Single molecular insight into steric effect on C-terminal amino acids with various hydrogen bonding sites
Yunzhi Xie,Chunhu Liu,Linxiu Cheng,Yuln Fn,Huifng Li,Wei Liu,Lei Zhu,Xun Li,Ke Deng,Qingdo Zeng,Shouf Hn,*
a State Key Laboratory for Physical Chemistry of Solid Surfaces,Department of Chemical Biology,College of Chemistry and Chemical Engineering,The Key Laboratory for Chemical Biology of Fujian Province,The MOE Key Laboratory of Spectrochemical Analysis &Instrumentation,and Innovation Center for Cell Signaling Network,Xiamen University,Xiamen 361005,China
b Jiangxi Provincial Key Laboratory of Low-Carbon Solid Waste Recycling,School of Geography and Environmental Engineering,Gannan Normal University,Ganzhou 341000,China
c Jiangxi Key Laboratory of Organo-Pharmaceutical Chemistry,Chemistry and Chemical Engineering College,Gannan Normal University,Ganzhou 341000,China
d CAS Key Laboratory of Standardization and Measurement for Nanotechnology,CAS Center for Excellence in Nanoscience,National Center for Nanoscience and Technology (NCNST),Beijing 100190,China
Keywords:C-terminal amino acids Steric effect Bonding site Scanning tunneling microscopy DFT calculations
ABSTRACT Amino acids are basic units to construct a protein with the assistance of various interactions.During this building process,steric hindrance derived from amino acid side groups or side chains is a factor that could not be ignored.In this contribution,adsorption behaviors of C-terminal amino acid derivatives with amino acid residues fused in 3,4,9,10-perylenetetracarboxylic dianhydride were investigated by scanning tunneling microscopy (STM) and density functional theory (DFT) calculations at various liquid/solid interfaces.STM results at 1-phenyloctane/HOPG interface show that N,N’-3,4,9,10-perylenedicarboximide(GP) and N,N’-methyl-3,4,9,10-perylenedicarboximide (AP) formed linear and herringbone structures,respectively.The driving force could be attributed to different H-bonding sites induced by steric hindrance at side groups.N,N’-Benzyl-3,4,9,10-perylenedicarboximide (PP) generates both linear and herringbone structures because steric hindrance changes the H-bonding sites between PP molecules,whereas N,N’-isopropyl-3,4,9,10-perylenedicarboximide (LP) failed to be imaged because of strong steric hindrance coming from larger side group.To further investigate the impact of steric hindrance,we utilized octanoic acid (OA) as solvent to capture the adsorption details of LP and PP.We found that OA molecules drag PP and LP molecules in a different direction to generate linear structure,impeding the molecular rotation.The structure-solvent relationship shows that the steric hindrance is brought by the large side group,which makes it easier to recognize OA molecules at the interface.These results demonstrate that steric effect plays a significant role in altering interaction sites of the compounds during the adsorption process at the liquid/solid interface.
As one of the most important constituents of living organisms,amino acids play a vital role in the living process.Amino acids are perfect for building thousands of structure-specific and different functional peptides or proteinsviaintermolecular interactions[1-4]such as electrostatic,hydrogen-bonding,hydrophobic,π-πinteraction,and other interactions [5-7].For example,various interactions mentioned above are involved in the formation of secondary structures of protein,such asα-helix andβ-sheet,of which steric hindrance [8-10]from amino acid side groups or side chains plays an important role during this building process.However,the influence and mechanism of steric hindrance are not quite clear.Therefore,establishing a research model about the adsorption of amino acids on surface both theoretically and experimentally is the first and vital step [11]to understand steric hindrance effect,and explore the relationship between molecular interactions and the function of peptides or proteins.
However,the challenge is how to immobilize amino acids on surface under ambient conditions and maintain stability.To overcome the adsorption barriers,perylene core with flatπsystems[12,13]is introduced to fix amino acids on graphite throughππinteraction between perylene core and graphite.This perylene core is aπ-πconjugated organic semiconductor named 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA).PTCDA has been proved to be an excellent model system to investigate the growth process of organic thin films at different surface of substrates [14-21].Additionally,the derivatives of PTCDA have also been demonstrated to possess features of functional diversity [22].PTCDA is prone to disperse and adsorb on the substrate to generate herringbone structure with the assistance of H-bonding and interfacial interactions,hence the introduction of PTCDA into our system is a superior idea to uncover the steric hindrance effect of amino acids.
Scanning tunneling microscopy (STM) [23-32]has turned out to be extremely useful analytical technology to probe adsorption behaviors of amino acids on surface.STM with high spatial and electronic resolution has advantage over visualizing two-dimensional assembled structure and surface electronic structures [33-35].It has been utilized to investigate chemical reactions,conformation,and properties of self-assembled materials [36].Hence,STM is considered to be an effective means in the field of surface and nanoscale science [37-44].
In this work,we adopt a well-established synthetic strategy not only based on a series of C-amino acids (Gly,Ala,Leu-and Phe),but introducing a strongπ-πconjugated 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) core,to prepare Cterminal amino acid derivatives and further explore the steric effect of C-terminal amino acid residues on two-dimensional (2D)surface at ambient conditions.
In this work,Gly,Ala,Leu,and Phe-functionalized PTCDA compounds were investigated: GP,AP,LP and PP.The amino acid derivatives were synthesized according to supporting information.Chemical structures of these compounds are shown in Scheme 1.Prior studies showed that the preferential alignment of organic semiconductor compound PTCDA was herringbone structure with two molecules per unit cell when vapored on HOPG substrate [45].The arrangement of PTCDA does not depend on the lattice constant of the substrate but apt to slightly misalign from a symmetry axis to reduce strain [45].
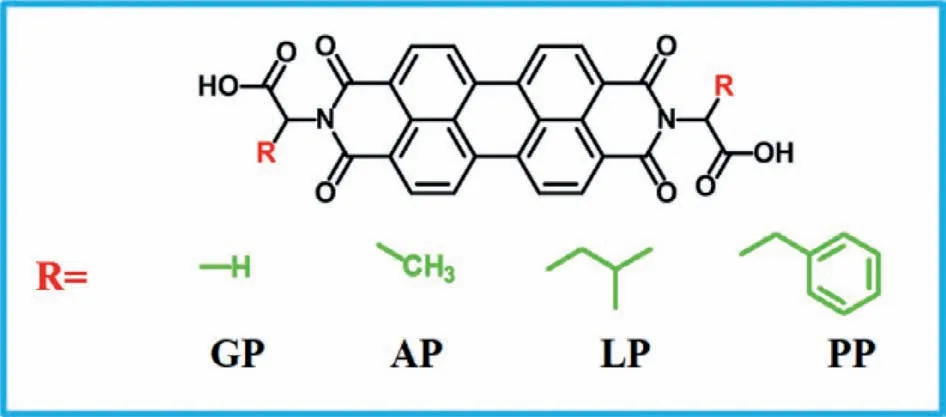
Scheme 1.Chemical structures of N,N’-R-3,4,9,10-perylenedicarboximide (GP,AP,LP and PP).
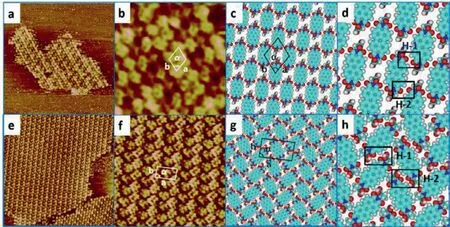
Fig.1.(a) Large-scale STM images of GP molecules at 1-phenyloctane/HOPG interface,the imaging parameter is 50.9 nm×50.9 nm,Iset=708.0 pA,Vbias=749.8 mV.(b) High-resolution STM image (10.6 nm×10.6 nm,Iset=708.0 pA,Vbias=749.8 mV).(c) Corresponding model of GP assembly structures.(d) Hydrogen bonding interactions between GP molecules,which are marked by black rectangles.STM images of AP at 1-phenyloctane/HOPG: (e) (50.3 nm×50.3 nm,Iset=487.1 pA,Vbias=639.5 mV) showing large-scale STM image.(f) High-resolution STM image(12.6 nm×12.6 nm,Iset=487.1 pA,Vbias=697.3 mV).(g) Suggested model of AP assembly structures.(h) Hydrogen bonding interactions between AP molecules,which are marked by black rectangle.
From the chemical structures exhibited in Scheme 1,amino acids (Gly,Ala,Leu-and Phe) were fused intoπ-πconjugated PTCDA core at the N-terminal position of amino acids to investigate steric effect of C-terminal amino acid residues on surface at ambient conditions.The only structural difference for these compounds exists in the C-terminal R groups.To probe the influence of steric effect from the C-terminal-R groups,we investigated the adsorption behaviors of these amino acid derivatives at two liquid/HOPG interfaces—1-phenyloctane/HOPG interface and octanoic acid (OA)/HOPG interfaceviaSTM imaging and DFT calculations.We initially explored adsorption behaviors of these four derivatives (GP,AP,LP and PP) at the 1-phenyloctane/HOPG interface.The results show that GP,AP and PP could self-assemble into ordered structures while LP failed to form a monolayer.According to DFT calculations,the calculated unit cell parameter for simulated structures agrees well with the parameter measured from highresolution images (Table 1).GP molecule with the simplest side group constructs a large condensed linear structure (Fig.1a).According to the molecular dimension of GP from the high-resolution image displayed in Fig.1b,we find that one bright spot represents one GP molecule.These molecules self-assemble into periodic paralleled arrangement with the unit cell parameter measured to bea=1.3±0.1 nm,b=1.4±0.1 nm,andα=71° ± 1.0° (Fig.1b).The driving forces involved in this linear structure are two kinds of hydrogen-bonding interactions existing in Ar-H···O-Ar between adjacent GP molecules (Fig.1c).These two types of H-bonds with an average bond length of 3.53 ˚A are named H-1 and H-2 (marked by a black rectangle in Fig.1d).The H-bonding energies for H-1 and H-2 are-4.232 kcal/mol and-4.481 kcal/mol (Table S2 in Supporting information).
Though these H-bonds are weak,every molecule connects with four adjacent molecules,with the driving force born at two sides of the molecule keeping balance.The side group is too tiny to induce steric effect thereby the terminal-COOH of GP molecules fails to generate H-bonding interactions with adjacent molecules in the absorption process,and consequently lead to the formation of ordered linear structure.The occurrence of linear structure is the consequence of releasing the stress induced by GP molecules to the binding sites of the HOPG surface,differing from that of PTCDA.
When the side group is replaced by methyl,surprisingly,a long-range-ordered herringbone structure is observed at the 1-phenyloctane/HOPG interface (Fig.1e).According to the unit cell marked in Fig.1f,the parameters are measured to bea=2.3±0.1 nm,b=1.3±0.1 nm,andα=87° ± 1.0° The arrangement of this close-packed array composed of AP molecules is different from that of GP arrays.As shown in Fig.1g,the neighboring arrays show slight rotation compared with the linear structure of GP.Two kinds of hydrogen-bonding interactions are involved in the herringbone structure with the bonding sites appearing at Ar-H···O-Ar between adjacent AP molecules.The H-bonding energies of these two categories of H-bonds with a bond length of~3.48 ˚A are-8.629 kcal/mol (H-1) and-6.595 kcal/mol (H-2),respectively (Fig.1h and Table S2).Comparing with the H-bonding sites of GP linear structure,the disparate H-bonding sites of AP herringbone structure imply that larger side group methyl induces steric hindrance.The appearance of methyl groups leads to the rotation of AP molecule to connect with neighboring molecules in the same array to form Ar-H···O-Ar H-bonds,whereas the adjoining AP array rotates in opposite direction to interact with adjacent AP molecules in the same array,leading to the formation of herringbone structure.Therefore,the participation of methyl groups changes the H-bonding sites,breaks the original balance analogous to that of the GP configuration,and establishes a new equilibrium.As a result,AP molecules assemble into a herringbone structure of which the morphology is identical to the structure of PTCDA.
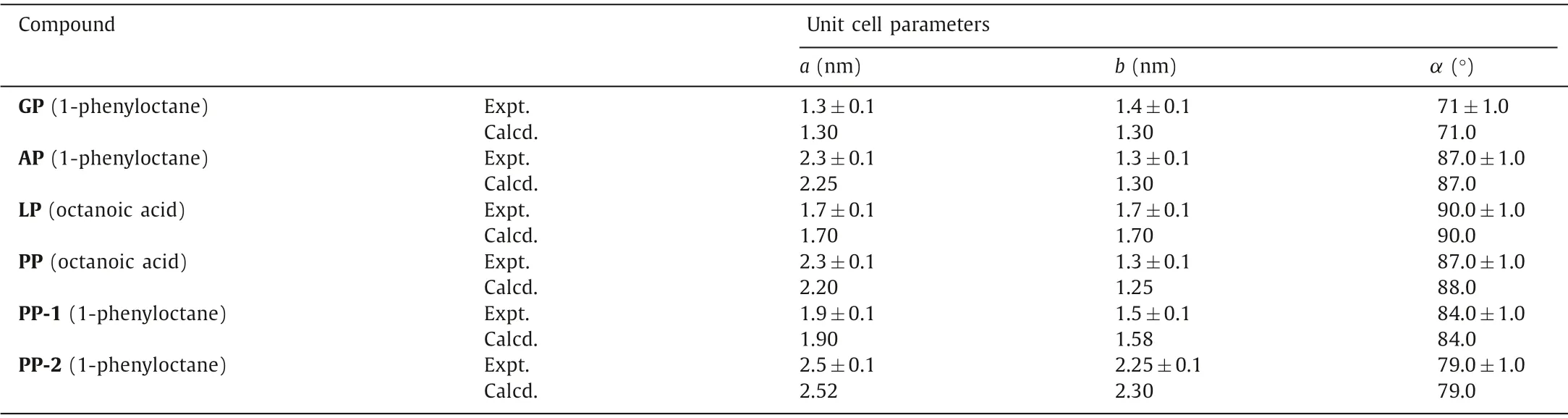
Table 1.Experimental (Expt.) and calculated (Calcd.) lattice parameters for assembly structures on HOPG.
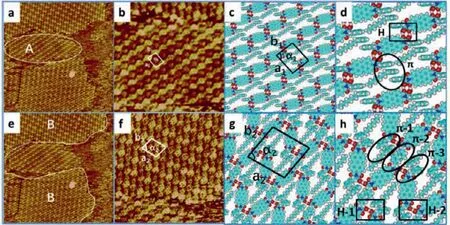
Fig.2.STM images for PP-1 and PP-2 structures at 1-phenyloctane/HOPG: (a)Large-scale STM image of PP-1 in domain A (49.5 nm×49.5 nm,Iset=592.4 pA,Vbias=749.8 mV).(b) (16.5 nm×16.5 nm,Iset=592.4 pA,Vbias=749.8 mV) showing high-resolution STM image of the domain A.(c) The tentative 2D packing model.(d) Interactions between PP molecules,the detail is marked by rectangle and circle.STM images for PP-2 structure in domain B at 1-phenyloctane/HOPG: (e)49.5 nm×49.5 nm,Iset=592.4 pA,Vbias=749.8 mV,showing large-scale STM image.(f) 17.7 nm×17.7 nm,Iset=592.4 pA,Vbias=749.8 mV,showing high-resolution STM image.(g) The corresponding 2D packing model.(h) Interactions between PP molecules,the detail is marked by rectangle and circle (black rectangle for H-bond,black circle for π-π interactions).
When the side group is isobutyl,the leucine functionalized compound LP fails to adsorb at 1-phenyloctane/HOPG interface.The failure of adsorption could be attributed to steric hindrance coming from the bulky isobutyl group.As the side group becomes large enough,steric effect is strong enough to prevent LP molecules from interacting with adjoining molecules to generate ordered nanostructure at the 1-phenyloctane/HOPG interface.
Whereas the side group is replaced by benzyl group,two different morphologies—PP-1 (in domain A) and PP-2 (in domain B) are visualized in Fig.2.The difference between these two structures exists both in the packing pattern and packing density.The largescale STM image of the self-assembled adlayer of PP-1 (in domain A) is exhibited in Fig.2a.PP-1 is composed of alternately packed bright and dark strips with four bright spots surrounding a dark spot to construct a supercell.The dimension of unit cell which is marked by a black quadrangle is determined to bea=1.9±0.1 nm,b=1.5±0.1 nm,andα=84° ± 1.0° As shown in Fig.2c,the bright spots (Fig.2b) represent the PTCDA cores,whereas the dark spots representπ-stacked benzyl and phenyl groups.One dark spot contains three benzene rings with two benzyl groups coming from neighboring PP molecules and one phenyl group coming from the 1-phenyloctane molecule.This speculation is confirmed by the appearance of the STM image,characteristics of its orientation,physical dimension,and DFT calculation result.As exhibited in Fig.2d,hydrogen bonding interaction andπ-πstacking are involved in this structure.Molecules interact with neighboring molecules in the same string to generate-COOH···O-Ar-hydrogen-bonding interaction with bond length measured to be ~2.66 ˚A (marked by a black rectangle in Fig.2d).Furthermore,molecules from adjacent strings interact with the phenyl group from 1-phenyloctane to generateπ-πinteraction.The energies of H-bonding andπ-πstacking are-19.087 kcal/mol and-12.077 kcal/mol,respectively (Tables S2 and S4 in Supporting information).
The other kind of configuration (PP-2) in domain B is herringbone arrangement which is similar to the herringbone structure constituted by AP molecules.Large domains of periodically arranged strings with adjacent strings behaving slightly differently are observed from the large-scale image in Fig.2e.From highresolution image,details of the herringbone structure are captured after careful observation.As shown in Fig.2f,we find that every two strings with identical orientations are separated by one string with a different orientation.These two kinds of strings adopt alternative arrangements to obtain periodical herringbone structure with the unit cell parameter measured to bea=2.5±0.1 nm,b=2.25±0.1 nm,andα=79° ± 1.0° The supercell which is drawn out with a white quadrilateral is constructed by six molecules with four molecules keeping one orientation and another two molecules keeping other orientation (Fig.2g).Detail of the driving force for the herringbone structure could be acquired from the simulated structure.As shown in Fig.2h,various kinds of hydrogen bonding interactions andπ-πstacking interactions are involved in this structure.Molecules in one string interact with adjacent string to generate two kinds of hydrogen-bonds (H-1 and H-2)via-COOH···HOOC-H-bonding interactions with an average bond length calculated to be 2.50 ˚A.The energies of these two types of double H-bonds are-40.946 kcal/mol and-42.251 kcal/mol,respectively (Table S2).Furthermore,π-πstacking interaction occurs between PP and 1-phenyloctane.On the one hand,PP connects with neighboring PP owing toπ-πinteraction between benzyl groups (π-2).On the other hand,PP interacts with 1-phenyloctane to formπ-πinteraction between benzyl and phenyl groups (π-1,π-3).The energies of these three categories ofππinteractions are-8.958 kcal/mol (π-1),-3.409 kcal/mol (π-2),and-6.367 kcal/mol (π-3),respectively (Table S4).New species of interactions—π-πinteractions are involved in the adsorption process of PP molecules.The related interactions and binding sites of these two types of morphologies constructed by PP molecules are different from the structures constituted by GP,AP and LP.The introduction of benzyl groups at the side group leads to the occurrence ofπ-πinteractions which enhance the stability of structures,albeit the side group is larger than LP.Comparing with AP,the benzyl group of PP could generateπ-πinteractions.Therefore,the H-bonding sites of PP linear and herringbone structure are distinctive from the H-bonding sites of AP herringbone structure,elucidating the larger side group benzyl groups assist the adsorption behaviors.
Table 2.Total energy and energy per unit area for amino acid-functioned systems.a.
The benzyl groups at the-R group site play two roles: steric effect andπ-πinteractions.On one hand,the bulky-R group may induce the rotation of PP molecules thereby changing the Hbonding sites of the molecules.On the other,the benzyl groups tend to interact with 1-phenyloctane and adjacent PP molecules.Combining these two factors,linear alignments and herringbone structures are observed during the adsorption process of PP.Owing to theπ-πinteractions,the competitive steric effect turns into an accelerative factor for PP molecules to generate highly ordered configurations.The herringbone structure is more stable than the linear structure with energy per unit area of the herringbone structure (-0.471 kcal mol-1˚A-2) lower than the linear structure (-0.364 kcal mol-1˚A-2) (Table 2),indicating the herringbone structure is the inherent advantageous structure for PP molecules.To some degree,the linear structure tends to turn into a herringbone structure.
The adsorption behaviors of GP,AP and LP show that steric hindrance becomes obvious increasing when the-R group are hydrogen atom,methyl,and isopropyl.Nevertheless,as for PP,the steric effect becomes an advantageous factor because the-R group contains benzyl groups which could formπ-πinteractions with adjacent molecules.
As LP fails to immobilize at the 1-phenyloctane/HOPG interface,and PP could assemble into both linear structure and herringbone structure,the influence of steric hindrance is unclear.To further investigate how steric hindrance affected the self-assembly process,we utilize octanoic acid (OA) as solvent to capture the adsorption details of these two compounds.According to the STM results,adsorption behaviors of LP and PP at OA/HOPG interface are inconsistent with those of these two compounds at the 1-phenyloctane/HOPG interface.As shown in Fig.3a,large-scale assembly structures constructed by LP molecules are visualized at OA/HOPG interface.Molecules assemble into linear packed geometry in Fig.3b as expected with the parameters yield to bea=1.7±0.1 nm,b=1.7±0.1 nm,andα=90° ± 1.0° The corresponding molecular model is exhibited in Figs.3c and d with the hydrogen-bonding interactions indwelling in carboxyl groups of LP and OA molecules.According to DFT calculations,the Hbonding energies for the linear-type configuration are calculated to be-43.952 kcal/mol and-43.628 kcal/mol (Table S2).The invasion of OA molecules into the system elucidates that the steric hindrance deeply influences the assembly behavior of LP compounds,resulting in enough space to trap and co-assemble with solvent molecules.It is speculated that the existence of steric hindrance changes the H-bonding sites of LP molecules,showing the tendency of molecular rotation of LP to fit the HOPG surface to generate herringbone structure.Whereas the participation of OA molecules impedes the rotation of LP by dragging LP from a different direction,resulting in the formation of linear structure.
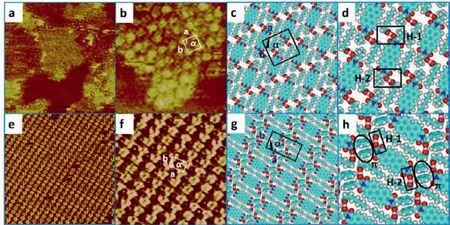
Fig.3.(a) Large-scale STM images of LP self-assembly structure at the OA/HOPG interface: 73.0 nm×73.0 nm,Iset=750.7 pA,Vbias=493.5 mV.(b) High-resolution STM image (12.3 nm×12.3 nm,Iset=750.7 pA,Vbias=493.5 mV).(e) STM images of PP assembly structure at OA/HOPG interface: 28.7 nm×28.7 nm,Iset=711.1 pA,Vbias=499.9 mV,showing large-scale STM image.(f) High-resolution STM image:11.0 nm×11.0 nm,Iset=711.1 pA,Vbias=499.9 mV.(c,g) show corresponding assembled model of LP and PP molecules,respectively.(d,h) Interactions for LP and PP assembly structures are marked by rectangle and circle,respectively (black rectangle for H-bond,black circle for π-π interactions).
To further gain insight into the impact of substituted groups on hydrogen-bonding sites for the self-assembled structure,we investigated the adsorption of PP compound at OA/HOPG interface.Fig.3e exhibits a large domain of periodic molecular arrays constructed by densely packed molecules.According to the highresolution image (Fig.3f),the molecular arrays are constructed by bright spots and dark space with the parameters measured to bea=2.3±0.1 nm,b=1.3±0.1 nm,andα= 87° ± 1.0° On basis of the observation,model building and DFT calculation were carried out with the results showing that the calculated parameter is identical to the measured one (Table 1).From the simulated structure,we could acquire information about the driving force and interaction sites for this linear structure.As shown in Figs.3g and h,hydrogen bonding interactions andπ-πstacking interactions are vital in the molecular packing process and structural stability.The interaction between PP molecules from adjacent arrays could be attributed toπ-πstacking between benzyl groups (π) with the energy calculated to be-6.414 kcal/mol (Table S4).Furthermore,participation of OA leads to the formation of H-bonding interactionsvia-COOH···HOOC-between PP and OA with the average bond length calculated to be 2.46 ˚A.The energies of these two Hbonds are-50.781 kcal/mol (H-1) and-46.899 kcal/mol (H-2),respectively (Table S2).The co-assembly of PP and OA exhibits that introduction of OA stabilizes the whole system with the combination of H-bonds andπ-πstacking,in which steric effect is a favorable factor for molecular arrangement.Similar to the co-assembly behavior of LP and OA molecules,the invasion of OA molecules into the system implies that the steric hindrance deeply affects the assembly behavior of PP,making enough space to trap and coassemble with solvent molecules.The occurrence of linear structure could be attributed to the steric effect and H-bonds coming from PP/OA system.It is speculated that steric hindrance changes the H-bonding sites of PP molecules,leading to molecular rotation of PP molecules to construct herringbone structure.However,the participation of OA molecules drags PP molecules in a different direction,impeding the molecular rotation.Consequently,PP molecules co-assemble with OA molecules to generate a linear structure.
Adsorption behaviors of C-terminal amino acid derivatives GP,AP,LP and PP were investigated at the 1-phenyloctane/HOPG and OA/HOPG interface.The above results indicate that side groups play significant roles in the adsorption process.Although GP and AP could form monolayers at the 1-phenyloctane/HOPG interface,the bonding sites of driving forces are disparate.Molecules in GP monolayers bond with four adjacent moleculesviaH-bonds with the bonding sites distributed in the molecular shoulders.However,molecules in AP monolayers could only interact with two adjacent moleculesviaH-bonds with the bonding sites locating at the molecular shoulders.The other two binding sites at the shoulder of the molecule fail to interact with adjacent molecules,which is due to the steric hindrance of methyl groups.In a word,the formation of herringbone structure is attributed to the steric effect from bulky methyl groups.GP and AP monolayers at 1-phenyloctane/HOPG interface exhibit their intrinsic binding sites because 1-phenyloctane fails to interfere in the formation of GP and AP monolayers.Interestingly,LP with isopropyl group which is larger than methyl group fails to generate herringbone structure.Two binding sites of LP are inclined to form H-bonds.Nevertheless,the other two biding sites at the shoulder of the LP molecule fail to interact with adjacent molecules because of the strong steric hindrance at the sopropyl group,failing to fix on the HOPG surface.Especially,PP with bulky side groups successfully self-assembles into two species of nanostructures,because 1-phenyloctane participates in the molecular assembly.The coassembly of 1-phenyloctane and PP molecules throughπ-πinteraction enhances the stability of the linear and herringbone structures.
To further investigate the influence of steric hindrance on the molecular self-assembly process,we utilized octanoic acid as a solvent to capture the adsorption details of LP and PP.According to the high-resolution STM images,LP and PP both arrange into linear structures due to their larger C-terminal amino acid residues.This phenomenon elucidates that when the side group is larger,the bonding sites of driving forces have been changed,leading to the co-assembly of amino acid derivatives with OA molecules.The differentiation of the interaction sites suggests that steric effect deeply influences the adsorption behaviors of amino acid derivatives.It is speculated that steric hindrance could change the Hbonding sites of LP (PP),leading to molecular rotation of LP (PP).However,OA molecules are involved in the self-assembly behavior by generating H-bond LP (PP) in a different direction,preventing molecular rotation.Therefore,LP (PP) interacts with OA molecules to form a linear structure.
In DFT calculations [46-48],molecule-molecule interactions and molecule-substrate are taken into consideration.Herein,only energy per unit area is discussed in this manuscript [49-52].The optimal structures are closely related to the molecule-substrate interactions,π-πinteraction,intermolecular hydrogen bonding interactions,and other interactions.Furthermore,steric hindrance deeply influences the intermolecular interactions,which leads to different assembly morphologies and energies per unit area.The energy per unit area of AP herringbone structure is lower than that of GP linear structure with the energies calculated to be-0.444 kcal mol-1˚A-2(AP) and-0.325 kcal mol-1˚A-2(GP),demonstrating that herringbone structure is more stable than linear structure(Table 2).The energy per unit area for PP compounds at the 1-phenyloctane/HOPG interface shows that the herringbone structure(PP-2,-0.471 kcal mol-1˚A-2) is stable than the linear structure(PP-1,-0.364 kcal mol-1˚A-2) (Table 2).These results illustrate that herringbone structure is more stable than linear structure thereby herringbone structure is the intrinsic and advantageous structure when adsorbed on HOPG surface.This phenomenon is identical to the adsorption behavior of the PTCDA core on HOPG surface.Due to the participation of OA,PP linear structure at OA/HOPG interface with the energy per unit area calculated to be-0.792 kcal mol-1˚A-2,is more stable than structures (PP-1,PP-2) formed at the 1-phenyloctane/HOPG interface (Table 2).For the same reason,LP molecules (-0.637 kcal mol-1˚A-2,Table 2) assemble into linear structures at OA/HOPG interface but fail to be imaged at 1-phenyloctane/HOPG interface.These situations elucidate that OA molecules could stabilize the adsorption system.
Considering that the London dispersion interaction in van der Waals interaction,we have further performed DFT-D3 calculations for all the systems.Total energy and energy per unit area for amino acid-functioned systems,the hydrogen-bonding interactions andπ-πinteractions by DFT-D3 method are listed in Tables S1,S3,S5(Supporting information),respectively.It should be noted that because of the dispersion corrections,all the values are decreased.However,the relative trend of the DFT-D3 results is the same as the DFT results.
In summary,we performed a systematic investigation on the steric effect of C-terminal amino acid derivatives GP,AP,LP and PP by STM and DFT calculations at the liquid/solid interface.2D supramolecular self-assembly structures were realized by adsorption of GP,AP,LP and PP at the 1-phenyloctane/HOPG and OA/HOPG interface.Depending on the different solvents utilized,the steric hindrance effects of amino acid residues were revealed utilizingin-situSTM.The results show that GP self-assembles into linear structure due to two categories of hydrogen-bonding interactions.AP arranges into herringbone structure based on hydrogen-bonding interaction which is controlled by steric effect from the methyl group.PP adopts linear and herringbone packing geometries at different solvent/HOPG interfaces since solvents are involved in the adsorption of PP molecules.LP with bulky side groups could form a linear structure with the assistance of OA molecules.As for GP and AP,the bonding sites of driving forces are disparate,albeit both of them could generate monolayers at the 1-phenyloctane/HOPG interface.The binding sites occur at the molecular shoulders with one GP molecule connecting with four adjacent moleculesviaH-bonding interactions.Owing to the steric hindrance of methyl groups,one molecule in the AP herringbone structure could interact with only two adjacent moleculesviaHbonds at the molecular shoulders,with the other two binding sites at the shoulder of the molecule failing to participate in the assembly.LP molecules with isopropyl groups larger than methyl groups are unable to immobilize at 1-phenyloctane/HOPG interface.Whereas the participation of OA leads to the formation of Hbonding interactions and alters the bonding sites of driving forces among LP molecules.Compared with that of LP,the molecular behavior of PP shows a slight difference since the benzyl groups participate in the assemblyvia π-πinteraction.The participation of 1-phenyloctane/OA into the PP system changes the interaction sites of PP molecules,resulting in the occurrences of different nanostructures.In a word,as the side group became bulky,the Hbonding sites from Ar-H···O-Ar turned to-COOH···O-Ar-,and finally transformed into-COOH···HOOC-.Furthermore,when enlarger the side group,the gap between molecules increases,leading to the transformation of H-bonding sites.Moreover,when the side group becomes bulkier,the solvent molecule could trap in the gap between molecules,leading to the construction of different structures.This phenomenon suggests that steric effect deeply influences the adsorption behaviors of amino acid derivatives.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Ministry of Science and Technology (No.2017YFA0205000),National Natural Science Foundation of China (Nos.21303024,21365003,21463003,51478123,21962003,21902033),the National Key Basic Research Program of China (No.2012CB933001) and the Chinese Academy of Sciences (No.YZ201318).The Jiangxi Provincial “Ganpo Talents 555 Projects”,Jiangxi Provincial Education Department Fund (No.KJLD13080),Jiangxi Provincial Funds for Distinguished Young Scientists (No.20153BCB23001),Jiangxi Provincial Project of Scientific and Technological Innovation Team (No.20152BCB24008),Jiangxi Province Youth Science Foundation Project (No.20192BAB216013),and Science and Technology Project of Jiangxi Province Education Department (No.180775) are also gratefully acknowledged.We thank Prof.Mingdong Dong for STM analysis.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.12.056.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
