Reversible aqueous zinc-ion battery based on ferric vanadate cathode
Wang Yang,Wu Yang,Yongfeng Huang,Chengjun Xu,Liuing Dong,Xinwen Peng
a State Key Laboratory of Pulp and Paper Engineering,South China University of Technology,Guangzhou 510640,China
b Tsinghua Shenzhen International Graduate School,Tsinghua University,Shenzhen 518055,China
c College of Chemistry and Materials Science,Jinan University,Guangzhou 511443,China
Keywords:Aqueous zinc-ion battery Ferric vanadate cathode Reversible phase transformation Zinc ion storage mechanism Biomass-based flexible quasi-solid-state battery
ABSTRACT Rechargeable aqueous zinc-ion batteries have attracted extensive interest because of low cost and high safety.However,the relationship between structure change of cathode and the zinc ion storage mechanism is still complex and challenging.Herein,open-structured ferric vanadate (Fe2V4O13) has been developed as cathode material for aqueous zinc-ion batteries.Intriguingly,two zinc ion storage mechanism can be observed simultaneously for the Fe2V4O13 electrode,i.e.,classical intercalation/deintercalation storage mechanism in the tunnel structure of Fe2V4O13,and reversible phase transformation from ferric vanadate to zinc vanadate,which is verified by combined studies using various in-situ and ex-situ techniques.As a result,the Fe2V4O13 cathode delivers a high discharge capacity of 380 mAh/g at 0.2 A/g,and stable cyclic performance up to 1000 cycles at 10 A/g in the operating window of 0.2-1.6 V with 2 mol/L Zn(CF3SO3)2 aqueous solution.Moreover,the assembled Fe2V4O13//Zn flexible quasi-solid-state battery also exhibits a relatively high mechanical strength and good cycling stability.The findings reveal a new perspective of zinc ion storage mechanism for Fe2V4O13,which may also be applicable to other vanadate cathodes,providing a new direction for the investigation and design of zinc-ion batteries.
Currently,Lithium-ion batteries (LIBs) have been successfully used in the field of electronics market such as mobile phones and computers owing to the high energy density.But the safety issues caused by flammable properties of the organic electrolyte,as well as the huge cost of lithium resources are still the most critical concerns to be solved [1-4].Aqueous rechargeable divalent-ion batteries (e.g.,Zn2+,Mg2+and Ca2+) have been intensively investigated because of their low cost,high safety,and environmental friendliness [5-8].In addition,aqueous electrolytes can deliver much higher ionic conductivity (ca.1 S/cm) than organic electrolytes (ca.1-10 mS/cm),which endows the aqueous battery with superior rate capability and power density [9-11].Among various aqueous rechargeable batteries,rechargeable aqueous zinc-ion batteries (ZIBs) are receiving significant attention owing to certain benefits from zinc anode: high theoretical capacity (820 mAh/g),low redox potential (-0.76 Vvs.standard hydrogen electrode),superior stability in water,and nontoxicity [12-15].Based on the comprehensive advantages of zinc anode,much attention has been paid to the new cathode materials with fast and durable Zn2+storage,and fundamental insights of zinc ion storage mechanism in cathode materials.
To date,various kinds of cathode materials have been demonstrated enabing reversibly store zinc ions for rechargeable aqueous ZIBs,such as manganese oxides [16-24],Prussian blue analogsbased materials [25-27],vanadium-based materials [28-44]and other types of materials [45-47].MnO2was the first cathode material proposed for aqueous ZIBs,in which Zn2+can achieve reversible intercalation/deintercalation for certain cycles [6].Then different MnO2polymorphs such as (α-,δ-,γ-) were investigated and deliver different mechanisms such as Zn2+and H+cointercalation or conversion rather than the typical intercalation of zinc ions [18-20].However,these cathode materials usually suffer from poor cycling stability due to partially irreversible phase transition and manganese dissolution during charge/discharge processes [17].Although the Prussian blue analogs-based cathodes exhibit stable zinc ion storage,they generally possess very limited reversible capacities of less than 100 mAh/g [25-27].Compared with the above two kind of cathodes,vanadium-based compounds have been widely studied recently because of their higher reversible capacity,better rate capability,and more stable cycle life [28].Various vanadium-based compounds,such as layered or tunnel vanadium oxides and vanadates,were designed as Zn2+host materials[48-57].Particularly,pure V2O5possess a high theoretical capacity of 589 mAh/g based on V5+/V3+redox couple.However,the cycle stability issue is another concern that occurs in acidic solution [42].The common strategy to improve its electrochemical performance is inserting metal ions (Zn2+,Ca2+,Mg2+,Ag+,Mn2+,etc.) or water molecules as “pillars” between [V-O]polyhedron layers [58-61].However,some key issues and energy storage mechanism are complicated and debatable.Besides,various metal vanadate such as lithium,potassium,sodium vanadate has also been employed for ZIBs,and these alkali vanadates evidently exhibit a high capacity and acceptable cyclability [32-34,38,50-52].Furthermore,a 3D printed composite (Fe5V15O39(OH)9·9H2O/rHGO) was explored as cathode for aqueous ZIBs,which achieved excellent rate performance and stable cycling stability even at a high mass loading [53].The strength of an ionic bond is associated with the valence and the electronegativity of the metal,stronger ionic bonds can further expand the size of the cavity between [V-O]polyhedron layers [56-58,62-65].Compared with monovalent alkali metal cations (e.g.,Li+,Na+,K+),the multivalent metal ions bonded with oxygen atoms can generate different ionic bonds and size of layers.Therefore,new metal vanadate with better crystal structure still needs to be explored for reversible zinc ion storage.
In this work,Fe2V4O13(FeVO) with tunnel structure was synthesizedviaa facile solution chemical reaction method and employed as a new cathode material for aqueous ZIBs.Its large tunnel structure containing VO4tetrahedron and FeO6octahedron makes it possible and beneficial for reversible zinc ion intercalation and diffusion.Herein,we investigate the morphological,structural,and valence changes of Fe2V4O13cathode upon charge/discharge process.Moreover,ex-situX-ray diffraction (XRD),ex-situscanning electron microscopy (SEM) andin-situRaman spectroscopy reveal the storage mechanism of zinc ions.Quite interestingly,two zinc ion storage mechanism can be observed simultaneously,i.e.,classical intercalation/deintercalation storage mechanism in the lattice structure of Fe2V4O13,and reversible phase transformation from ferric vanadate to zinc vanadate.As a result,the Fe2V4O13cathode delivers a capacity of 380 mAh/g at 0.2 A/g as well as good rate performance and long-term cycling stability.Furthermore,the assembled Fe2V4O13//Zn flexiblequasi-solid-state battery also exhibits a relatively high mechanical strength and good cycling stability.
The structure of obtained FeVO sample was confirmed by XRD characterizations.As shown in Fig.1a,the diffraction peaks are well indexed to monoclinic Fe2V4O13phase (space group: P21/c,JCPDS card No.04-011-4796),and the crystal structure of Fe2V4O13(Fig.1a inset and Fig.S1 in Supporting information) exhibits a framework witha=8.313 ˚A,b=9.406 ˚A,c=14.577 ˚A (α=γ=90°).This tunnel structure is formed by VO4tetrahedron and FeO6octahedron.Figs.1b and c depict SEM images of FeVO at different magnifications,showing a nanosheet morphology with an average length of 500 nm.The interconnected nanosheet without stacking structure not only ensures the good electrical conductivity but also enhances electrolyte ions infiltration and integral mechanical stability.To obtain the in-depth morphology and crystalline features of FeVO nanosheet,low-and high-resolution transmission electron microscopy (HRTEM) micrographs as well as selected-area electron diffraction pattern (SAED) were also carried out.TEM images(Fig.1d and Fig.S2a in Supporting information) affirm the interconnected nanosheet structure with only about 10 nm in thickness,which can significantly shorten the ion diffusion distance in solid phase.HRTEM image (Fig.1e) clearly represents lattice fringes with a measured inter-planar distance of 0.28 nm corresponding to the (024) plane of monoclinic Fe2V4O13.However,particularly necessary to point out that the high-resolution image in Fig.1e and SAED pattern in Fig.S2b (Supporting information) of a single nanosheet reveals clearly distinguishable lattice fringe regions separated from certain portion of amorphous domains.This observation demonstrates that these nanosheets are composed of crystalline and amorphous domains,which can be attributed to the relatively low reaction temperature.In addition,the homogeneous distributions of Fe,V and O were further evidenced by elemental mapping images in Figs.1f-i.
The electrochemical performance of FeVO as cathode for ZIBs was investigated in assembled coin cells using 2 mol/L Zn(CF3SO3)2aqueous solution as electrolyte [66].Cyclic voltammetry (CV)curves of FeVO at 0.5 mV/s was shown in Fig.2a.We can see that after the first cycle,the shapes of the redox couples show no obvious changes in the subsequent cycles,indicating a highly reversible Zn insertion/extraction process [57].The galvanostatic charge-discharge (GCD) curves of FeVO at different current densities from 0.2 A/g to 10 A/g are demonstrated in Fig.2b.Clearly,it exhibits platform-like discharge-charge curves and even maintains well at high current density,demonstrating its excellent rate capability.As shown in Fig.2c,the FeVO cathode could exhibit reversible capacities of 380 mAh/g at 0.2 A/g and maintain 200 mAh/g at high current density of 10 A/g,when the current changes to 0.2 A/g,the capacity can recover and display a relatively stable cycling capability in the following dozens of cycles.In addition,galvanostatic intermittent titration technique (GITT) was also applied to study the electrochemical reaction kinetics of the cathode.As displayed in Fig.2d,the Zn2+diffusion coefficient (D) is estimated to be 10-8-10-10cm2/s (calculation method is given in Supporting information).Obviously,the fast Zn2+mobility in the cathode is conducive to achieving superior rate capability and high power output [61].Furthermore,FeVO cathode exhibits good cycling performance,exhibiting a reversible capacity retention of 83%after 1000 cycles at 10 A/g (Fig.2e).It should be noted that,at the beginning of the cycle,the capacity shows a tendency of decrease and then gradually increase,which possibly due to an activation process of the cathode.The electrochemical impedance spectroscopy (EIS) result (Fig.S3 in Supporting information) also indicates that the electrode delivers a decreased charge transfer impedance during charge/discharge process.The low impedance of FeVO electrode mainly results from the thin sheet morphology,which is beneficial to improve the wettability of the material in the electrolyte.As shown in Fig.S4 (Supporting information),compared to commercial V2O5electrode,the FeVO electrode exhibits much lower static contact angles in 2 mol/L Zn(CF3SO3)2aqueous electrolyte.CV curves at different scan rates from 0.1 to 1 mV/s were performed to further understand the electrochemistry of the FeVO cathode,as depicted in Fig.2f.As previous reported,the peak current can be related with scan rates by Eq.1:

whereaandbare adjustable parameters.whenbis close to 0.5,the main contribution oficomes from faradic intercalation process and controlled by ion-diffusion;and ifbapproaches 1,a surfacecontrolled capacitive response is dominant.The three peak current values were utilized as theivalues in the equation.Fig.2g shows the linear relationship between logiand logv,and the correspondingbvalues of peaks 1-3 are 0.92,0.5 and 0.65,respectively,suggesting that the charge storage process is synergistically controlled by the capacitive and diffusion behaviors [35].This phenomenon leads to a fast Zn2+diffusion kinetics,enabling the high-rate performance.The contribution ratios between two different processes at various scan rates,which can be calculated by Eq.2:

wherei(V),k1ν,k2ν0.5andνrepresent the peak current,the capacitive and diffusion-controlled currents and scan rate,respectively.The capacitive contribution raises from 60% to 74% as the scan rate increases (Fig.2h),indicating that the electrochemical behaviors is mainly dominated by the capacitive-controlled effect,which implies that the FeVO cathode possess favorable charge transfer kinetics and high-rate performance.With the increase of scan rate,there is no significant deviation with the shape and peak position of CV curves (Fig.S5 in Supporting information).The first and second cathodic peak potential both shifts less than 0.08 V when scan rate ≤1 mV/s,which further confirms the good rate performance of FeVO cathode [36].
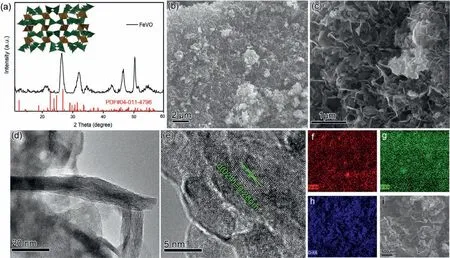
Fig.1.(a) XRD pattern of FeVO.(b,c) SEM images of FeVO at different magnifications.(d,e) TEM and HRTEM images of FeVO.(f-i) EDS mapping of FeVO.
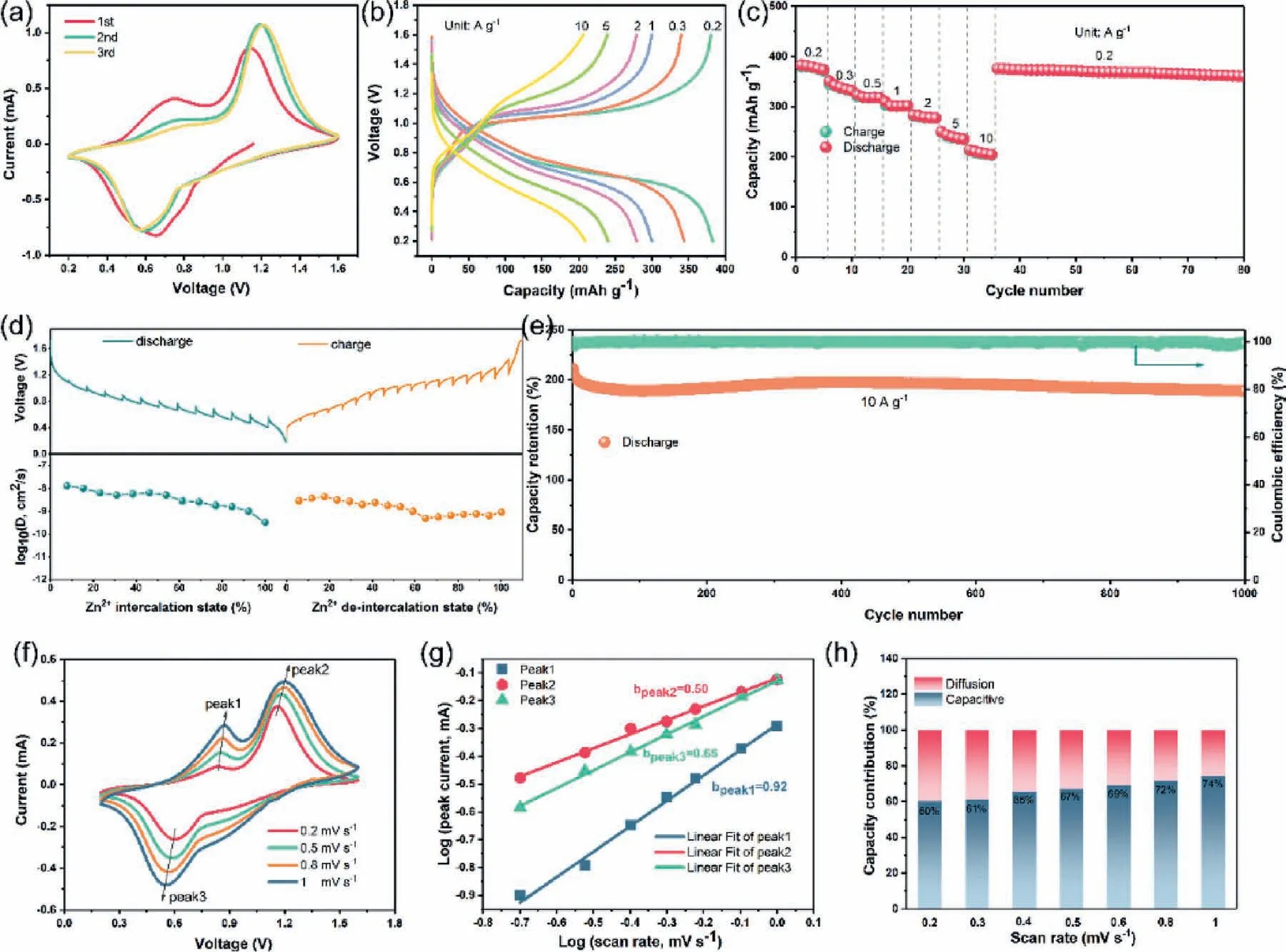
Fig.2.Electrochemical performance of FeVO as cathode for aqueous ZIBs.(a) CV curves at 0.5 mV/s.(b) GCD curves at different current densities.(c) Rate performance of FeVO at different current densities from 0.2 A/g to 10 A/g.(d) Discharge/charge GITT profiles and calculated Zn2+ diffusion coefficient (i.e.,D) in discharge-charge processes of the cathode.(e) Cycling performance at current densities of 10 A/g.(f) CV curves of FeVO at different scan rates from 0.2 mV/s to 1 mV/s.(g) Linear relationships between logi and logν of cathodic and anodic peaks.(h) The contribution ratio of capacitive capacity and diffusion-controlled capacity at different scan rates.
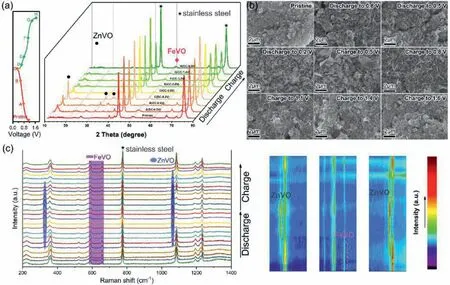
Fig.3.(a) Charge/discharge profiles for the initial cycle at 0.2 A/g and corresponding ex-situ XRD patterns of FeVO cathode during discharge-charge process.(b) Ex-situ SEM images of cathodes at various states: original state;the 1st discharge process;the 1st charge process.(c) In-situ Raman curves of FeVO cathode during discharge-charge process.
To visualize Zn2+-storage mechanism in the cathode host,exsituXRD analysis andin-situRaman test were conducted to detect the evolution of the crystal structure at different charge/discharge states,as shown in Fig.3.From XRD patterns in Fig.3a,we can see that during the discharge process,the intensity of FeVO peak gradually decrease,accompanied by the formation of a second phase of Zn3V2O7(OH)2·2H2O (JCPDS No.50-0570),which can be recovered upon charging,thus confirms the reversibility of the reaction.This is likely due to the strong electrostatic interaction (bonds) between the inserted Zn2+and the vanadium oxygen layers [41,67,68],which leads to the formation of Zn3V2O7(OH)2·2H2O.SEM images at different charge/discharge states also verify the structural evolution.In pristine state (Fig.3b),Fe2V4O13nanosheets,surrounded by conductive carbon black particles and binder,can be observed.When discharged from open circuit voltage (OCV) to 0.8 V,the cathode is covered by cross linked ultrathin sheets.Further discharged to 0.5 V and 0.2 V,the nanosheets became dense and the thickness also increased.During subsequent charge process,the nanosheets gradually disappeared.It is worth mentioning that this transformation is not complete,only partial transformation of ferric vanadate into zinc vanadate.Zinc ions can also be reversibly intercalated and de-intercalated in the tunnel structure of ferric vanadate,which can be verified by EDS analysis in supporting information (Figs.S6 and S7 in Supporting information).In-situRaman (Fig.3c) further demonstrate the reversible phase transformation during discharge-charge process.Typically,the band at 221,360,523,590,657,1085 and 1195~1234 cm-1corresponds to the O-V-O bridge,V-O stretching mode,and the stretching vibrations of the V-O-V bridges of original FeVO cathode [39].Upon charging,these peaks gradually move to the left and become weaker,accompanied by the appearance of new peaks at 328,577 and 1064 cm-1,which can be assigned to the V-O in zinc vanadate phase.As for the charging process,these new peaks gradually vanish,and the peak of ferric vanadate gradually increases and moves right to the original position.The Raman results further certify the highly reversible zinc ion storage in FeVO cathode.
Theex-situX-ray photoelectron spectroscopy (XPS) spectra at the initial state,discharged state and charged state were carried out to gain insight into the behavior of Zn2+storage in FeVO cathode.In the pristine state,no signal of Zn can be detected.The Zn 2p signals located at 1022.3 eV (2p3/2) and 1045.4 eV (2p1/2) were observed in the fully discharged state,accurately declaring the insertion of Zn2+ions into FeVO.The Zn 2p can be deconverted to two kinds of Zn,the main peak at 1022 eV corresponds to the intercalated Zn2+,and another weak signal at 1020.5 eV could be attributed to the absorbed Zn2+on the electrode surface.The weak signal of the Zn 2p at the charged state may be responsible for the remaining surface absorption (Figs.4a and S8 in Supporting information) [54].Besides,we can find that the valence of vanadium was reduced to V4+during the insertion of zinc ions and it can be reversibly re-oxidized to V5+at the charged state (Fig.4b).In the Fe 2p region (Fig.4c),the Fe signal maintained well and the valence did not change.Theex-situTEM analysis (Figs.4d and e)shows that new phase is observed at the fully discharged state,corresponding to the (111) planes of Zn3(OH)2V2O7·2H2O.In addition,the lattice spacing of ~0.28 nm at the fully discharged state matches the crystal lattice plane (024) of monoclinic FeVO phase.Due to its large tunnel structure,the lattice spacing did not change after zinc ion intercalation.Impressively,the crystal structure immediately recovered to its initial state (0.28 nm for (024) plane of FeVO) upon charged to 1.6 V.Based on the above investigation,the crystal structural changes during the electrochemical intercalation process are graphically summarized in Fig.4f,which illustrate the intercalation of Zn2+ions in the FeVO host accompanied by the formation of new phase (Zn3V2O7(OH)2·2H2O).Above discussions demonstrate that with the Zn2+intercalation in the discharge process,partial original FeVO transform to Zn3V2O7(OH)2·2H2O and simultaneously the rest FeVO serve as the host for Zn2+storage.In following charge processes,the Zn2+reversibly deintercalated from FeVO and the new phase Zn3V2O7(OH)2·2H2O reversibly transform to original FeVO.The electrode reactions can be illustrated as follows:
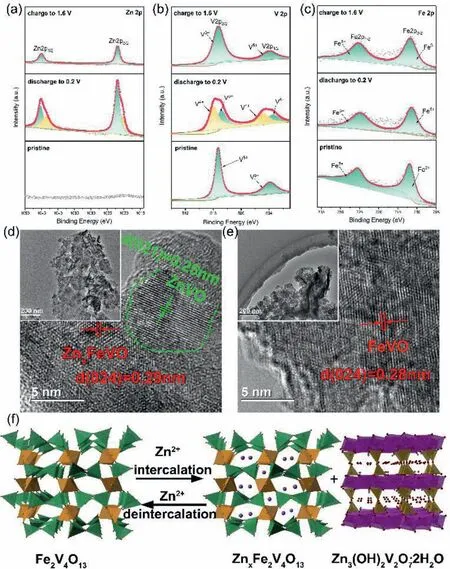
Fig.4.(a-c) Ex-situ high-resolution XPS spectra of Zn 2p,V 2p and Fe 2p at pristine,the fully discharge,and charge state.(d) HRTEM images of the electrodes at the fully discharge and (e) charged state;(f) scheme showing the intercalation of Zn2+ insertion/extraction process in FeVO during cycling.
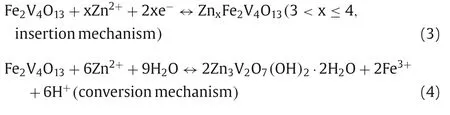
The cathode exhibits good reversibility and stability,as shown in Fig.S9 (Supporting information).The original sheet morphology was maintained even after 1000 cycles.
As a proof of concept,cellulose-based flexible Zn/FeVO batteries were assembled by sandwichingquasi-solid-state ployacrylamide (PAM)/cellulose nanofibrils (CNF)/Zn(CF3SO3)2electrolyte between the FeVO positive electrode and Zn foil,and then sealed by Al-plastic films (Fig.5a).Although the performance of thequasi-solid-state Zn/FeVO batteries cannot touch that of batteries based on aqueous electrolyte due to the degraded ionic conductivity ofquasi-solid-state electrolyte,they still display the excellent capacities of 340 mAh/g at 0.2 A/g,based on the mass of FeVO in positive electrode (Fig.5b).In addition,the corresponding charge/discharge curves are similar to those in aqueous Zn(CF3SO3)2electrolyte,indicating that thequasi-solid-state electrolyte nearly has no influence on the reaction mechanism of Zn/FeVO systems.In order to demonstrate the viability of ourquasi-solid-state Zn/FeVO batteries as flexible energy storage devices,we tested the cycling performance of a representative battery at different bending states.As shown in Fig.5c,during such a bending process,the battery is always able to charge/discharge well with only a slight capacity fading,displaying the high stability of thequasi-solid-state Zn/FeVO batteries as flexible energy storage devices.To demonstrate the flexibility of the resultantquasi-solid-state Zn/FeVO batteriesviaa simple visual cue,we integratedquasi-solid-state Zn/FeVO batteries in series.They can light up light-emitting diodes with a shape of “SCUT” even under bending state (Fig.5d),illustrating the practical application potential of our cellulose-based flexiblequasi-solid-state Zn/FeVO batteries.
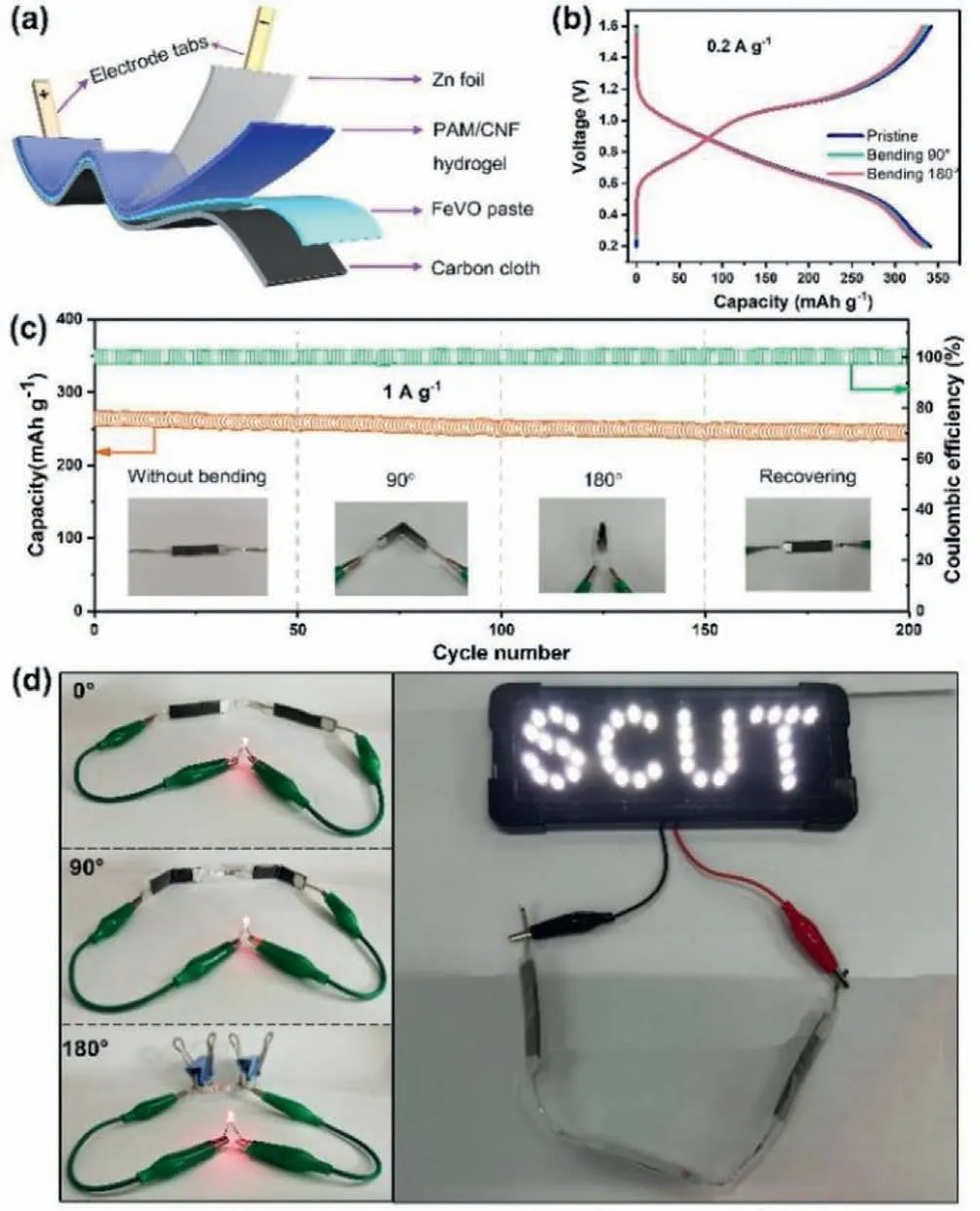
Fig.5.Configuration and performance of cellulose-based flexible quasi-solid-state Zn/FeVO batteries.(a) Schematic diagram of a cellulose-based flexible quasi-solidstate Zn/FeVO battery.(b) GCD curves under different bending states (0.2 A/g) of the cellulose-based flexible quasi-solid-state Zn/FeVO battery.(c) Cycling performance under different bending states (1 A/g) of the cellulose-based flexible quasisolid-state Zn/FeVO battery.The insets show the optical images of the quasi-solidstate Zn/FeVO battery at corresponding bending states.(d) LED array powered by Zn/FeVO batteries under bending state.
In summary,Fe2V4O13was synthesized using simple solution chemical reaction method.When employed as cathode for aqueous zinc-ion batteries,it delivers a capacity of 380 mAh/g at 0.2 A/g as well as good rate performance long-term cycling stability (83%capacity retention after 1000 cycles at 10 A/g).Extensivein-situandex-situcharacterization of the cathode material at different charge/discharge states revealed that the structure of the Fe2V4O13cathode is highly reversible.The phase transformation combined with intercalation mechanism demonstrated here is quite different from the previously reported metal vanadate,which provides more information and gives a new insight into the reactions of aqueous ZIBs.Moreover,the assembled Fe2V4O13//Zn flexiblequasi-solidstate battery also exhibits a relatively high mechanical strength and good cycling stability.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the China Postdoctoral Science Foundation (Nos.2020M682710,2020M682711,2019M652882 and 2019T120725),Guangdong Basic and Applied Basic Research Foundation (No.2020A1515110705),National Program for Support of Top-notch Young Professionals (No.x2qsA4210090),National Natural Science Foundation of China,(No.31971614),and State Key Laboratory of Pulp and Paper Engineering(No.2020C03).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.12.049.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
