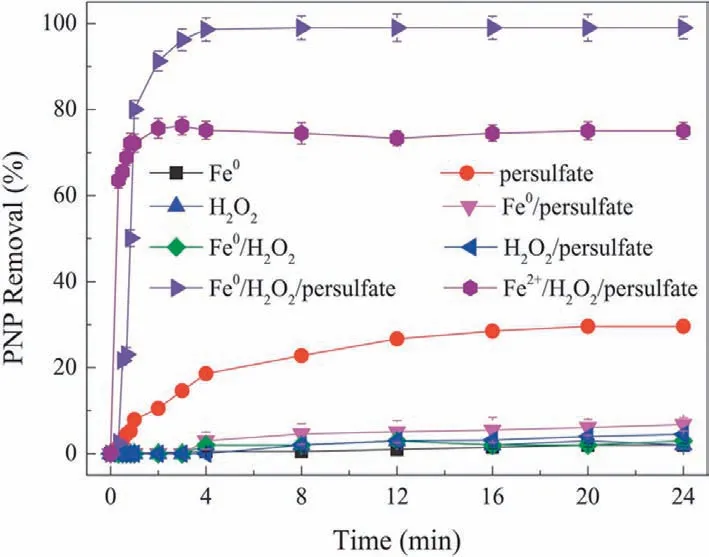
Fig.15.The degradation of PNP by the ZVI/H2O2/PDS system ([PNP]=500 mg/L;[ZVI]=1.3 g/L;[H2O2]=24.8 mmol/L;[PDS]=6.7 mmol/L).Reproduced with permission [180].Copyright 2017,Elsevier.
(2) Eosinophilic organism.This is a microbiological process that removes sulfates.Biological sulfate reduction in high pH value can be inhibited due to the deficiency of H+[182].pH value is a key factor.Therefore,adjusting pH to acidic condition and choosing an eosinophilic organism is beneficial to sulfate reduction.In a microbial electrolytic cell,an eosinophilic organism—a sulfate-reducing bacterium—acts as the cathode for the cell,and organics act as anodes for the cell.The main mechanism of microbial electrolytic cell is that the cathode accepts electrons and reduces sulfate to sulfide,thus achieving the removal of sulfate.For example,Teng’s team studied a kind of biological electrolysis cell with eosinophilic organisms as the cathode,and the reduction rate of sulfate reached 32 g m-3d-1,which better removed SO42-from water [183].Similarly,Liu’s group used a microbial electrolytic cell to remove SO42-,where autotrophic sulfate-reducing bacteria act as a cathode to accept electrons to reduce SO42-to sulfides.The mechanism is shown in Fig.16.The maximum sulfate reduction rate (39% ± 9.2%) was achieved at-0.9 V [184].In conclusion,eosinophilic organisms have good application potential in sulfate removal.
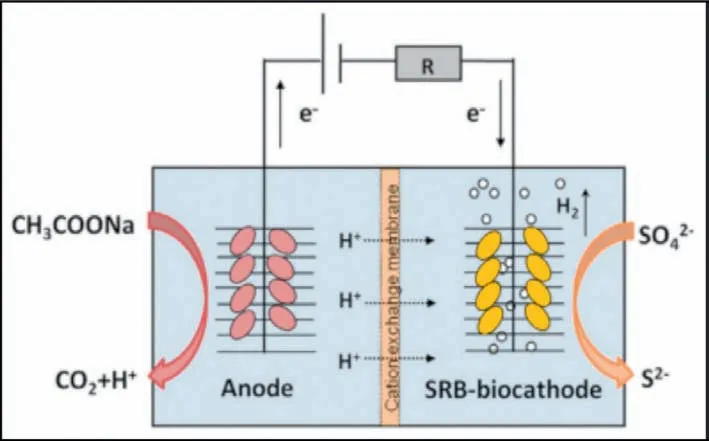
Fig.16.The mechanism of biological electrolysis cell.Reproduced with permission[184].Copyright 2014,Elsevier.
(3) Strong basic ion exchange resin.The positive group of the resin can be combined with anion adsorption,resulting in anion exchange.A strong base (such as NaOH) is generally used for the regeneration of resin [185].Öztürk’s team showed that strong alkaline ion exchange resin can remove more than 90% of sulfate in wastewater within 5 min,with high adsorption kinetics (the maximum adsorption capacity can reach 181.8 mg/g) [186].Strong basic ion exchange resin is an effective process for the removal of sulfate ions with strong regeneration ability.
4.2.Coexisting factors
4.2.1.Coexisting inorganic anions
The composition of actual wastewater is always complex,and it contains a variety of coexisting anions and other substances.The common coexisting anions are Cl-,NO3-,CO32-,HCO3-,and SO42-.In the process of PDS-based AOP degradation of pollutants,coexisting ions interfere with and affect the process of wastewater treatment,playing a certain role in promoting or inhibiting [187].
The coexisting CO32-and HCO3-can be regarded as effective scavengers of SO4·-and·OH and react with SO4·-and·OH to produce the subspecies CO3·-and HCO3·,respectively (Eqs.23-26) [188].According to the research of Shanet al.,in the process of nZVI-Ni@BC/PDS-mediated degradation of TCE,both CO32-and HCO3-can react with SO4·-and·OH to produce CO3·-and HCO3·,respectively,which inhibit the reaction process[151].In the degradation of TC by the nZVI/BC/PDS system,the coexisting HCO3-reacts with SO4·-to form HCO3·with weak redox ability,which inhibits the degradation of TC [94].Similarly,Luet al.also found that the presence of CO32-and HCO3-would inhibit the reaction when using a UV/PDS system to degrade diclofenac [19].It is worth noting that Fuet al.found that the presence of CO32-promoted the reaction when using the Cu2+/PDS system to degrade SMZ,which was attributed to the rapid reaction rate between CO3·-and the aniline moiety of SMZ (108mol L-1s-1) [33].
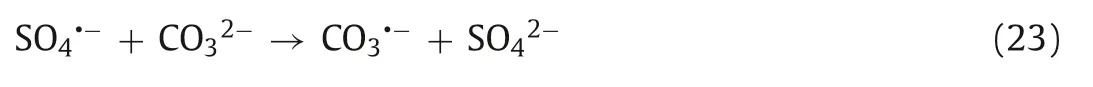
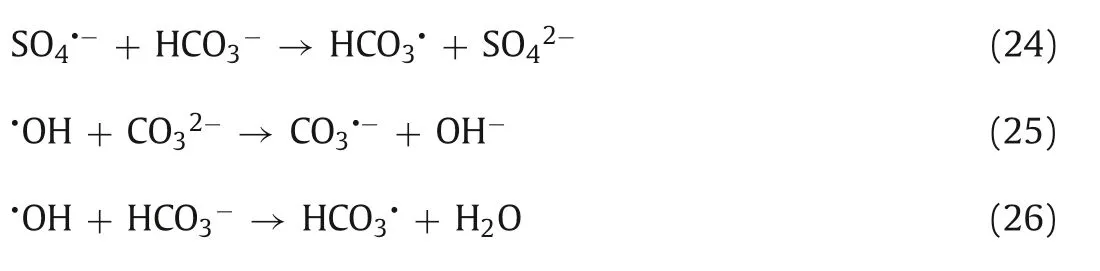
The effect of Cl-on the reaction is controversial.Jawad’s research indicates that the presence of Cl-can quickly react with SO4·-and·OH,and more active species were formed by the further reaction between Cl·(Eqs.27-31) [28,189].Some studies have shown that the inhibitory effect on the reaction still exists even at very low Cl-concentrations [190].For example,in the process of TC degradation,Shaoet al.found that Cl-reacted with SO4·-to form Cl2·-with low oxidation ability,which inhibited the degradation of TC [94].Vasseghianet al.also pointed out that Cl-could inhibit the degradation of malathion in a UV/Fe2+/PDS system [191].In addition,another study pointed out that the existence of Clhas a positive effect on the reaction system [192].For instance,in the system of Fe2+/HA/PDS,adding trace Cu2+to degrade orange II,it was found that the addition of Cl-can effectively promote the degradation of orange II.This result occurs because although Clreacts with certain SO4·-to form Cl·-with weak oxidation ability,Cu2+/Cu+reacts with SO4·-to form Cu3+in the reaction system.Cu3+is also an oxidizing active substance,but its oxidation ability is weaker than Cl·-,so the existence of Cl-will compete with Cu2+/Cu+for SO4·-to form Cl·-to promote the degradation of orange II [53].Ghanbariet al.found that the presence of Cl-can promote the decolorization efficiency of dye molecules,and the production of chlorine active substances such as Cl·and Cl2can give full play to their bleaching ability [189].
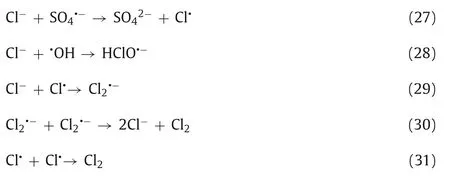
The presence of NO3-reacts with SO4·-and·OH to produce NO3·,and its oxidation activity is weaker than that of SO4·-(Eqs.32 and 33) [144,193].Vasseghianet al.indicated that the presence of NO3-inhibited the degradation of malathion in a UV/Fe2+/PDS system [191].Similarly,in the process of TC degradation,Shaoet al.found that coexisting NO3-reacted with SO4·-to form NO3·with low oxidation ability,which inhibited the degradation of TC [94].It is worth noting that Leeet al.found that the presence of NO3-can promote the reaction rate when using a UV/PDS system to degrade BP3;because NO3-can react with SO4·-and·OH to produce NO3·,NO3·can reproduce in water to produce secondary free radical NO2·,which can accelerate the degradation of phenolic compounds [144].
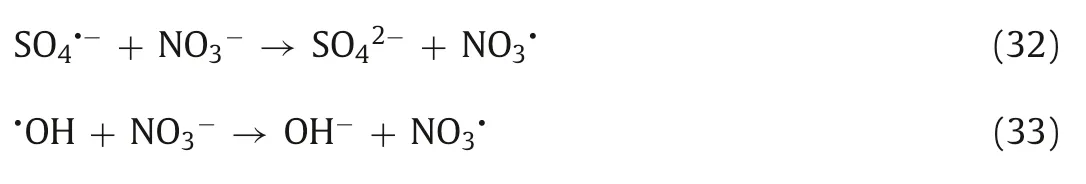
In addition,SO42-itself does not react with SO4·-,so it will not affect the system [151].This conclusion is consistent with that of Hu’s research [188].Currently,the study on the influence of coexisting anions on the system is not very comprehensive,and there is no unified method to overcome its effects,but we can also have a further study according to some characteristics of different ions.For instance,carbonate radicals have a very high reaction rate with aniline,so they can be introduced to promote the reaction when aniline or aniline-containing substances are degraded in future studies.In addition,the activity of Cl·is higher than that of Cu3+.Therefore,in future studies of the activation of PDS by transition metal ions,it can be considered whether the presence of chloride ions can also play a certain promoting role.However,for some systems in which the reaction process was inhibited due to the presence of coexisting ions,more persulfate can be added to offset the interference so that the target pollutants can be better removed.In future research,the influence of coexisting ions needs to be further explored.
4.3.Natural organic matter
In the actual water matrix,natural organic matter (NOM) is very common.Humic acid (HA),as an example,is discussed in the process of pollutant degradation.In general,HA may interact with target pollutants through catalytic reaction,adsorption,competition and other mechanisms to affect their degradation effect [194].Luet al.found that the presence of HA would reduce the removal of diclofenac in the process of UV-activated PDS for degradation of diclofenac.The mechanism is that HA has a strong absorption of UV photons at 254 nm,thus reducing the UV penetration,leading to the reduction of sulfate radicals and the reduction of direct UV photodecomposition of DCF [19].Shanet al.explored the effect of NOM on TCE removal in nZVI-Ni@BC/PDS system,and the results showed that TCE removal decreased with the increase of NOM.It was attributed to the fact that NOM is a strong radical scavenger,the electronic part existing in NOM structure is easily decomposed by sulfate radicals and hydroxyl radicals,which competes with TCE for free radicals and reduces the removal of TCE [151].Wuet al.also pointed out that in the degradation process of TCE by ferrous ion activated persulfate system,humic acid may play the role of free radical scavenger by competing with target pollutants for sulfate radicals and hydroxyl radicals,thus reducing the removal of target pollutants [155].However,when using Fe3O4/MW/PDS system to degrade PNP,Huet al.found that the influence of humic acid on PNP degradation was negligible,highlighting the selectivity of Fe3O4/MW/PDS system [70].It is a positive reference for overcoming the influence of NOM in the system in the future.
4.4.Energy consumption
EEO(electrical energy per order) refers to the electrical energy required to remove one order of magnitude of pollutants in a unit volume,kWh/m3[195],which has been widely used to compare the power cost of different advanced oxidation technologies.In practical applications,the highest acceptable value of energy consumption is 2.5 kWh/m3[196].The calculation is as follows (Eq.34) [197]:

wherePelis the combined power of the MW oven and UV light(kW),tis the reaction time (min),Vis the volume of the treated wastewater sample (L),C0andCare the initial and final concentrations of the treated sample (mg/L),respectively,and the constant“60′′ converts min to h.
In the PDS-based AOP process,with the assistance of MW and UV,the removal efficiency of pollutants is significantly improved.Compared with alkali-activated persulfate,the reaction time of MW activation and UV activation are very short,and some pollutants can even be completely removed in a few minutes;thus,these approaches are efficient and promising processing technologies [21,163].However,one of the limitations is that both approaches require certain energy consumption,especially in MWactivated persulfate,and the energy consumption is generally at 1000 kWh/m3or higher,which is far beyond the acceptable energy consumption value in practical applications.The energy consumption of UV-activated persulfate varies with the power and reaction time (Table 5) [67,112,144,163,177,197-199].In the actual process of wastewater treatment,we should consider not only the water treatment effect but also the energy consumption.
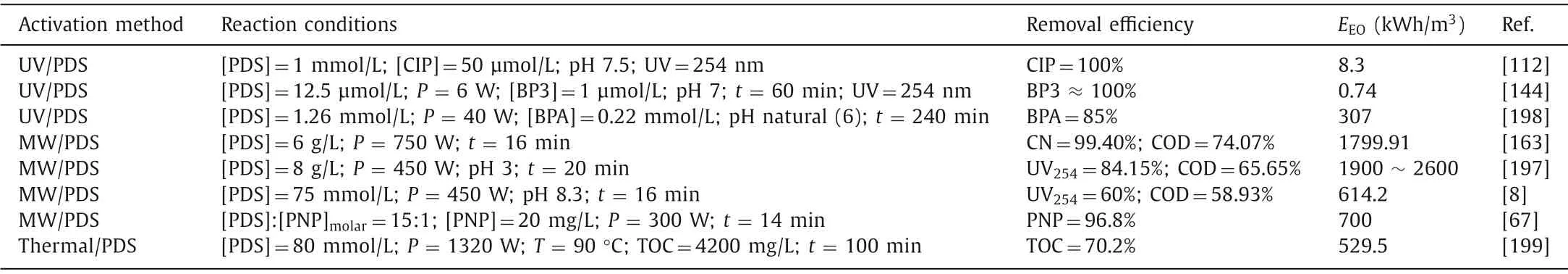
Table 5 Energy consumption of persulfate degradation in different ways.
As seen from Table 5,MW activation has a good effect on removing chromaticity,COD and UV254when treating wastewater,and the reaction time is short.However,due to the characteristics of MW heating (fast and uniform heating),the applied power is very large (usually a few hundred watts).Substituting the power value into the above energy calculation formula,it is found that its energy value is very large,far higher than the acceptable energy consumption value (2.5 kWh/m3mentioned above),which is a challenge.In light of the problem of the high microwave activation energy,we can consider starting by reducing the volume of the instrument,making full use of microwaves,and expecting that more mature microwave technology can be used for practical wastewater treatment as soon as possible.In addition,we can take full advantage of the rapid reaction of microwaves.For example,because a large amount of wastewater needs to be treated in a short time,microwave heating is obviously better than the traditional heating activation method.
For UV activation,the UV/PDS system can effectively degrade refractory organic pollutants in wastewater,and some of them can almost completely mineralize pollutants.In the laboratory simulation experiment,the output power of UV is lower than that of the microwave or traditional heating method,and its energy consumption is relatively low compared with the other two methods.In addition,ultraviolet light also performs the functions of disinfection and sterilization [200].With respect to protection,ultraviolet operation is relatively safe,and its degradation effect is very efficient.
Both MW and UV activation require a certain amount of energy beyond the maximum acceptable value.However,MW and UV activation are both better choices for specific water bodies,such as those where heavy metals cannot be introduced.In future research,this technology still needs to be further improved.
5.Conclusions
Persulfate oxidation as an advanced oxidation method,can be activated by many methods,and the advantages of sulfate radicals have allowed the use of persulfate oxidation not only to degrade insoluble organic pollutants but also to effectively remove emerging pollutants,making it a promising approach for wastewater treatment.PDS-based AOPs can treat different types of organic wastewater,such as antibiotic wastewater,PPCP wastewater,dye wastewater,EDC wastewater,chlorine-containing organic pollutants,and phenolic wastewater,and PDS-based AOPs have shown good effects on all these types of wastewater.Currently,PDS-based AOPs have not been put into practical application.(1) The use of persulfate leads to a large amount of sulfate ions in the effluent,which makes it difficult to meet the water quality requirements of drinking water and is harmful to humans and animals.Subsequent methods,such as the double oxidant process,eosinophilic organisms,and strongly basic anion exchange resins,can be used to remove sulfate ions.(2) The actual water body is very complex,including coexisting inorganic anions and natural organic matter.The existence of coexisting ions interferes with the effective degradation of the target pollutants by persulfate.However,coexisting ions also have a positive side.Therefore,this positive factor should be considered in future research.For natural organic matter,the effect of which can be overcome by improving the selectivity of the reaction system.(3) The energy consumption in the degradation process,which has always been a major limitation of MW and UV activation,should be considered.For MW activation,we can consider reducing the volume of the instrument to make full use of microwaves.In addition,the characteristic of a short time with MW can be better used in rapid wastewater treatment processing.For UV activation,the energy consumption was relatively low and has excellent mineralization efficiency.In addition,UV light also has a certain sterilizing effect.PDS-based AOPs are a very effective and advanced oxidation technology and seeking an acceptable and sustainable activation method is needed.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors sincerely appreciate the National Natural Science Foundation of China (No.51678185) and Talents of High Level Scientific Research Foundation of Qingdao Agricultural University (No.6651120004).
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
- A review on treatment of disinfection byproduct precursors by biological activated carbon process
