In situ localization of BiVO4 onto two-dimensional MXene promoting photoelectrochemical nitrogen reduction to ammonia
Demei Zhng,Shiyu Yng,Xioyu Fng,Huifeng Li,*,Xuebo Chen,Dongpeng Yn,
a Beijing Key Laboratory of Energy Conversion and Storage Materials,Key Laboratory of Radiopharmaceuticals,Ministry of Education,College of Chemistry,Beijing Normal University,Beijing 100875,China
b Key Laboratory of Theoretical and Computational Photochemistry,Ministry of Education,College of Chemistry,Beijing Normal University,Beijing 100875,China
c Green Catalysis Center,College of Chemistry,Zhengzhou University,Zhengzhou 450001,China
Keywords:Photoelectrocatalysis Nitrogen reduction reaction BiVO4 2D materials MXene
ABSTRACT The existing industrial ammonia synthesis usually adopts the Haber-Bosch process,which requires harsh conditions of high temperature and high pressure,and consumes high energy.Under this circumstance,photoelectrochemical (PEC) catalysis is regarded as a promising method for N2 reduction reaction (NRR),but bears problems of low efficiency and yield.Thus,exploring active catalysts remains highly desirable.In this work,BiVO4@MXene hybrids have been facilely synthesized by a hydrothermal route.The heterojunctions by the in situ growth of BiVO4 onto two-dimensional (2D) MXene greatly increase the NRR efficiency: under photoelectric conditions,the optimized NH3 yield is 27.25 μg h- 1 cm-2,and the Faraday efficiency achieves 17.54% at-0.8 V relative to the reversible hydrogen electrode (RHE),which are higher than most state-of-the-art NRR (photo) electrocatalysts.The mechanism speculation shows the enhanced light absorption range and the heterojunction formation largely promote the separation and the transfer efficiency of photogenerated carriers,thereby improving the PEC catalytic ability.Therefore,this work provides a hybrid route to combine the advantages of photo and electric catalysis for effective artificial nitrogen fixation.
Synthetic ammonia is a major breakthrough in the history of modern technology and has alleviated the problem of food shortages [1].Ammonia has become one of the most produced chemical compounds in the world due to its wide range of uses,e.g.,more than 80% of the ammonia is used to make nitrogenous fertilizers [2].Since 1913,the industrial ammonia synthesis has adopted the Haber-Bosch process [3].With the advancement of technology and the renewal of equipment,the process conditions for industrial ammonia synthesis have been optimized.At present,the conversion rate of each reactant in industrial ammonia synthesis is between 25%-35%,depending on the design and configuration of the reactors [4,5].There are still two problems to be solved: (1) The process needs to consume nearly 1.2% of the world’s energy [6],including 5% of natural gas,because the raw material H2comes from steam reformed natural gas [7];(2) The process emits 1.5% CO2gas in the atmosphere every year,which affects the environment and climate [8].
The electrocatalytic nitrogen reduction reaction (NRR) at room temperature and atmospheric pressure has zero emission in the whole process,and the raw material H+comes from an abundant aqueous solution [9-11].In this context,electrocatalysis is very promising in the next generation of ammonia synthesis process.However,high bond energy and potential together with low yield and Faraday efficiency (FE) have limited the development of the electrocatalytic NRR to some extent.Thus,the design of new catalysts,which inhibit hydrogen evolution reaction (HER) activity to boost NRR process,is highly desirable [12-14].From the view of clear energy,in photoelectrochemical (PEC) cells,semiconductor materials are usually used as photoelectrodes,which play an important role in light absorption and photocatalysis,and also effectively promote the electrocatalytic process with the aid of light[15,16].It has been reported that the p-type semiconductor as photocathode can catalyze the NRR [17].By modifying the surface state of the electrode,such as lattice defects and specific surface area,the PEC activity can be improved [18].A typical PEC catalysis introduces light energy to the electrocatalysis equipment [19,20],which combines advantages of photocatalysis and electrocatalysis to ensure commercial ammonia production [21].The key to PEC NRR also includes selectively increase of the N2adsorption and effectively decrease of the activation energy barrier in each intermediate reaction.
In addition to the broadly applicable precious metals (such as Pt[22,23]and Au [24-26]),transition metals (including nickel [27,28],iron [29-32],cobalt [33],zinc [34],molybdenum [35,36]and bismuth [37-42]) systems also serve as NRR catalysts [43-46].For example,the BiVO4catalyst with oxygen holes breaks the kinetic limit and promotes the adsorption and reduction potential of N2.The amorphization helps to increase the activity relative to the crystalline counterpart of electrocatalytic NRR [47].Due to the extremely low solubility of nitrogen in aqueous solutions,the adsorption of nitrogen at the surface of the catalyst becomes a difficulty[48].The high specific surface area of two-dimensional (2D) materials,with effective electron transport channels and a large number of surface unsaturated atoms,can increase the catalytic active sites [49].For example,the full exposure of edge in bismuth nanosheets and effectivep-orbital electron delocalization facilitate the N2adsorption [50].In addition,the semiconductor properties that limit the accessibility of surface electrons can improve the FE of the nitrogen fixation reaction [51].
Among the 2D catalysts,MXene has large specific surface area,excellent metallic conductivity,and large number of unsaturated atoms on the surface which can stably adsorb positive charges,but performs weak nitrogen fixation ability [52-55].It has been reported that MXene can be combined with semiconductors to form a Schottky junction for efficient photocatalytic hydrogen evolution,because it can generate the built-in electric field to effectively improve carrier seperation [56,57].In this work,we used acid etching to obtain 2D layered MXene,and then hydrothermally grew BiVO4on the surface of MXenein situ.The different ratios of BiVO4@MXene led to tunable PEC NRR properties,with the optimized NH3yield of 27.25 μg h-1cm-2and FE of 17.54% at-0.8 Vvs.RHE under photoelectric conditions.The high PEC performance can be related to the Schottky junction formed between BiVO4and MXene,in which the photogenerated electrons excited by light can promote charge transfer,and the electron transport channel built by the heterostructure can effectively reduce the resistivity of the catalysts.Thus,we propose that hybridization of electrocatalysts and photoactive semiconductors can serve as an efficient way to realize the high-efficiency catalytic nitrogen fixation utilizing both light and electric energy.
Titanium aluminum carbide (Ti3AlC2,98%),bismuth(III) nitrate pentahydrate (Bi(NO3)3·5H2O,99%),vanadium nitrate (NH4VO3,99.5%),sodium hydroxide (NaOH,99%),sodium dodecylbenzenesulfonate (SDBS,95%),Nafion (5 wt%) were purchased from Beijing InnoChem Science &Technology Co.,Ltd.Hydrofluoric acid(HF,40%) and nitric acid (HNO3,AR) were purchased from Beijing Tong Guang Fine Chemicals Company.All the reagents were used without additional purification.Deionized (DI) water (H2O) were used in the research.
Firstly,5 g of Ti3AlC2was ball milled for 6 h to become the fine powder.Secondly,1.2 g of the prepared Ti3AlC2powder was corroded with 20 mL of 40 wt% HF solution for 24 h under stirring to completely remove the Al layer.Finally,the solution was rinsed with H2O to remove unnecessary HF until the pH of the solution reached 5-6,and the residue was dried under vacuum at 80°C for 24 h.
BiVO4nanoparticles were prepared by a hydrothermal method.Typically,2.45 g of Bi(NO3)3·5H2O and 0.58 g of NH4VO3were dissolved in 10 mL of HNO3(4 mol/L) solution and NaOH (2 mol/L) solution,respectively.Then,0.25 g of SDBS was added to the above two solutions under vigorous stirring.After stirring for 0.5 h,two solutions were mixed.2 mol/L NaOH was added to adjust the pH to 7,and then the mixture was continuously stirred for 0.5 h.After that,the suspension was transferred to a 50 mL stainless steel autoclave lined with Teflon and placed in an oven at 200°C for 3 h.After the autoclave was cooled to room temperature,the resulting bright yellow sample was centrifuged,washed several times with water and ethanol,and then dried at 100 °C for 4 h.BiVO4@MXene samples were prepared in the same way,except that Bi(NO3)3·5H2O and MXene were dissolved in 10 mL of HNO3(4 mol/L) solution.The molar ratio of Bi(NO3)3·5H2O and NH4VO3was 1:1,while SDBS were added in proportion to the weight of BiVO4and Mxene was added to obtain BiVO4@MXene samples with weight ratios of 0:1,1:5,1:2,1:1,2:1,5:1 and 1:0,respectively.
Before electrocatalytic and PEC measurements,5.0 mg catalyst was dispersed in a mixed solution containing 800 μL deionized water,isopropanol (200 μL) and 30 μL Nafion solution (5.0 wt%).Ultrasound for at least 1 h produced a stable suspension.Next,200 μL of the suspension was loaded on a carbon paper electrode with an area of 1×1 cm2and dried under ambient conditions to obtain a working electrode.All electrochemical measurements were tested by a potentiostat (CHI 660E) through a three-electrode system.The working electrode,reference electrode and counter electrode corresponded to carbon paper supported catalyst,Ag/AgCl saturated electrode and Pt wire electrode,respectively.Nafion 211 membrane was used after treatment.An open beaker containing a 0.1 mol/L HCl solution around the test equipment was used to keep the concentration of ambient ammonia in the solution below the detection limit to avoid the impact on ambient ammonia.Before each electrolysis,N2gas was slowly bubbled for at least 30 min until the N2was saturated.In addition,during the entire NRR electrolysis process,N2gas was continuously bubbled into the electrolyte 0.1 mol/L K2SO4for 2 h.Under the applied potential ofiR(irefers to the current,whileRis the overall resistance,including contact resistance,charge-transfer resistance,and intrinsic resistance) compensation (95%),linear sweep voltammetry (LSV) was used with a rate sweep of 2 mV/s.The calibration of all potential measurements was based on the following Nernst equation (Eq.1)[53]:
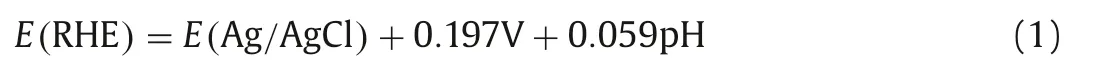
For PEC process,carbon paper supported catalyst,Ag/AgCl saturated electrode and Pt wire electrode were working electrode,reference electrode and counter electrode,respectively.Additional light illumination supplied by a 300 W Xe arc lamp (Newport Corporation) was fitted with an AM 1.5 G filter at the cathode of the electrolytic cell.According to theCdl=Ic/v,we tested the electrochemical double-layer capacitance at a potential of-0.05 V to 0.25 V relative to the RHE,so as to compare the actual electrochemical active surface area (ECSA) of the catalyst.In the formula,Cdl,Icandvare the double-layer capacitance of the catalyst (F/cm2),charging current (mA/cm2) and scan rate (10,20,40,60,80 and 100 mV/s),respectively.Electrochemical impedance spectroscopy (EIS) was obtained at a voltage of 1.8 V relative to RHE,with a frequency range of 0.01-100 kHz,and an amplitude of 5 mV/s [54].
The obtained NH3was determined by the indoxyl blue method.Usually,2 mL electrolyte of electrochemical cell was taken from the cathode compartment.After that,2 mL of 1 mol/L NaOH solution containing salicylic acid (5 wt%) and sodium citrate (5 wt%),1 mL of 0.05 mol/L sodium hypochlorite (NaClO) and 0.2 mL of nitroferricyanide sodium (C5FeN6Na2O,1 wt%) were added.After standing at room temperature for 2 h,the absorbance was measured using an ultraviolet-visible spectrophotometer.The produced indoxyl blue can be quantitatively measured by the equation conversion of the absorbance value at a wavelength equal to 655 nm.Using standard NH4Cl solutions with different concentrations,a standard curve was established in the K2SO4electrolyte to obtain the calibration curve fitting equation required to calculate the NH3content.
The Watt and Chrisp method was used to measure the concentration of N2H4in a 0.1 mol/L K2SO4solution.We mixed 4 mL of the catalyzed electrolyte solution with an equal volume of color developer.The developer was a mixed solution of 4 g ofp-(dimethylamine)benzaldehyde (p-C9H11NO) dissolved in concentrated HCl (15 mL) and anhydrous C2H5OH (150 mL).After standing for 0.5 h,we measured the absorbance of the yellow solution by the UV-vis spectrum at a wavelength of 455 nm.Finally,using the absorbance of standard hydrazine dihydrochloride solutions with different concentrations,a calibration fitting equation for calculating the N2H4content can be established.
The yield of NH3can be determined by the formula: Yield rate=(C(NH3)×V)/(t×A),where C(NH3) is the measured NH3concentration,Vis the electrolyte volume,tis the reaction time,andAis the surface area of the working electrode.FE is the percentage of electrons consumed during the formation of a given product,which is directly related to specific product selectivity.Assuming that three electrons are needed to generate an NH3molecule,the FE calculation formula is: FE=(3F×C(NH3)×V)/Q.
50 mL of electrolyte solution obtained by PEC catalysis under-0.8 V with14N2and15N2gas was steamed respectively,until 5 mL of solution remained.2 mL of concentrated sample,2 mL of phenol solution (mixed with 6.0 g phenol,3.0 g Na3PO4·12H2O,3.0 g trisodium citrate dihydrate,0.3 g of ethylenediaminetetraacetic acid anhydrous disodium salt,0.02 g of Na2[Fe(CN)5NO]·2H2O in 100 mL solution),1 mL NaClO solution (100 mL aqueous solution containing 2 mL of 5 wt% NaClO and 1.6 g of NaOH),and 1 mL of 0.5 wt%formic acid solution were mixed and stirred for 1 h.Then we used the LTQ-XL linear ion trap mass spectrometer from Thermo Scientific (USA) for further analysis.
The preparation of catalysts was based on the use of the 2D MXene material for the dispersion of the BiVO4.HF acid etching was used to form a multilayer 2D MXene (Scheme 1),and then thermally combined with vanadium-bismuth metal salt to synthesize BiVO4@MXene [58].The hybrid catalysts with different proportions were hydrothermally synthesized to further optimize the PEC NRR performances.
Scheme 1.Schematic diagram of the synthesis process of BiVO4@MXene heterojunction.
Fig.1a showed the XRD profiles of BiVO4@MXene hybrids with different ratios (0:1,1:5,1:2,1:1,2:1,5:1 and 1:0).In each case,the peak positions at 18.9°,28.8° and 30.5° corresponded to the(011),(112) and (004) crystal planes of BiVO4,respectively [59].The diffraction peaks before 10° (dashed frame) were attributed to the MXene,which proved the effective combination between BiVO4and MXene [60].It was observed that as the ratio of BiVO4increased,the main peak intensity of MXene decreased.The disappearance of the main peak of MXene occurred in the sample BiVO4@MXene (5:1).The morphological characterizations of BiVO4@MXene with different ratios were shown in Fig.S2 (Supporting information),which showed that the surface of MXene was gradually affected by the increase of BiVO4.MXene was covered completely in the sample BiVO4@MXene (5:1),which was consistent with the observation of XRD pattern.XPS was used to detect the element and valence changes in BiVO4@MXene.As shown in Fig.1b,all signals of Bi,V,O,C and Ti can be observed clearly in the survey XPS spectrum of BiVO4@MXene.For the Ti 2p pattern (Fig.1c),six peaks at 455.07,456.24,459.53,460.97,462.13 and 465.85 eV can be deconvoluted and ascribed to Ti(III) 2p3/2,Ti(II) 2p3/2,Ti(IV) 2p3/2,Ti(II) 2p1/2,Ti(III) 2p1/2and Ti(IV) 2p1/2,respectively [57].After combined with BiVO4,the corresponding low-valence species disappeared and only two peaks corresponding to Ti(IV) 2p3/2and Ti(IV) 2p1/2can be observed,which may be due to some redox reaction occurred during the synthesis process.It is worth noting that the binding energy of Ti(IV) 2p3/2and Ti(IV)2p1/2in BiVO4@MXene had shifted negatively compared with MXene.Figs.1d-f compared the valence changes of BiVO4after being combined with MXene.164.02 eV and 158.82 eV represent the 4f5/2and 4f7/2of the Bi element,respectively,and 516.22 eV represents the 2p3/2of the V element [61].After combined with MXene to generate BiVO4@MXene (1:1),binding energy of Bi 4f,V 2p and O 1s moved positively,which was opposite to that of Ti.Therefore,it can be inferred from the XPS characterization that the electron transfer path was from BiVO4to MXene,which indicated Schottky junction was formed in the BiVO4@MXene composite [56].
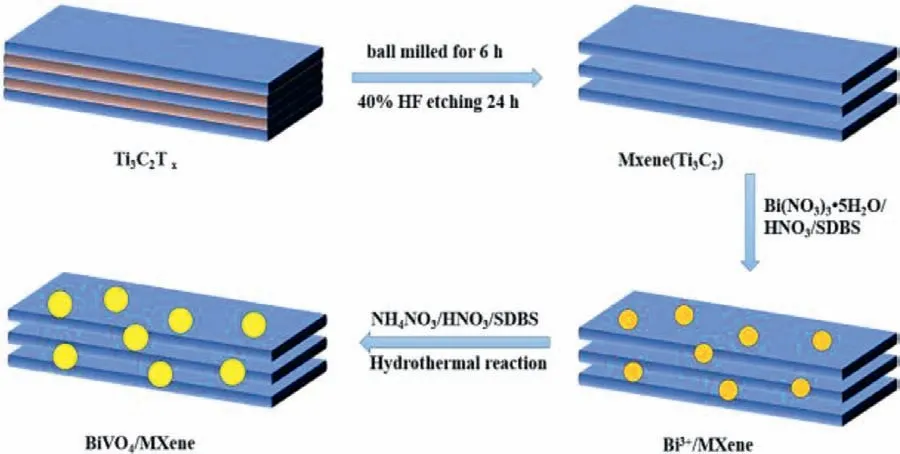
The MXene synthesized by etching has been shown in Fig.2a,from which an obvious layered structure can be observed.Fig.2b showed that the side length of the single-layer MXene was about 150 nm and the thickness was 3 nm.For the composite (Figs.2c and d),the BiVO4was uniformly dispersed on the surface and between layers of MXene in the BiVO4@MXene (1:1).According to TEM image (Figs.2e and f),BiVO4@MXene had obvious crystalline and amorphous interface.The interplanar spacing of 0.226 nm corresponds to the (211) crystal plane of BiVO4,and the amorphous section belonged to MXene.The element mapping(Figs.2g-l) showed that the Bi,V,O,Ti,C and F elements were evenly distributed,which was consistent with XPS,indicating that BiVO4and MXene were tightly coupled together,forming a crystalline/amorphous Schottky junction.
Considering that K+ion can effectively block the collision probability between water molecules and the catalyst,and reduce the activity of the hydrogen evolution reaction (HER),the K2SO4solution was selected as the electrolyte in this work.The calibration curve for calculating the yield was shown in Figs.S3 and S4 (Supporting information).From the LSV curve in Fig.S5 (Supporting information),it can be observed that the current curve of BiVO4@MXene under photoelectric conditions was higher than the value without light,which indicated that BiVO4@MXene hybrid was more active upon light irradiation.Fig.3a showed the chronocurrent graph of BiVO4@MXene (1:1) at different potentials,in which the more negative the potential,the greater the current value.Fig.3b was the bi-coordinate diagram of ammonia yield and FE of BiVO4@MXene,in which the NH3yield was 27.25 μg h-1cm-2and FE was 17.54% at-0.8 Vvs.RHE.The stability of the catalyst was also tested.Fig.3c showed the chronocurrent curve of BiVO4@MXene under photoelectric condition in continuous 5 times at-0.8 Vvs.RHE.Calculated from the UV absorption diagram (Fig.S7 in Supporting information),the NH3yield was relatively stable (Fig.3d),and the specific values were shown in Table S1 (Supporting information).Under the same conditions at-0.8 Vvs.RHE,the NH3yield and FE values of BiVO4@MXene with different ratios (Fig.S6 in Supporting information) were 2.35,2.94,9.41,14.90,10.59,and 5.10 μg h-1cm-2and 0.40%,1.93%,5.20%,11.87%,5.11%,1.11%,respectively.The yield (Fig.4a) and FE (Fig.4b) values of BiVO4@MXene with various ratios showed a volcano pattern trend,in which BiVO4@MXene (1:1) had the highest activity in PEC NRR.Upon removal of light,the chronocurrent curve(Fig.4c) and low NH3yield (7.84 μg h-1cm-2) and FE (4.03%) values verified BiVO4@MXene does exhibit high photoelectric activity(Fig.4d).Such PEC performance can be further confirmed by the relatively low yield and FE of pristine MXene and BiVO4at-0.8 Vvs.RHE (Table S2 in Supporting information).

Fig.1.(a) XRD diagrams of BiVO4@MXene with different ratios (0:1,1:5,1:2,1:1,2:1,5:1,1:0) in curves a to g.(b) Survey XPS profile of as-prepared catalysts.(c) Highsolution XPS spectra of Ti 2p of MXene and BiVO4@MXene.(d) High-solution XPS spectra of Bi 4f.(e) V 2p and (f) O 1s of BiVO4 and BiVO4@MXene.
Fig.2.(a) SEM and (b) AFM images of MXene.(c,d) SEM and (e) TEM images of BiVO4@MXene (1:1).(f) The enlarged area of the selected area in (e).(g-l) The element mappings of Bi,V,O,Ti,C and F elements of BiVO4@MXene (1:1).
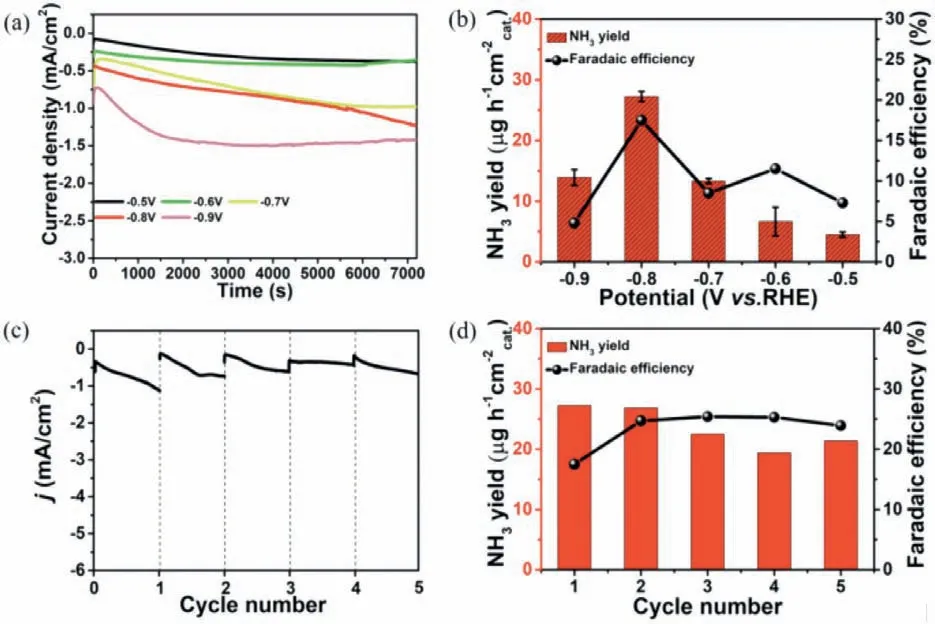
Fig.3.(a) Chronocurrent graph.(b) Ammonia yield and FE of BiVO4@MXene (1:1)under photoelectric conditions at different potentials.(c) Chronocurrent curve.(d)Ammonia yield and FE of BiVO4@MXene with continuous 5 times under PEC condition at-0.8 V vs.RHE.
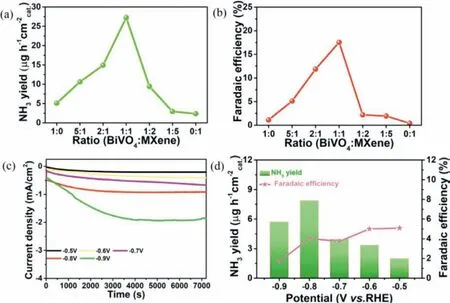
Fig.4.(a) The NH3 yields.(b) FE of various ratios under PEC condition at-0.8 V vs. RHE.(c) Chronocurrent curve.(d) NH3 yield and FE of BiVO4@MXene under no light condition.
In order to further explore the PEC mechanism,we explored the impact of the photoelectric response on the catalyst.As shown in Fig.5a,under light irradiation,the photocurrent appeared instantaneously,but the photocurrent dropped instantly when turning off the light source.Moreover,the photocurrent value of BiVO4@MXene was higher than that of the pristine BiVO4and MXene,which proved that the combination with MXene can effectively improve the light response of BiVO4.Fig.5b showed that photoelectron of BiVO4@MXene had a much longer life span than BiVO4,while that of MXene was almost negligible.The solid ultraviolet absorption pattern of BiVO4@MXene (Fig.S9 in Supporting information) was converted into a semiconductor band gap(Eg) of 1.84 eV (Fig.5c).Compared with BiVO4with a band gap of 2.22 eV,the band gap was reduced,which means BiVO4@MXene can absorb a wider range of visible light.This also explained the higher photocurrent and photovoltage of BiVO4@MXene.From XPS(Fig.S10 in Supporting information),valence band (EVB) of BiVO4and BiVO4@MXene was determined to be 1.94 and 1.38 eV,respectively.The corresponding conduction band (ECB) values were calculated to be-0.37 eV and-0.46 eV according to the formulaEg=EVB-ECB.The more negative ofEVBis,the more thermodynamically conducive to N2reduction.The emission intensity of BiVO4@MXene in the fluorescence spectrum (Fig.S11 in Supporting information) was lower,which was attributed to the electron transfer through the heterojunction,thus reducing electron-hole recombination.
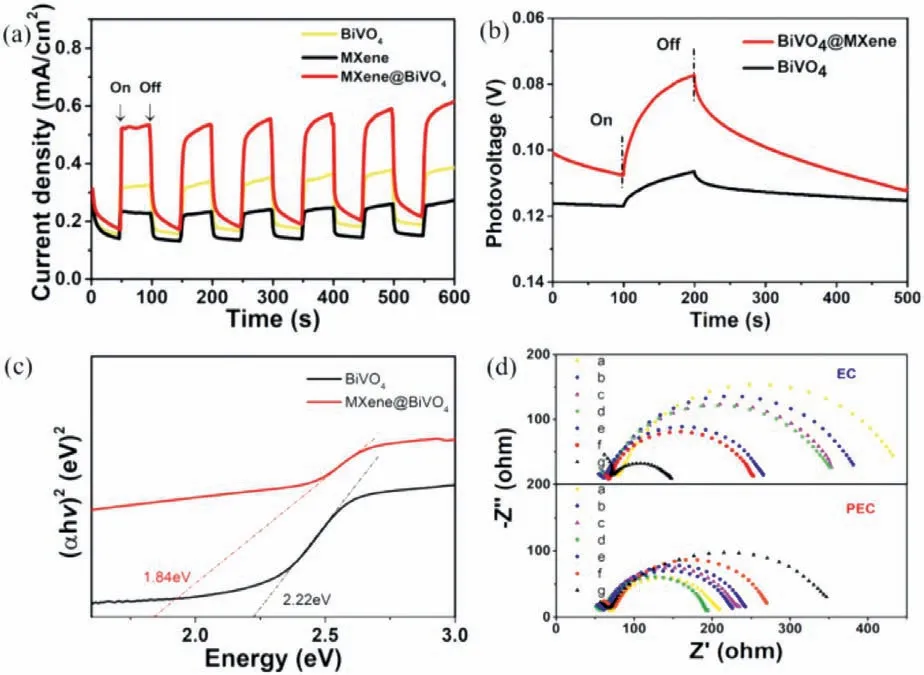
Fig.5.(a) Photocurrent under light on and off conditions.(b) Photovoltage change dependent on the time.(c) Semiconductor band gap of the BiVO4@MXene and pristine BiVO4.(d) Nyquist diagrams of BiVO4@MXene with different ratios (0:1,1:5,1:2,1:1,2:1,5:1 and 1:0 for curves a-g) in 0.1 mol/L K2SO4 solution at 1.8 V vs. RHE with a frequency ranging from 0.1 Hz to 100 kHz with 5 mV/s.
The charging current density differenceΔjplot against scan rate of BiVO4@MXene with different proportions was shown in Fig.S13 (Supporting information).As the content of MXene gradually increased,the slope became higher,which was consistent with the high surface area of 2D MXene material.Fig.5d showed the Nyquist diagrams of the catalysts loaded on GCE in 0.1 mol/L K2SO4solution at 1.8 Vvs.RHE with the frequency ranging 0.1-100 kHz.TheCdlvalue can be calculated to represent the ECSAs.It can be seen that under light condition,the resistance of BiVO4@MXene(1:1) was the lowest.The number of carriers in semiconductor is inversely proportional to the resistivity,so a high number of photogenerated electrons corresponds to a low resistivity.Moreover,mass spectrometry was used to prove the source of ammonia.As shown in Fig.S14 (Supporting information),94.6% of phenol blue containing14N2was detected in the14N2electrolyte solution.Correspondingly,75.0% of phenol blue containing15N2was detected in the15N2electrolyte solution.The results of mass spectrometry proved that ammonia was obtained from NRR catalytic process.
Based on the above experimental results,we confirm that assynthesized BiVO4@MXene has a superior PEC NRR performance.It was proved that V5+provides the main adsorption and active sites due to its more negative adsorption energy in BiVO4[62,63].However,if the numbers of photogenerated electrons is greater than those of N2adsorption and desorption,rest photogenerated electrons will recombine with holes,resulting in the loss of photogenerated electrons.We propose the PEC mechanism of BiVO4@MXene as follows (Fig.6): The localization of BiVO4is dispersed onto 2D MXene matrix,and the superior conductivity also enhances the electron transport ability.Under light irradiation,the photogenerated electrons from BiVO4can be transferred to MXene through the heterojunction interface.Then,N2is further reduced on the 2D surface of MXene,which greatly reduces the recombination probability of photogenerated electrons and holes.In addition,it adjusts the conductive band position of BiVO4to move it in the direction that is more conducive to light absorption,which improves the light response capability and the PEC NRR efficiency.In other words,BiVO4serves as the light response center for effective electron-hole separation,and MXene acts as the electrocatalytic active sites.
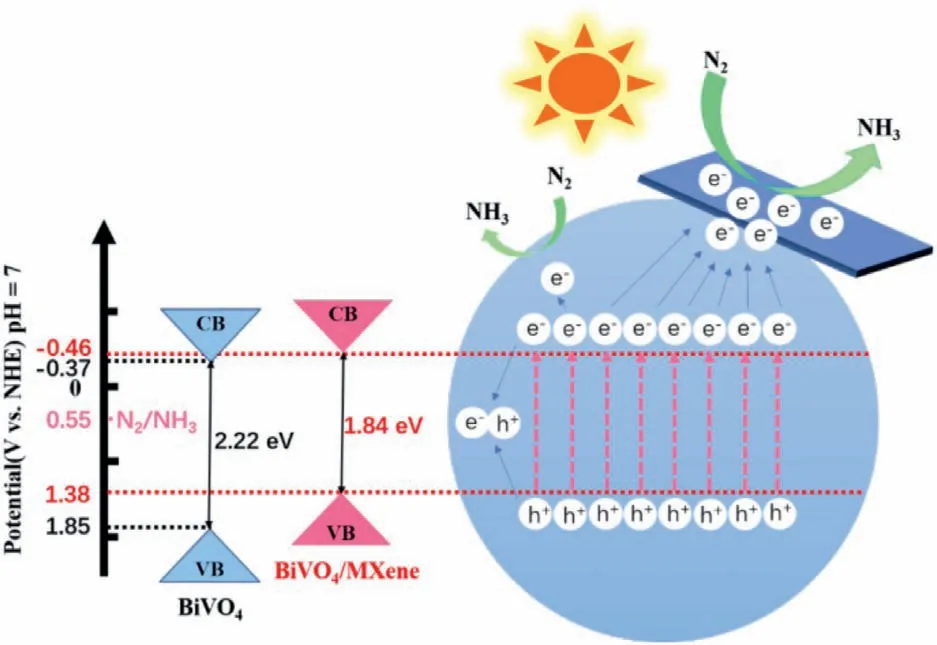
Fig.6.Photoelectrocatalytic mechanism of N2 to NH3 by BiVO4@MXene.
In summary,in situgrowth of BiVO4onto 2D layered MXene results in the formation of uniform Schottky junction structure.The optimized NH3yield of BiVO4@MXene (1:1) is 27.25 μg h-1cm-2,and the optimized FE is 17.54% at-0.8 Vvs.RHE under PEC condition.Such NRR performances outperform most of state-of-the-art NRR electrocatalysts (Table S3 in Sopporting information).The photogenerated electrons excited by light can promote charge transfer,and the electron transport channel established by the heterojunction can effectively reduce the resistivity of the catalyst,thereby effectively improving the PEC NRR properties.Therefore,we propose a promising strategy to combine semiconductor BiVO4and highconductivity MXene towards artificial nitrogen fixation application using primary energy solar energy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21771021,21822501,21725303,22061130206 and 22120102005),the Beijing Municipal Natural Science Fundation (No.JQ20003),the Newton Advanced Fellowship award(No.NAFR1201285),the Fok Ying-Tong Education Foundation(No.171008),the Measurements Fund of Beijing Normal University,and the State Key Laboratory of Heavy Oil Processing.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.02.001.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
