A review on treatment of disinfection byproduct precursors by biological activated carbon process
Jie Fu,Ching-Hu Hung,Chenyun Dng,Qilin Wng
a School of Environmental Science and Engineering,Huazhong University of Science and Technology,Wuhan 430074,China
b School of Civil and Environmental Engineering,Georgia Institute of Technology,Atlanta,GA 30332,United States
c Center for Technology in Water and Wastewater,School of Civil and Environmental Engineering,University of Technology Sydney,Sydney,New South Wales 2007,Australia
Keywords:Disinfection byproduct precursor Biological activated carbon Formation potential Natural organic matter Empty bed contact time Ozonation
ABSTRACT Disinfection by-products (DBPs) in water systems have attracted increasing attention due to their toxic effects.Removal of precursors (mainly natural organic matter (NOM)) prior to the disinfection process has been recognized as the ideal strategy to control the DBP levels.Currently,biological activated carbon(BAC) process is a highly recommended and prevalent process for treatment of DBP precursors in advanced water treatment.This paper first introduces the fundamental knowledge of BAC process,including the history,basic principles,typical process flow,and basic operational parameters.Then,the selection of BAC process for treatment of DBP precursors is explained in detail based on the comparative analysis of dominant water treatment technologies from the aspects of mechanisms for NOM removal as well as the treatability of different groups of DBP precursors.Next,a thorough overview is presented to summarize the recent developments and breakthroughs in the removal of DBP precursors using BAC process,and the contents involved include effect of pre-BAC ozonation,removal performance of various DBP precursors,toxicity risk reduction,fractional analysis of NOM,effect of empty bed contact time (EBCT) and engineered biofiltration.Finally,some recommendations are made to strengthen current research and address the knowledge gaps,including the issues of microbial mechanisms,toxicity evaluation,degradation kinetics and microbial products.
1.Introduction
Disinfection byproducts (DBPs) are generated from reactions of organic and inorganic matter in water with chemical agents during the water disinfection process.Chlorine,ozone,chlorine dioxide,chloramines and peracetic acid (PAA) are among the commonly used disinfectants for water/wastewater treatment today,and each produces its own suite of DBPs,with overlapping constituents [1].Based on the disinfectant type,the DBPs can be categorized into chlorination DBPs and those from non-chlorinated disinfectants.The DBPs from chlorination may include those regulated for drinking water such as the trihalomethanes (THMs,e.g.,chloroform,bromodichloromethane,chlorodibromomethane and bromoform),haloacetic acids (HAAs,e.g.,chloro-,bromo-,dichloro-,dibromo-and trichloroacetic acids) and chlorite,and the so-called“emerging” DBPs such as halonitromethanes (HNMs),haloacetonitriles (HANs),haloacetamides (HAMs),halofuranones,iodotrihalomethanes (I-THMs),iodo-acids (I-HAAs),nitrosamines (NAs),and others.The DBPs from non-halogenated oxidants are mainly resulted from the application of ozone or PAA as the disinfectant,including the regulated bromate (from ozonation),and other various types of aldehydes (e.g.,formaldehyde),ketones,and carboxylic acids (from ozone and PAA) [2,3].The existence of elevated levels of DBPs in the drinking water systems worldwide puts risk to the public health due to their potential for carcinogenic,genotoxic,and negative reproductive/developmental effects [2,4].
So far,over 700 DBPs have been identified,and unfortunately,only a small part has been assessed either in quantitative detection or toxicity studies.More than half of the organic halogen generated by the chlorination [5]or aldehydes,bromate,and organic bromine generated by ozonation have not been reckoned as the known DBPs [6].Table S1 (Supporting information) illustrates typical DBPs and their toxicities in different treatment processes.Generally,the DBPs in drinking water have been quantified at sub-μg/L or low-to mid-μg/L levels [1,2,4,7],and many of them show considerable genotoxicity or carcinogenicity [2].
Based on the discoveries of DBPs,the developed nations have promulgated guidelines or regulations to manage the concentration level and health risk of DBPs.In 1979,the US Environmental Protection Agency (USEPA) published a regulation to guarantee the annual average level of Total THMs (TTHM,including chloroform,bromodichloromethane,dibromochloromethane,and bromoform) in drinking water under 100 μg/L [8].In 1998,the USEPA published the Stage 1 Disinfectants/DBP Rule to lower the maximum contaminant levels (MCLs) of TTHM to 80 μg/L;in addition,HAA5 (defined as chloroacetic,dichloroacetic,trichloroacetic,bromoacetic and dibromoacetic acids),bromate,and chlorite are regulated for the first time with the permissible levels at 60 μg/L,10 μg/L,and 1000 μg/L,respectively (Table S2 in Supporting information) [9].The European Union (EU) and World Health Organization (WHO) also published guidelines or standards to regulate DBPs (Table S2) [10,11].
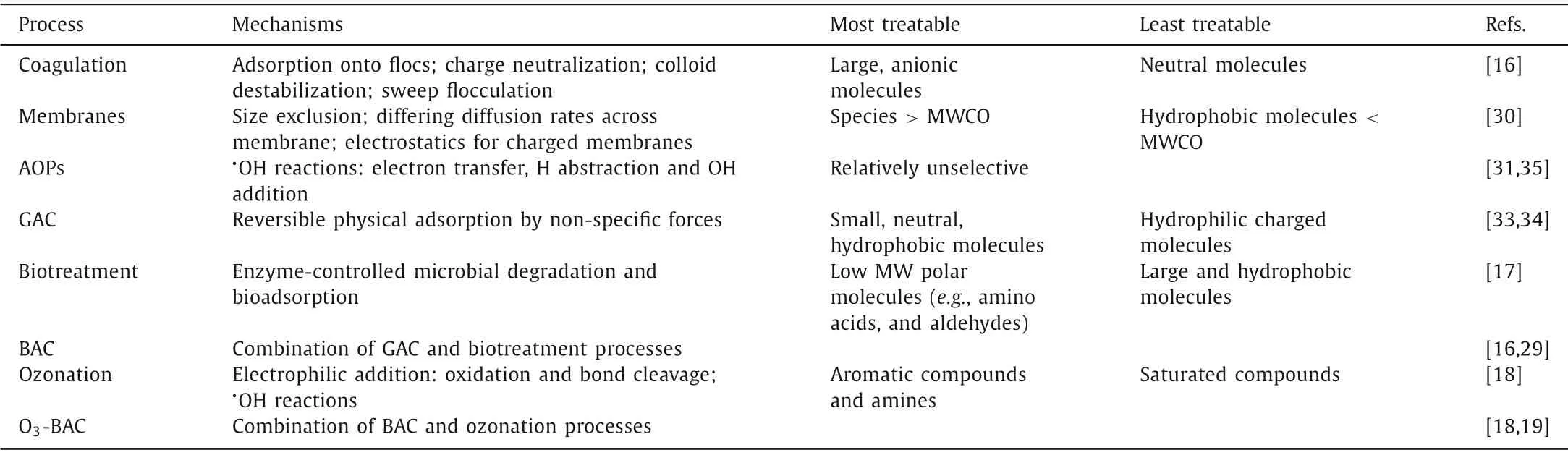
Table 1 Mechanisms and selectivity of different water treatment processes on removal of NOM.
To meet the stricter regulations,many drinking water plants have employed so-called alternative disinfectants (e.g.,ozone,chlorine dioxide,and chloramines) to replace chlorine.However,ozone disinfection would significantly enhance the production of aldehydes,bromate,and organic bromine,which are of carcinogenic concern [12].Chloramination would produce a considerable amount ofN-nitrosodimethylamine (NDMA),which is also a carcinogen classified by the USEPA [13].Another available strategy to reduce the DBPs levels is removal of precursors before the disinfection process,which can avoid the production of alternative DBPs by other disinfectants.
Natural organic matter (NOM) in water is generally the predominant precursor of DBPs [14],and represents a significant portion of total organic carbon (TOC) content.NOM is originated from vegetation and microbial degradation processes,and has pedogenic and aquagenic sources [15].The pedogenic NOM has a higher lignin content and aromatic fraction,whereas aquagenic NOM is low in phenolic and aromatic constituents [16].There are several available technologies to reduce the concentration of NOM including coagulation,anion exchange,membranes process,activated carbon adsorption,biotreatment,advanced oxidation processes (AOPs),and ozonation [17].
Of these technologies,biological activated carbon (BAC) process is an optimum process (see Section 3) and increasingly used in the last decade for treatment of DBP precursors.Two recent publications have reviewed the current knowledge of BAC for advanced water and wastewater treatment [18,19],however,the related issue and research progress on the treatment of DBP precursors by BAC process has not addressed.Liuet al.reviewed biofiltration processes for controlling disinfection by-products and their precursors,including BAC process [20].The rule of removing various disinfection by-products and the influence of process parameters were discussed in detail.Unfortunately,there are still many issues not involved,such as the selection of technology,removal of toxicity risk,fractional analysis of precursor components,and engineered biofiltration.To this end,this paper presents a thorough overview to summarize the recent developments and breakthroughs in the removal of DBP precursors using BAC process,including the fundamental knowledge of BAC process,selection of BAC process for treatment of DBP precursors from the perspective of removal mechanisms,the comprehensive performance of BAC process in applications,and discussion of the current knowledge gaps and further evolution of BAC process for the treatment of DBP precursors.
2.Biological activated carbon process
2.1.History of BAC process
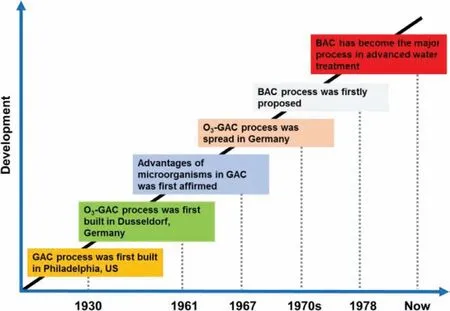
Fig.1.Developmental history of BAC process.
Fig.1 summarizes the important events for the development of BAC process.The BAC technology is derived from granular activated carbon (GAC) technology.The first water treatment plant using GAC adsorption tank was constructed in Philadelphia,US in 1930[18].In the initial applications,the prechlorination is often used prior to GAC treatment.As a result,the growth of microorganisms in the carbon layer is significantly inhibited.To improve the removal of recalcitrant macromolecules,ozonation is sometimes utilized as the preoxidation step of GAC treatment.The combination of ozonation and GAC process was firstly put into practice at Amstaad Water Plant in Dusseldorf,Germany in 1961 [21].The advantages of microbial activity in the activated carbon layer were first confirmed by Parkhrust and his colleagues in 1967 [22],which enabled the prolonging the operation life of GAC media.In the 1970s,the research and application of ozonation-GAC process were widely carried out in Germany.Until 1978,the BAC process was firstly defined by Miller and Rice [18].Since then,the BAC technology is widely spread and used,and the treatment itself is perfected gradually.By now,BAC has become the prevalent process in advanced water treatment worldwide,and moreover,the process is also used in sewage reclamation and industrial wastewater treatment.
2.2.Basic principles of BAC process
BAC process utilizes the synergistic effect between physicalchemical adsorption and biological-oxidation degradation.Activated carbon has good adsorption capacity with different sizes of micropores,mesopores and macropores [23],and different sized organic molecules and dissolved oxygen (DO) can enter these pores[24].However,the adsorption sites would be occupied by adsorbatesvianon-specific mechanisms,including hydrophobic interactions,dipole interactions and van der Waals forces [25].For BAC technology,microorganisms on activated carbon will extend the adsorption capacity of activated carbon by regenerating the adsorption sites through the biodegradation of organic adsorbates[26].The BAC technology is based on the interaction between activated carbon,microorganisms,organic matters (OM) and DO (Fig.S1 in Supporting information) [18].Activated carbon is used as a carrier of microorganisms,which will reproduce and grow on the activated carbon to form BAC by feeding on sufficient OM and DO.The biofacies on activated carbon consist of bacteria,fungi,algae,protozoa and metazoan.
2.3.Typical BAC process flow
To date,the application of BAC process is in the following three areas: the advanced treatment of drinking water and sewage reclamation,and treatment of industrial wastewater.Fig.S2 (Supporting information) presents the typical processes of the advanced treatment of drinking water or sewage reclamation.The processes are based on conventional coagulation-sedimentation-filtration way.To improve the biodegradation efficiency,pre-ozonation prior to BAC,i.e.,O3-BAC,is widely applied.The refractory organics are oxidized into small molecules by ozone,which are more prone to be degraded by microorganisms on the activated carbon;meantime the oxygen derived from the decomposition of ozone will increase the level of DO and promote the biological-oxidation degradation [27].In practice,O3-BAC can be placed after sedimentation and before filtration as shown in Fig.S2a.In this process,a relatively higher ozone dosage is required to guarantee the quality of the effluent.The sand filter in the end can remove the tiny carbon particles and fallen microorganisms in the outflow of BAC.Alternatively,O3-BAC can be placed after the filtration as shown in Fig.S2b.Compared with process A,a lower ozone dosage is needed because the ozone depleting substances will be removed by filtration.However,the frequent backwash on the activated carbon is required to alleviate the leaping of micro carbon particles and microorganisms from BAC [18].
2.4.Basic operational parameters of BAC process
For the design of a BAC system,the characteristics of water quality,water amount and some certain operational parameters are necessary to be considered.Table S3 (Supporting information)summarizes the typical operational parameters of BAC process.First of all,the GAC material selection is crucial for the treatment performance.GAC for water treatment typically has an average diameter of 1.2-1.6 mm.GAC pore size has a significant influence on the attachment of microbial cells,and highest biomass was observed with the pore size of 2-50 nm.For the BAC operation,flow rate,height of the carbon layer,empty bed contact time (EBCT)and gas-water ratio directly influence the effluent quality.In practice,the general flow rate is 8-15 km/h;height of the carbon layer is within 1.5-3 m;EBCT should be 6-30 min;and gas-water ratio could be (4-6):1 [28].The growth of microorganisms and suspended solids brought by the inflow on the long-term operation may block the carbon layer.Therefore,the carbon layer should be washed periodically,and related parameters for backwash are also shown in Table S3.
3.Selection of BAC process for treatment of DBP precursors
Table 1 summarizes the mechanisms of dominant water treatment technologies as well as the most or least treatable fraction of NOM.Coagulation is the most commonly used process to remove NOM through principally electrostatic mechanisms [16].Coagulation can remove a large part of the NOM and especially the hydrophobic fraction,containing more aromatic and larger molecules in comparison to hydrophilic fraction.However,significant DBP formation potential (DBPFP) is still retained in the residual NOM after coagulation [29].Therefore,advanced treatment is needful for the removal of residual NOM components.Membrane process is an increasingly used water treatment,including microfiltration,ultrafiltration (UF),nanofiltration (NF) and reverse osmosis (RO).In general,owing to the small size of DBP precursors,NF membranes with a molecular weight cut-off (MWCO) of below 200 Da are required for successful precursor removal [30],which greatly increase the investment and operation cost,thus limiting their large scale application.Advanced oxidation processes (AOPs),based on thein situgeneration of hydroxyl radicals (·OH),is an effective technology to non-selectively remove various NOM compositions[31].Nevertheless,the breakdown intermediates by AOPs tend to increase the downstream DBPFP [32].Moreover,higher energy and chemical input is generally needed for AOPs to guarantee the treatment efficiency.GAC is a more ideal technology for control of DBP precursors in larger systems [33].GAC has very high adsorption capacity for small,neutral,and hydrophobic molecules,however,the adsorption capacity of GAC for hydrophilic and charged molecules is relatively lower [34].In addition,the routinely replacing the GAC media due to adsorption saturation has become a major constraint in operating GAC contactors [16].BAC process can extend GAC media lifevia in situregeneration of adsorption sites through biodegradation,which will substantially reduce the GAC replacement costs [35].Moreover,the biological process in BAC can enhance the treatment performance of GAC on low molecular weight (MW) hydrophilic compositions,e.g.,amino acids,and aldehydes [17].Especially,combined with pre-ozonation,the biodegradation performance of BAC process can be significantly promoted due to the enhanced biodegradability of NOM by transforming the large aromatic compositions into small MW and oxygen-containing molecules [18].Therefore,BAC/O3-BAC process is the best choice for advanced water treatment.
In terms of removal mechanisms,treatability of NOM is largely determined by physical properties,especially size,charge and hydrophobicity (Table 1).Thus,assigning the NOM groups with discriminative properties is necessary to optimize the processes to remove the DBP precursors.Based on several assumptions,NOM can be classified to several groups,i.e.,humic substances,carboxylic acids,amino acids,proteins and carbohydrates (Table 2)[36].Humic substances are the largest,most hydrophobic and highly charged of the NOM groups,which make up 40%-60%of the dissolved organic carbon (DOC) in natural surface water[36]and are directly proportional to specific ultraviolet absorbance at 254 nm (SUVA254) [37].The high DBPFP of humic substances is well known,and they are the main sources of THM and HAA precursors [29].Carboxylic acids (e.g.,acetate,glycolate,butyrate,and formate acids) in NOM are assumed to be smaller and more hydrophilic than humic substances,of which properties are consistent with the NOM transphilic fraction [38].Although the percentage of carboxylic acids in raw water is small,the concentration of carboxylic acids can be significantly increased during the oxidation process (e.g.,ozonation) [39],which constitute the important sources of THM and HAA precursors [17].Glutamic acid,glycine,serine and aspartic acid are the most abundant aqueous amino acids and they are usually present in a combined form [36].Amino acids are the considered to be the major source of nitrogencontaining DBP (N-DBP) precursors [17],and many of them (e.g.,tryptophan,tyrosine,aspartic acid and asparagine) are also known to be reactive HAA precursors [40].Proteins in water often originate from algae or phytoplankton and can include phenol,pyridine,toluene and styrene groups [41].The concentration of proteins will correlate to N-DBP formation [42],and proteins also show high HAA formation potential (HAAFP) [43].Carbohydrates (e.g.,glucose,arabinose and mannose) are neutral,relatively hydrophilic and relatively small molecules [36].Typically,carbohydrates have a relatively low DBPFP [43].
Based on the physiochemical properties of NOM groups,the treatability of different processes can be proposed (Table 2).Coagulation is normally the first step of conventional water treatment[16].Coagulation can remove most of humic substances,which may be sufficient for controlling the regulated DBPs,because humic substances are the main sources of THM and HAA precursors.Unfortunately,some fragments of lower size and charge remain in the residual humic substances that retain considerable DBPFP.What is worse,the other hydrophilic NOM groups including carboxylic acids,amino acids,proteins,and carbohydrates,which are important sources of DBP (especially N-DBP) precursors,are recalcitrant for the coagulation treatment [17,29].Post-treatment is thereby employed to further dislodge residual NOM compositions to control the DBPFP level.Among the available technologies including membranes,AOPs,GAC and (O3-)BAC,BAC is a more ideal choice for the control of DBP precursors based on not only the economical consideration but also removal mechanisms.The size exclusion is the major mechanism for rejection of molecules by membrane process,and typically,only the NF membranes with the MWCO of below 200 Da have the considerable efficacy for DBP control [44].AOPs have strong oxidation ability of non-selectivity,which can degrade all kinds of organic compounds.However,since the produced intermediates during the oxidation process may provide new DBP precursors,AOPs always show a limited removal of DBPFP.Moreover,AOPs are generally not economically feasible.GAC prefers to adsorb small,neutral,and hydrophobic molecules relative to hydrophilic and charged molecules [34].Compared with GAC,BAC process could enhance the removal of small compounds with low aromaticity and high nitrogen content in NOM (e.g.,carboxylic acids or amino acids) through biodegradation [45],and reduce the formation potential of HAA and N-DBP [17].Combined with pre-ozonation,the biodegradation performance of BAC process can be further enhanced.Based on the above discussion,it can be concluded that BAC/O3-BAC process is an ideal and feasible choice for treatment of various DBP precursors,especially the emerging ones such as N-DBP precursors.
4.Treatment of DBP precursors by BAC process
Table S4 (Supporting information) summarizes the studies on treatment of DBP precursors by BAC process.The earliest study was reported by Sketchellet al.in 1995 [46],where the effects of DOC and bromide on the formation of THM were investigated through a laboratory-scale BAC column test.After 2005,more and more studies have been carried out on the removal of DBP precursors by BAC process (Fig.2A).In the early studies,researchers focused on the regulated DBPs,i.e.,THMs and HAAs.In 2012,Chu and the colleagues first extended the target regulated DBPs to some NDBPs including two HAMs,four HANs and trichloronitromethane(TCNM) [47].Since then,the emerging DBPs have gained increasing attention and been widely studied.Based on the literature statistics (Fig.2B),it can be found that BAC technology is mainly used in drinking water treatment (accounting for 94%),while only three publications reported controlling the DBP precursors in sewage reclamation with BAC process [48-50].In terms of the study scale,pilot-scale study is most commonly conducted relative to laboratory-or full-scale study.This may be due to that the pilot experiment is close to the actual situation and convenient to evaluate the process feasibility and optimize the process parameters.As for the study geography,almost half of the researches come from China with a variety of water sources such as Taihu Lake [51,52],Yangtze River [53],Yellow River [54]and Huangpu River [55].The deteriorating water pollution and eutrophication in these surface waters are threatening local water quality safety [56].Especially,the algal blooms caused by eutrophication release large quantities of algal organic matters into water such as oligosaccharides,polysaccharides,proteins,peptides,amino acids,as well as other traceable organic acids [57],which provide considerable DBP precursors.Therefore,BAC technology is widely employed in China’s drinking water treatment plants (DWTPs) to control the effluent DBPFP [51].Since the relatively lower proportion of biodegradable fractions in NOM (biodegradable DOC (BDOC)/DOC = 0.1-0.3) [58],the single BAC process may not well play the biodegradation function.Thus,the pre-filter treatment has been employed to change the NOM compositions and enhance the BDOC fraction such as ozonation [48],AOPs (e.g.,O3/H2O2[59],ultraviolet (UV)/O3[60],vacuum UV (VUV) [24],and UV/H2O2[32]),biofiltration [29],and membrane process [61].Of these pretreatments,ozonation is recognized as the optimal procedure and has been widely used in many DWTPs around the world.
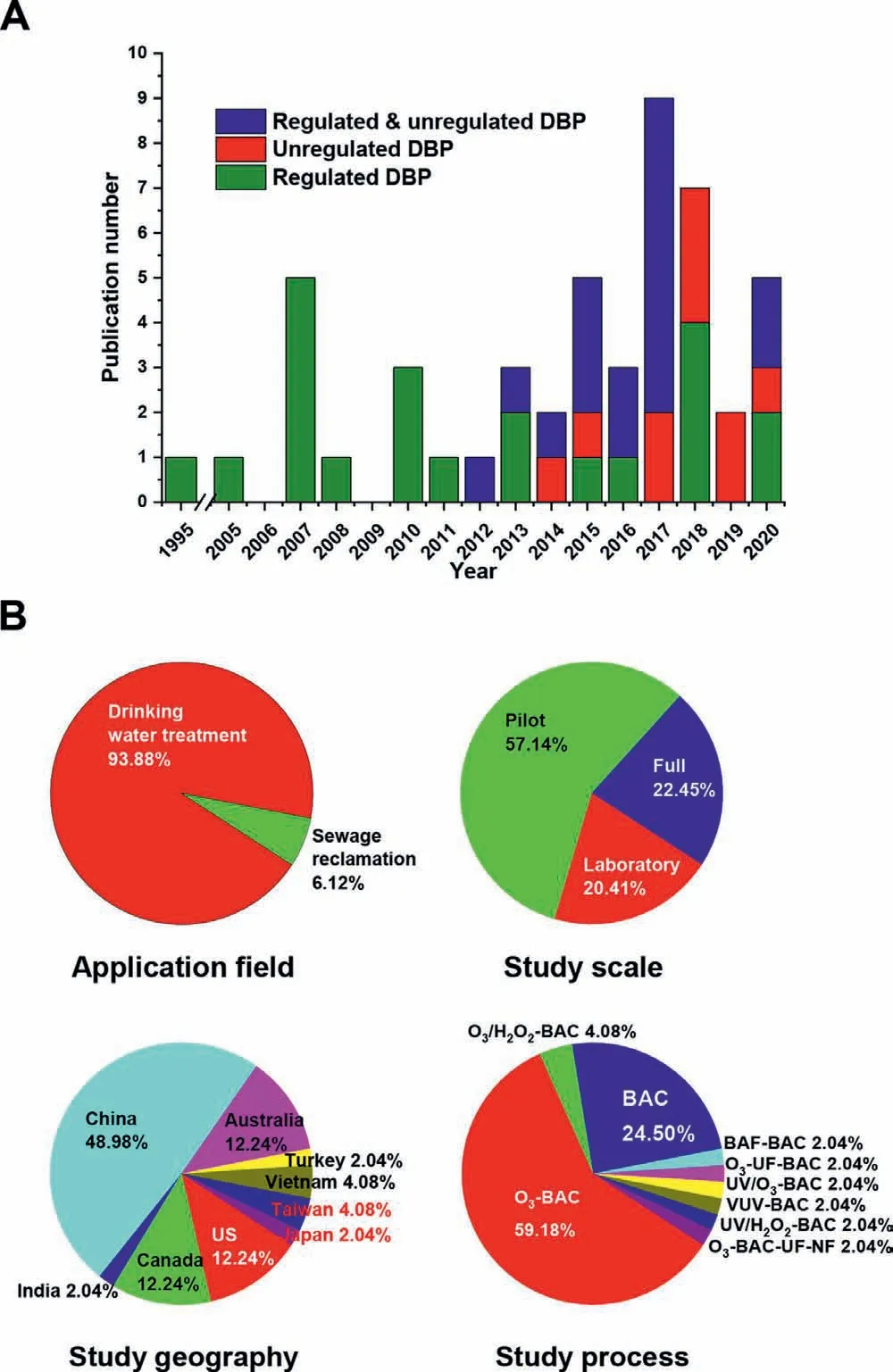
Fig.2.Studies on treatment of DBP precursors by BAC process: (A) publication number versus publication year;(B) publication distributions based on application field,study scale,geography and process.The target regulated and unregulated DBPs in each publication were marked with different color in (A).Abbreviations:BAF,biological anthracite filter;NF,nanofiltration;UF,ultrafiltration;VUV,vacuum ultraviolet.
4.1.Effect of pre-BAC ozonation
Ozonation is usually applied before the BAC process (Table S4),and the typical ozone dose and contact time are 1.5-2.5 mg/L and 10-30 min.Researches indicate that ozonation can significantly increase the small MW fractions,breakdown the large aromatic structures,and increase the BDOC/DOC ratio of the NOM in water[18].For example,Chen and the co-workers conducted a comparative pilot study to evaluate the effects of ozonation on the NOM biodegradability and removal of DBP precursors by following BAC process [62].The results indicated the ozonation prominently increased BDOC from 0.25 mg/L to 0.72 mg/L,resulting in a 67.4% of HAAFP removal by BAC.However,without pre-ozonation,the BAC process could remove only 24.5% of HAAFP.
The alteration of NOM characteristics by ozonation is due to the reaction of ozone towards the electron-rich moieties of NOM which include activated aromatic systems,olefins,and nonprotonated amines [63].Fig.3 illustrates the typical reaction paths as well as the impact on the formation of different types of DBPs during post-chlorination.Ozone reacts with phenolic compounds(Fig.3A)viaan ozone adduct which proceeds primarily to ring cleavage,formation of muconic-type compounds,and eventually resulting in aliphatic aldehydes,ketones and carboxylic acids [64].These formed aliphatic products can be biodegraded during the subsequent BAC process [65],which are expected to reduce the formation potential (FP) of THMs,HAAs,chloral hydrate (CH) and haloketones (HKs).For olefins (Fig.3B),the ozone reaction occursviathe Criegee mechanism that involves cleavage of the C=C double bond and formation of carbonyl compounds [66],which are biodegradable for BAC process,and ultimately reducing the FP of THMs,HAAs,CH and HKs.For amines (Fig.3C),an ozone adduct on the nitrogen atom leads to formation ofN-oxide for tertiary amines and hydroxylamine for primary and secondary amines [67],and further ozone oxidation can form nitro compounds [68].Biodegradation of these ozonolysis products of amines in BAC process is beneficial for the reduction of N-DBPFP such as HANs,HAMs,and HNMs.
Fig.4 summarizes the treatment performance of pre-BAC ozonation on bulk water quality parameters and various structural types of DBP precursors in literatures (Table S4).Ozonation had a significant effect on UV254removal (average 29%),but limited or negligible effect on DOC (5.7%) and DON (dissolved organic nitrogen) removals (11.5%).This can be explained from the reaction mechanism that certain UV absorbing units of NOM can be partially oxidized and transformed to lower MW compounds by ozonation rather than being mineralized (Fig.3) [63].In addition,in the full-scale operation,the particulate matter leaked from presand filtration could be oxidized to the dissolved organic matter by ozone,which leads to the increase of DOC level after postozonation [69].For the DBP precursors,ozonation shows a significant effect on decrease of THMFP (18.3%),HAAFP (14.7%),HAMFP(20.4%),HANFP (23.3%) and NAFP (26.7%),but limited effect on HKFP removal (11%) and negative effect on HALFP (haloacetaldehyde formation potential) (-5%) and HNMFP removals (-136.9%).The dramatic increase of HNMFP after ozonation is due to the oxidation of amino groups,leading to pronounced formation of nitro compounds (Fig.3C).
4.2.Removal of various DBP precursors
Fig.5 illustrates the treatment performance of BAC process on bulk water quality parameters and various structural types of DBP precursors based on the literature data (Table S4).BAC process can remove approximately 30.3% of DOC on an average level,decreasing from 3.76 mg/L to 2.36 mg/L (Fig.5A).The TOXFP in the influent has an average level of 1 mg/L,accounting for 27% of DOC(Fig.5B),and BAC can averagely reduce 43.9% of TOXFP [47,63,70-72].This indicates the DBP precursors are relatively easier to be decreased in comparison to bulk DOC.The known DBPFP including THMFP,HAAFP,HALFP,HKFP,HAMFP,HANFP,HNMFP and NAFP in the influent has an average level of 390 μg/L and accounts for 38.7% of TOXFP (Fig.5B).The removal efficiency of these known DBPFPs across the BAC contactor (45.2%) is slightly higher than that of TOXFP,resulting in the weakly increased proportion of known DBPFP in the BAC effluent (Fig.5C).Of the known DBP precursors,THMs and HAAs,mid-μg/L concentration level (Fig.5A),are the predominant ones and they together account for over 90%of the identified DBPs in both influent and effluent of the BAC process.HALFP,HKFP,HAMFP,HANFP and HNMFP are usually at lowμg/L level in the influent,while NAFP is typically at sub-μg/L level.
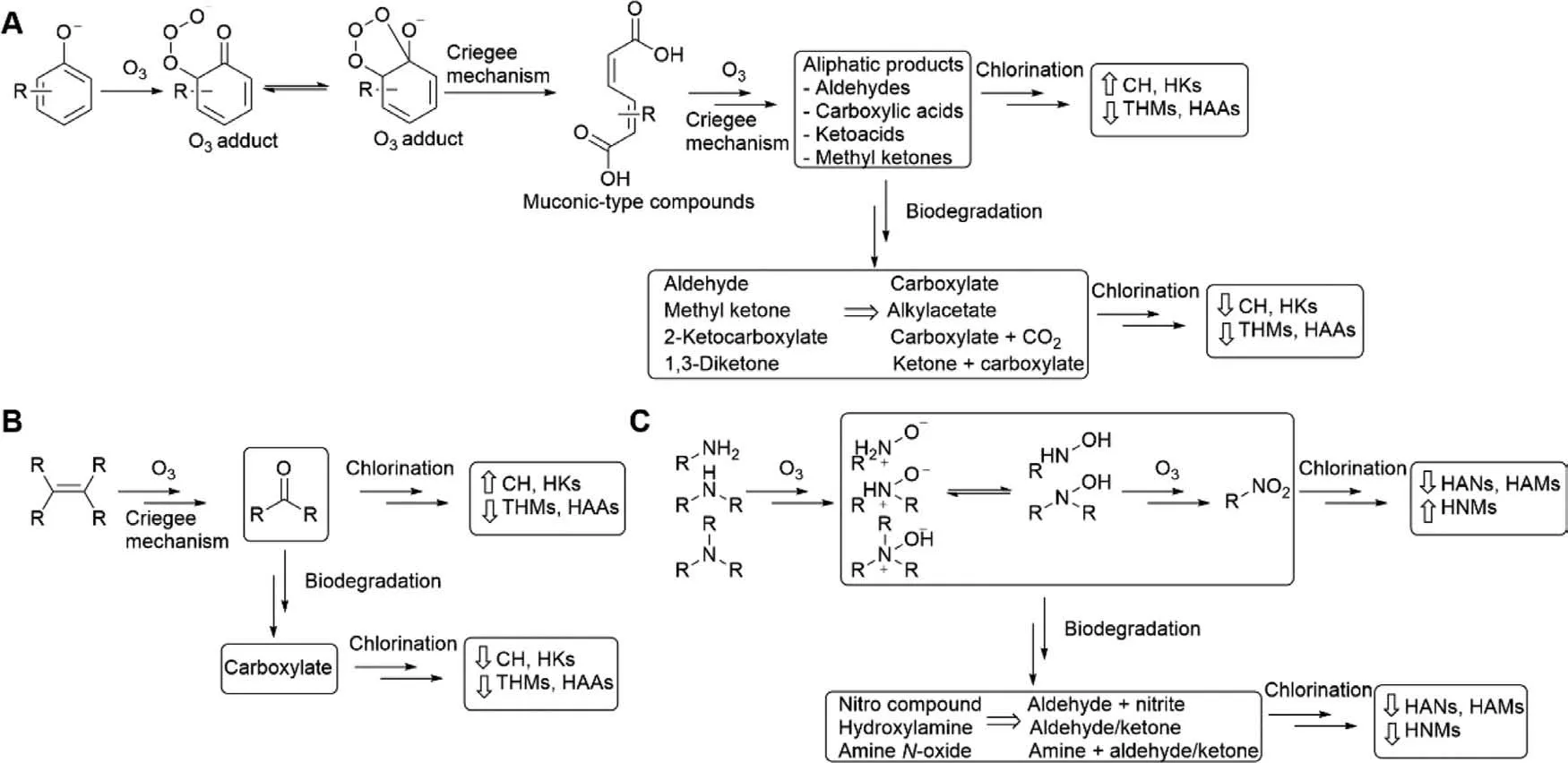
Fig.3.Impact of ozonation and biodegradation on DBP precursors: (A) phenolates,(B) olefins,and (C) amines.Abbreviations: CH,chloral hydrate;HAAs,haloacetic acids;HAMs,haloacetamides;HANs,haloacetonitriles;HKs,haloketones;HNMs,halonitromethanes;THMs,trihalomethanes.This figure is modified from literature [63].
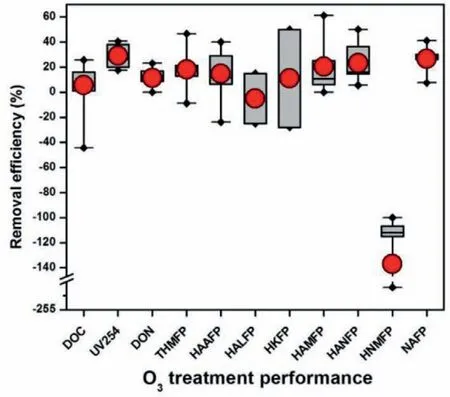
Fig.4.Effect of pre-BAC ozonation on the removal of bulk water quality parameters and various structural types of DBP precursors.Abbreviations: DOC,dissolved organic carbon;DON,dissolved organic nitrogen;FP,formation potential;HAA,haloacetic acid;HAL,haloacetaldehyde;HAM,haloacetamide;HAN,haloacetonitrile;HK,haloketone;HNM,halonitromethane;NA,nitrosamines;THM,trihalomethane;UV254,ultraviolet at 254 nm.The detailed literature data can be found in Table S4.
For different types of DBP precursors,the average removal efficiency across the BAC process is in the order of HKFP (72.5%)>HALFP (69%)>HNMFP (66%)>NAFP (57.7%)>HAMFP (51.3%)>HAAFP (48.1%)>HANFP (46%)>THMFP (37.4%) (Fig.5A).The direct precursors for HKs and HALs are ketones and aldehydes,which are prone to removal by biodegradation (Table 1 and Fig.3).In addition,HKs and HALs,as intermediate DBPs,are rapidly formed as soon as the water is chlorinated,and finally converted to THMs or HAAs due to hydrolysis or reactions with residual chlorine [73],which also contributes the high reduction of HKFP and HALFP.Non-aromatic amines of small size are the most important precursors to form HNMs,which are highly biodegradable [17].Thus,high FP removal of HNMs across the BAC process has been observed.NAs formation seems to be complex,and early mechanistic research suggested the nucleophilic substitution reaction between unprotonated secondary amines and chloramines was the most important NA formation pathway [74].The biological degradation of secondary amines [65]and biological oxidization of ammonia [16]during the BAC process can effectively suppress the formation of NAs.Amino acids and aldehydes yield the highest HAM and HAN concentrations [74],and biodegradation plays the crucial role to breakdown of these precursors (Table 1).Relatively,the removal of THMFP and HAAFP is restrained compared with intermediate DBPs (HKs and HALs) and N-DBPs (NNMs,NAs,HAMs and HANs).This can be explained by the high contents of THM and HAA precursors (e.g.,humic substances) (Table 2) and replenishment from the conversion of intermediate DBPs (e.g.,HKs and HALs) [73].
Bromide ions are nearly ubiquitous in natural water sources,and chlorine can rapidly oxidize bromide ions to form bromine during the drinking water chlorination process [75],which shows a stronger halogenating activity than chlorine and can produce brominated DBPs (bromo-DBPs) [29].Because of their high molecular weights,bromo-DBPs can cause difficulties for utilities to meet the regulatory limits of TTHM and HAA5.Moreover,bromo-DBPs may present higher health risks than their chlorinated analogs based on some toxicological studies [75].Bromine incorporation factor (BIF) has been used by many researchers to evaluate bromine substitution of DBPs [75,76].Typically,the BIF across the water treatment processes (including BAC process) shows an increasing trend [46,76],because of non-affecting bromide ion concentrations [70,77].For instance,Kosakaet al.[76]observed the BIF in a DWTP increased from ~0.2 in raw water to ~0.8 in BAC effluents.However,although BAC may increase the bromo-DBP proportion,the overall reduction of DBPFP far exceeds this negative change,thereby producing a much less toxic effluent.I-THMs have the similar situation,and the average removal of I-THMFP across the BAC process is-13.8%,while their proportion in the TOXFP of effluent is only 0.8% [49,69,77].
4.3.Removal of toxicity risks of DBPFP
Above discussion indicates the BAC process can effectively remove various DBP precursors.Since different classes of DBPs have different toxicity levels,it seems more meaningful to evaluate the control effectiveness of DBPs by comparing their integrated toxicity risks,rather than merely investigating their FPs [69].Plewa and colleagues systematically investigated the cytotoxicity and genotoxicity of a broad range of DBPs,including THMs,HAAs,HANs,HNMs and HAMs,and established the combined toxicity value(CTV,L/mol) of each DBP [78,79].Based on these CTVs,Chu and colleagues calculated the integrated toxicity risk value (ITRV) of a specific DBP in DWTPs by multiplying its CTV by FP (nL/mol) [80].The ITRV for one class of DBPs can be calculated by summing values for each DBP.
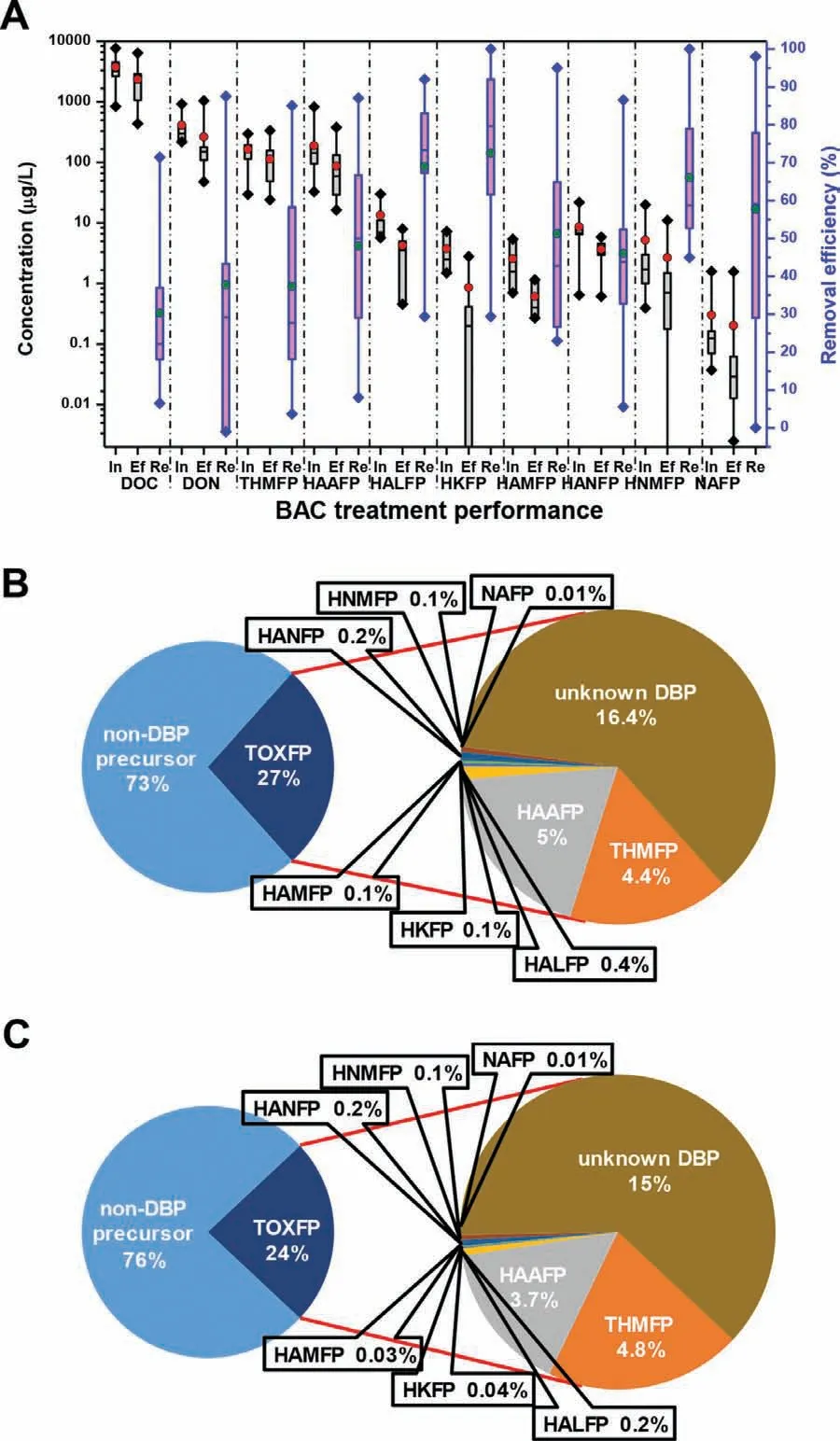
Fig.5.BAC treatment performance on the removal of bulk water quality parameters and various structural types of DBP precursors: removal amount and efficiency(A),and proportional distribution of DBP precursors in DOC before (B) and after (C)treatment.Abbreviations: DOC,dissolved organic carbon;DON,dissolved organic nitrogen;Ef,effluent;FP,formation potential;HAA,haloacetic acid;HAL,haloacetaldehyde;HAM,haloacetamide;HAN,haloacetonitrile;HK,haloketone;HNM,halonitromethane;In,influent;NA,nitrosamines;Re,removal efficiency;THM,trihalomethane;TOX,total organic halide.The detailed literature data can be found in Table S4.
We used the reported data from a full-scale study [69]to illustrate the removal of toxicity risks of DBPFP across the BAC process.As shown in Fig.6,the BAC process shows a good removal of all kinds of DBPFP with an average efficiency of 37.1%.However,the toxicity risks of BAC effluent actually slightly increase by 2.4%.Inspecting the DBP classes,we find the toxicity risk of HANs is significantly elevated by 42.4% across the BAC process,and moreover,the ITRV of HANs accounts for the largest proportion (>80%) in the ITRV of total DBPs.Therefore,HANs play the critical role for the toxicity risks of DBPs though their concentrations are much lower than those of THMs and HAAs.Further inspecting the HAN species,dibromoacetonitrile (DBAN) is caught out due to its dramatic increase of concentration (by nearly onefold) and high CTV (61,444 L/mol).Although the dichloroacetonitrile (DCAN) is the dominant HAN species and its concentration is reduced by 64%,the relatively lower toxicity (CTV = 712 L/mol)cannot compensate the increased risk induced by DBAN.Thus,the significant higher toxicity risk caused by HANs than other DBPs cannot be ignored.In fact,many studies have indicated HANs,especially DBAN,are the toxicity drivers among the known DBPs [81-83].Moreover,most of the target DBPs are aliphatic compounds,while many of more toxic aromatic DBPs have not been well identified,such as halo-benzoquinones,halo-hydroxybenzaldehydes,halo-hydroxybenzoic acids,halo-salicylic acids,halo-phenols,and halo-pyrroles [52,84,85].Therefore,the change of toxicity risks of DBPFP across BAC process is difficult to predict,and some toxic species may pose an important effect.
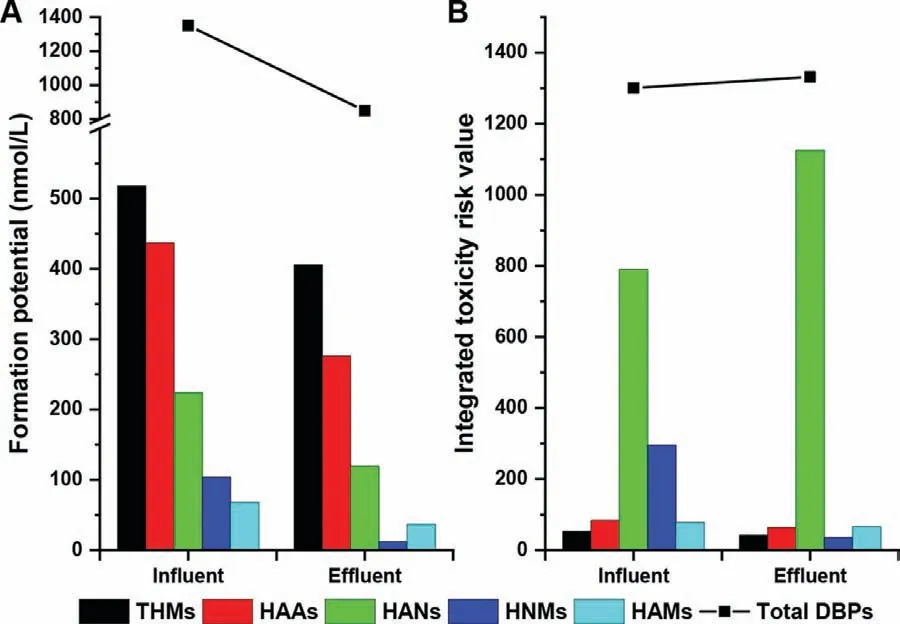
Fig.6.Removal of DBPFP (A) and integrated toxicity risks (B) by a full-scale BAC process.Abbreviations: HAA,haloacetic acid;HAL,haloacetaldehyde;HAM,haloacetamide;HAN,haloacetonitrile;HNM,halonitromethane;THM,trihalomethane.The used data are from literature [69].
4.4.Fractional analysis on NOM
Many researchers conducted the fractional analysis on NOM to explore the removal patterns of NOM and DBP precursors across the BAC process.The employed methods include composition characterization by liquid chromatography-organic carbon detection (LC-OCD) analysis [59,70-72]and fluorescence excitationemission matrix (F-EEM) [47,53,86],MW distribution analysis by high performance size exclusion chromatography (HPSEC) [63,87],MW fractionation through ultrafiltration [51,55,86,88],and polarity fractionation through solid-phase extraction [76]and resins[51,54,61,89].LC-OCD can characterize NOM into five fractions including biopolymers,humic substances,building blocks,low molecular weight (LMW) acids,and LMW neutrals [90].According to the fluorescence features of NOM,F-EEM may be divided into five parts [91]: (I) aromatic protein (represented by tyrosine);(II)aromatic protein (represented by tryptophan);(III) fulvic acid-like;(IV) soluble biological byproduct-like;(V) humic acid-like.HPSEC can be used to characterize the MW distribution in a series of MW gradients,such as<0.5 kDa,0.5-1 kDa,1-3 kDa and>3 kDa[87],while ultrafiltration can separate the samples into subgroups according to the MW gradients [92].Resins or solid-phase extraction are often adopted to fractionate NOM into different fractions based on the polarity,such as hydrophobic acid,base and neutral,weakly hydrophobic acid and hydrophilic matter [61].
Generally,the LMW fraction (<1 kDa) and hydrophilic fraction are the dominant compositions in NOM [92],which are the major sources of DBP precursors [51],and also preferable fractions for biodegradation (Table 1).For instance,the LMW fraction of<1 kDa in the Feng-shan Reservoir water of Southern Taiwan was 1.40 mg/L,and accounted for 38% of DOC.This fraction caused a THMFP of 222.79 μg/L,accounting for 53.1% of the THMFP for total dissolved organic matter (DOM) [93].BAC can preferably remove the LMW fraction,resulting in the relatively higher removal of DBP precursors relative to that of bulk DOC across BAC process (Fig.5A).For instance,a pilot-scale study indicated the removal efficiency of LMW (<3 kDa) DOC by BAC was 30%,while the removal of high molecular weight (HMW) fraction (>3 kDa) was only 8% [55].FEEM studies indicated BAC could effectively decrease the fractions of aromatic proteins and soluble biological byproducts [89],which are significant precursors of NDBPs (Table 2).Therefore,high removal efficiency of NDBPFP can be achieved by using BAC technology (Fig.5A).
4.5.Effect of EBCT
Of the BAC studies for removing DBP precursors (Table S4),the typical EBCT is 12-22.5 min.Extending the EBCT can enhance the removal of organics,but it may sacrifice efficiency and increase cost.Thus,exploring the relationship between removal of organics and EBCT is very important for BAC process.Many studies have indicated the effluent DOC along increased EBCT during biofiltration process shows a fast decrease in a characteristic initial period followed by a period of slow decrease [94].de Vera and colleagues also observed a similar trend for the decrease of DBP precursors across BAC process [63].The relationship between effluent DOC or DBPFP and EBCT can be described by a first-order kinetic equation[63,94]:

wheretis the EBCT (min),kis the specific first-order rate constant(min-1),Ptis the concentration at timet(mmol/L),Pbiodegis the biodegradable concentration (mmol/L),andPfis the minimum DOC concentration or DBPFP (mmol/L) after a certain EBCT.
Table S5 (Supporting information) summarizes the model parameters for reduction of DOC and DBPFP across BAC process in de Veraet al.’s study [63].The results indicated a good fitting of Eq.1 for various DBPFP as well as DOC with a coefficient of determination (R2) of 0.9268-0.9993.kcan be used to evaluated the sensitivity of different classes of DBP precursors for the EBCT,and a largerkvalue indicates a faster decrease with increasing the EBCT.The calculatedkfor different classes of DBP precursors is in the order of HAL>HK>HNM>HAN>THM>HAM>HAA,which is basically consistent with their removal efficiency (Fig.5A).For a desired DBPFP level,the needed EBCT can be calculated using the deformation of Eq.1,t= [ln (Pbiodeg)-ln (Pt-Pf)]/k.For example,if we want an effluent THMFP of 1.5 μmol/L,the needed EBCT is calculated to be 11.4 min.
4.6.Engineered biofiltration
Currently,the biofiltration (mainly BAC) process is generally working in a passive pattern,and the treatment performance and stability are largely dependent on media configuration,backwash strategy and loading rate [95].The microbial activity plays a crucial role in the performance of a biofilter including removal of NOM and inorganic contaminants,reduction of turbidity,underdrain fouling,media clogging and filter head loss.Recently,“engineered biofiltration” has been proposed to change the passive process to a purposefully operated biological system.The goal of engineered biofiltration is to improve the water quality or hydraulic performance by providing specific conditions to enhance the biological performance.So far,two engineered strategies are prevalent: nutrient enhancement and peroxide enhancement.
Nutrients are important for the microbial production and secretion of extracellular polymeric substances (EPS),which consists of exopolysaccharides and other biopolymers [96].EPS are the structural materials of microbial biofilms,where microorganisms can establish stable intercellular arrangements and function as synergistic microbiota.EPS also constructs protective barrier for microbes to resist various environmental stress [97,98].In a biofilter,the ratio of C (bioavailable organic carbon,BOC):N (ammonium nitrogen,NH3-N):P (orthophosphate phosphorus,PO4-P),is important for the microbial growth [95],and a molar ratio of C:N:P = 100:10:1 is generally recommended in an aerobic process[99].However,the nutrient concentrations of NH3-N and PO4-P are usually are limitation factors for microbial EPS production and activity levels [100].Therefore,replenishing nutrients in the feed water has been considered as an effective way to promote the microbial growth in the biofilter,and enhance the microbial activity for removal of NOM and DBP precursors.
A number of researches have been conducted to evaluate the nutrient enhancement on the performance of BAC process[16,29,71,95,101,102].A pilot-scale study investigated the method of nutrient enhancement to improve the performance of BAC process at the John F.Kubala Water Treatment Plant (JKWTP).The BAC contactor with nutrient enhancement was found to decrease terminal head loss by approximately 15% relative to a control BAC contactor without nutrient enhancement.Nutrient enhancement also decreased breakthroughs of DOC as well as 2-methylisobornel(MIB),and manganese (Mn) [95].Nemani and colleagues claimed phosphorus addition to the BAC influent had a small impact on DOC (3%) and THMFP (5%) reduction when compared to control without nutrient enhancement [101].However,other studies suggested the nutrient addition had no significant effect on enhancing the biological performance of BAC for removal of DOC and DBP precursors [29,71].A possible explanation is the biodegradable carbon fraction was limited in their study and addition of nitrogen or phosphorus would not further enhance the biological performance.
For peroxide enhancement,hydrogen peroxide (H2O2) is commonly applied to the biofilters,which can not only provide microorganisms with an additional source of dissolved oxygen,but induce the expression of peroxidase-family oxidoreductase enzymes in microorganisms to catalyze the oxidation of organic matters [103].Studies indicated low dosages of peroxide (<1 mg/L)may effectively mediate the oxidation of inactive biomass and EPS[104,105].EPS are potential foulants of biofilters and play a significant role in media clogging,underdrain fouling,and filter head loss [106].Therefore,removing the excessive EPS can effectively improve the operational performance of biofilters [95].
Lauderdale and the coworkers have conducted a series of pilot studies to investigate effect of H2O2on the operational performance of biofilters.A case study in JKWTP suggested H2O2increased oxidative action of BAC microorganisms and promoted the oxidation of inactive biomass;the addition of peroxide decreased terminal head loss up to approximately 60% relative to the control biofilter [95].In Azzehet al.’s work [71],addition of 0.5 mg/L H2O2to BAC contactor decreased head loss by up to 45% without affecting removal of DBP precursors.The hydraulic improvement by peroxide is specific and dependent on multiple factors,such as temperature,source water,microbial ecology and upstream treatment.
5.Conclusions and perspectives
DBP issue is a relatively troublesome problem in drinking water treatment.One reason is that DBPs are very toxic and pose a threat to human health,and the other is that the DBPs have a broad of precursors,which are difficult to remove.NOM in surface water and especially the LMW polar fractions (e.g.,amino acids,aldehydes and ketones) play a critical role in the formation of DBPs during the post-chlorination process.Unfortunately,the conventional treatment processes,i.e.,coagulation-sedimentationfiltration,preferably remove HMW hydrophobic fractions of NOM,and therefore,considerable DBPFP still exists in the effluent.Advanced treatment is needed to further remove the recalcitrant NOM compositions.Based on the detailed analyses on DBPFP of different NOM groups and the mechanisms and selectivity of different water treatment processes,BAC process is the optimal choice for controlling the DBP levels in the finished water,especially when combined with pre-ozonation,which can significantly increase the small MW fractions,breakdown the large aromatic structures,and increase the BDOC/DOC ratio of the NOM in water.
Typically,BAC process has a better treatment performance on DBP precursors compared with the bulk DOC.It is gratifying that BAC process can effectively decrease the FP of N-DBPs,which are considered to be more toxic than the carbonaceous DBPs (C-DBPs).These removal patterns of DBP precursors can be well explained by the fractional analysis on NOM.EBCT is an important parameter for BAC’s performance,and a first-order kinetic equation can be used to determine the optimal EBCT with the goal of decreasing suffi-cient DBPFP.In addition,the recently proposed engineered biofiltration with nutrient or peroxide enhancement seems to achieve considerable hydraulic improvement but limited effect on DOC or DBPFP removal.
O3-BAC/BAC process is a mature technology and widely used to control the DBP levels in many DWTPs and some sewage treatment plants for potable water.Although large numbers of researches have been conducted and the treatment performance of BAC on DBP precursors removal has been recognized,there are still many knowledge gaps especially microbial mechanisms needed further study to fill up.The following issues may be constructive for the future BAC study on the removal of DBP precursors:
(1) Microbial mechanisms.Microbes in BAC play the key role for removing the organic and inorganic contaminants,where biodegradation of organics and nitrification of ammonium are the dominant processes.Previous researches rarely involve microorganisms.Only few studies measured the biomass or characterize the bacterial community structure.The biochemical mechanisms on the degradation of various DBP precursors have not been addressed.The recent prevalent metagenomics technique is a powerful tool that can map the profiles of microbial community structure and functions.Applying metagenomics may be helpful to construct the enzyme-based metabolic pathway for the transformation and degradation of DBP precursors during BAC process.
(2) Toxicity evaluation.The toxicity of DBPs is the major concern,while the current regulation of water quality is based on the mass weight of target DBPs.As discussed in the review,the change of toxicity risks of DBPFP across BAC process is difficult to predict,and some toxic species may pose an important effect.Moreover,the DBPs are a mixture of various types of toxic compounds,and some synergistic and antagonistic effects may exist among these compounds.Therefore,it is particularly necessary to establish a simple and effective method for determining the water potential toxicity induced by the formation of DBPs.In addition,the pre-filter ozonation could generate large quantities of bromate,and organic bromine,promoting the production of bromo-DBPs which may present higher health risks than their chlorinated analogs.The potential heal risks of preozonation should be investigated in details.
(3) Degradation kinetics.The degradation kinetics of pollutants along BAC bed can reflect the relationship between microbial growth and degradation velocity of organic substrate,and the Monod equation is universally acknowledged as the practical model.The relationship between degradation velocity and biomass can be used to estimate the matrix potency;if the relationship is linear,the matrix has high organic degradation potency,while if the relationship is in exponential form,the matrix has low organic degradation potency.This model can be applied to BAC matrix to estimate its degradation potency for DBP precursors.
(4) Microbial products.The microorganisms in the BAC will produce some microbial products such as soluble microbial products (SMP) and EPS,when microorganisms degrade organics in water.These microbial products will generate new sources for DBP precursors.However,these DBP precursor sources are less concerned.Some researches can be conducted to characterize the microbial products in the BAC effluent and evaluate their contribution to the total DBPFP.The development of microbial metabolomics may provide a powerful tool for the screen and identification of the compounds derived from microbial metabolism.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
J.Fu acknowledges the support by National Natural Science Foundation of China (Nos.91851110 and 41701541),and Hubei Provincial Natural Science Foundation of China (No.2020CFA106).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.12.044.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
