Distachydrimanes A-F,phenylspirodrimane dimers and hybrids with cytotoxic activity from the coral-derived fungus Stachybotrys chartarum
Shuang Lin,Jianzheng Huang,Hanxiao Zeng,Qingyi Tong,Xueke Zhang,Beiye Yang,Ying Ye,Jianping Wang,Zhengxi Hu,Yonghui Zhang
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation,School of Pharmacy,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China
Keywords:Stachybotrys chartarum Distachydrimanes A-F Structure elucidation Cytotoxic activity Mechanism of action
ABSTRACT By integrating one strain-many compounds (OSMAC) and LC-MS-based molecular networking strategies,distachydrimanes A-F (1-6),six novel phenylspirodrimane dimers and hybrids representing two types of unprecedented terpenoid-polyketide hybrid skeletons,were isolated from the modified fermented rice substrate of a coral-derived fungus Stachybotrys chartarum.All the structures incorporating their absolute configurations were elucidated based on comprehensive spectroscopic analyses,mainly including HRESIMS and NMR data,single-crystal X-ray diffraction (Cu Kα),and comparison of the experimental electronic circular dichroism (ECD) data.Architecturally,compounds 1-6 represent an unprecedented class of dimeric phenylspirodrimanes with an unexpected C-18-C-23′ linkage,of which compounds 1-3 also feature an unexpected 5-methyl-1,3-benzenediol moiety via a carbon-carbon linkage.The bioactivity assay demonstrated that compounds 1,5 and 6 induced cell proliferation inhibition,G0/G1 cell cycle arrest,senescence and mitochondrial-mediated apoptosis in L1210 cells,highlighting their potentials as a new category of anticancer agents.
Cancer poses the greatest incidence and mortality rate worldwide.It was caused by an abnormal cell growth with the ability to invade and spread throughout the organism [1],accounting for an estimated 10 million deaths in 2020 [2].It is estimated that over the next 20 years,cancer cases are predicted to increase by 60%globally and by up to 81% in low-and middle-income countries[3].More than that,the current clinical cancer treatments are still faced with recurrence due to the residual cancer cells and minute lesions in surgery,chemotherapy and other treatments [4].Therefore,there is an urgent need to develop novel drugs for cancer therapy.
Natural products (NPs),isolated from plants,microbes,and even animals,are an immense reservoir of bioactive molecules that play an irreplaceable role in the discovery and development of innovative drugs [5-7].NPs are thought to have ushered a new golden age since the 2015 Nobel Prize in Physiology or Medicine was awarded to avermectin and artemisinin [8].Drugs developed from NPs have been confirmed to possess many beneficial pharmacological effects and are widely used to treat various human diseases [9,10],for cancer therapy,such as taxol [11]and doxorubicin[12]are successful precedents.
In recent years,our research team has performed several studies towards chemically novel and bioactive secondary metabolites from fungi inhabiting unique environments [13-18],and a coralderived fungusStachybotrys chartarumcame into our sight.Previously,we have isolated and identified six new dolabellanes and atranones from the title fungus [19].To tap into the fungus-stored biosynthetic potentials,we adopted the OSMAC strategy by adding 0.1% Fe2(SO4)3and 3% sea salt into the normal rice medium and employed LC-MS/MS-GNPS (Global Natural Product Social Molecular Networking) data to analyze the metabolites.By screening against GNPS spectral database (Fig.1),four nodes (parent ions atm/z429.218,471.240,387.217 and 371.139) were identified as stachybotrysin C [20],stachybotrysin H [21],stachybotrylactone[22],and mer-NF5003E [23],respectively,which have been isolated and characterized from theStachybotrysspecies as potential leads for anticancer,antivirus,anti-inflammatory,and avian myeloblastosis virus protease inhibitor drugs.These analytical data suggested the presence of more bioactive phenylspirodrimane congeners in the metabolites of fungusS.chartarum,which stimulated us to ulteriorly investigate this fungus by a large-scale culture [24],facilitating the isolation and characterization of six novel phenylspirodrimane dimers and hybrids,namely distachydrimanes A-F(1-6).Structurally,compounds 1-6 represent a series of skeletally unprecedented dimeric phenylspirodrimanesviaan unexpected C-18-C-23′ linkage,of which compounds 1-3 are uniquely defined by an unexpected 5-methyl-1,3-benzenediol moietyviaa carboncarbon linkage.Additionally,all six compounds showed certain cytotoxic activity,and notably compounds 5 and 6 showed significant cytotoxic activity against all cancer cell lines (<10 μmol/L).Herein,the detailed isolation,structure elucidation,cytotoxic activity and the preliminary mechanism of these compounds are described.
StrainS.chartarumwas cultured on potato dextrose agar (PDA)plates at 28 °C for 7 days to prepare the seed cultures.Agar plugs were cut into small pieces (approximately 0.2×0.2×0.2 cm3) and inoculated into 400 Erlenmeyer flasks (1 L),previously sterilized by autoclaving,each containing 250 g of rice and 250 mL of distilled water (adding 0.1% Fe2(SO4)3and 3% sea salt).All flasks were incubated at 28 °C for 30 days.The growth of fungus was stopped by adding 250 mL of 95% EtOH to each flask,and the solvent was decanted and removed under reduced pressure.The rice cultures were soaked with the recycled EtOH five times until the solvent extract was nearly colorless at room temperature.The concentrated extract was suspended in water (3 L) and extracted with EtOAc (1:1,v/v) three times to obtain a total residue of 400 g.
The EtOAc residue was fractionated on RP-C18silica gel CC eluted with MeOH-H2O (20%,40%,50%,60%,80%,and 100%) to give four main fractions (A-D).Fraction B (MeOH-H2O,60:40,v/v)was loaded onto Sephadex LH-20 (CH2Cl2-MeOH,1:1,v/v) and silica gel CC eluted with petroleum ether-ethyl acetate (5:1,2:1,1:1,and 0:1,v/v) to give seven main fractions (B1-B7) by TLC analysis.Fraction B2 was subjected to RP-C18column chromatography eluted with a step gradient of MeOH-H2O (from 20:80 to 80:20,v/v) to yield seven fractions (B2-1-B2-7) in total.Fraction B2-4 was separated by semi-preparative HPLC (MeCN-H2O,89:11,v/v;2.0 mL/min) to afford compound 5 (5.2 mg,tR30.1 min).Fraction B2-5 was subjected to semi-preparative HPLC (MeOH-H2O,88:12,v/v;2.0 mL/min) to afford compound 4 (4.9 mg,tR20.5 min).Fraction B5 was separated into four fractions (B5-1-B5-4) by Sephadex LH-20 eluted with CH2Cl2-MeOH (1:1,v/v).Fraction B5-1 was purifiedviasemi-preparative HPLC (MeCN-H2O,80:20,v/v;2.0 mL/min) to provide compounds 2 (3.9 mg,tR19.5 min) and 3(2.9 mg,tR30.5 min).Fraction B5-2 was consecutively separated by semi-preparative HPLC (MeOH-H2O,81:19,v/v;2.0 mL/min) to provide compound 1 (7.4 mg,tR12.0 min).Fraction B6 was applied to Sephadex LH-20 (CH2Cl2-MeOH,1:1,v/v) and silica gel CC eluted with petroleum ether-ethyl acetate (5:1,3:1,2:1,and 1:1,v/v) to obtain four main fractions (B6-1-B6-4) by TLC analysis.Fraction B6-2 (petroleum ether-ethyl acetate,3:1,v/v) was purifiedviasemi-preparative HPLC (MeCN-H2O,92:8,v/v;2.0 mL/min) to afford compound 6 (5.2 mg,tR35.8 min).
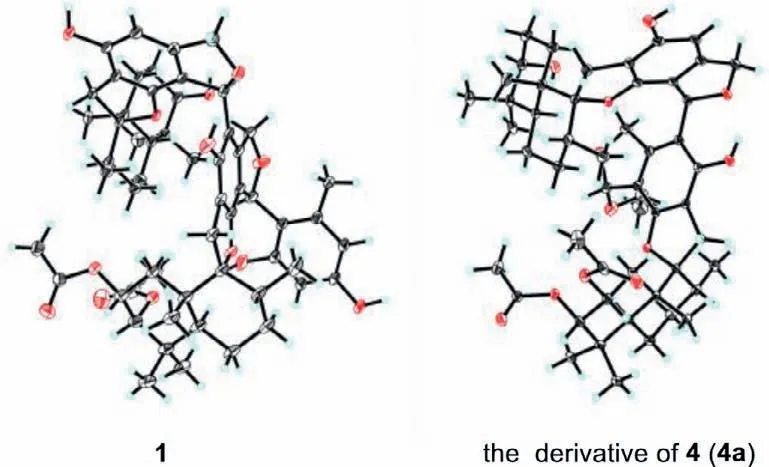
Fig.3.X-ray ORTEP drawings of compounds 1 and 4a.
Distachydrimane A (1) was obtained as a colorless crystal with a molecular formula of C57H72O13,as deduced from the HRESIMS data atm/z987.4848 [M+Na]+(calcd.for 987.4865),corresponding to 22 degrees of unsaturation.The IR spectrum of 1 showed absorption bands for hydroxy (3420 cm-1),ester carbonyl (1744 and 1719 cm-1) and double bond (1627 cm-1) groups.The13C and DEPT NMR data (Table S2 in Supporting information) revealed 57 carbon resonances,including 11 methyl carbons,11 methylene carbons (including two oxygenated),nine methine carbons (including five oxygenated),six quaternary carbons (including two oxygenated),18 aromatic carbons for three phenyl groups,and two ester carbonyl carbons.Combined with the1H NMR data (Table S1 in Supporting information) of compound 1,the typical signals including two oxygenated methylenes atδH5.32/5.68 and 4.99/5.09 and eight methyls atδH0.27,0.22,0.99,0.87,0.92,1.10,0.98,and 0.94 for a phenylspirodrimane dimer backbone were observed.Further analysis of 2D NMR data (Fig.2) validated the presence of two structurally similar phenylspirodrimane monomers,except that two acetyl groups were respectively attached to C-2 and C-3 in unit A and only one hydroxy group was attached to C-3′ in unit B.Moreover,the essential signals were carefully assigned by the HMBC correlations from H-23′ to C-18 and C-20′ (Fig.2),which established an unexpected C-18-C-23′ linkage between units A and B.Interestingly,an extra 4-substituted-5-methyl-1,3-benzenediol moiety (unit C) was attached to C-23,which could be discerned by the HMBC correlations from H-23 to C-3′′/C-5′′,from H-6′′ to C-1′′/C-2′′,from OH-3′′ to C-2′′/C-3′′,and from H3-7′′ to C-4′′/C-5′′/C-6′′ (Fig.2).Therefore,the planar structure of 1 was defined,which represents the first example of dimeric phenylspirodrimane featured by an unexpected C-18-C-23′ linkage (units A and B) and hybrid with an unusual 5-methyl-1,3-benzenediol moiety.
The relative configuration of 1 was determined by analysis of the coupling constant and NOESY data (Fig.S1 in Supporting information).With the fact that H-3 and H-3′ of phenylspirodrimanetype sesquiterpenoids maintained theβ-orientation [25],and the small coupling constant between H-2 and H-3 (J=2.5 Hz) inferred that they were in thecis-relationship,therefore H-2,H-3 and H-3′ were all assigned to beβ-oriented.The NOESY correlations of H3-13/H-2/H-3,H-2/H3-15,H3-15/H-8,H3-15/H-11b and H-8/H-11a in unit A,as well as H3-13′/H-2′β/H-3′,H-2′β/H3-15′,H3-15′/H-8′,H3-15′/H-11′b and H-8′/H-11′a in unit B,indicated that H3-13/13′,H3-15/15′,H-8/8′ and CH2-11/11′ wereβ-oriented just like H-2 and H-3/3′,whereas the NOESY cross-peaks of H3-14/H-5 and H3-14′/H-5′ suggested that H3-14/14′ and H-5/5′ were on the opposite side withα-orientations.These results were supportive of the common configurations at all corresponding chiral centers as reported phenylspirodrimane dimers [25].Crucially,the diagnostic NOESY correlations of H3-14′/H-23 and H-23′/H-22a/H-22b were observed,which implied theα-orientation of H-23/23′.
Regarding the novelty and complexity of 1,we attempted recrystallization in various two-phase or three-phase solvent systems to address the issue of growing suitable crystals for Xray diffraction experiment so as to furnish solid evidence for its structure.Fortunately,a high-quality crystal was obtained from CH2Cl2-EtOH-H2O (10:10:1),which was then successfully analyzed by single-crystal X-ray diffraction with Cu Kαradiation (Flack parameter=-0.04(6);Fig.3).Accordingly,the absolute configuration of 1 was unambiguously determined as 2R,3S,5S,8R,9R,10S,23S,3′R,5′S,8′R,9′R,10′S,23′S.
Distachydrimane B (2) was isolated as a colorless oil.Its molecular formula was determined as C61H76O16by the HRESIMS analysis (m/z1087.5095 [M+Na]+,calcd for 1087.5026),indicating 24 indices of hydrogen deficiency.The 1D NMR data (Tables S1 and S2) of 2 showed high similarities to those of 1,implying that 2 was also a phenylspirodrimane dimer.The obvious differences were that a methylene (δC24.3,C-2′) in 1 was replaced by an oxymethine (δC68.2,C-2′) in 2 and two acetyl groups were severally connected to C-2′ and C-3′,suggesting the replacement of unit B in 1 by unit A’in 2.This assumption was further confirmed by the key1H-1H COSY correlation of H-2′ (δH5.24)/H-3′ (δH5.03)and HMBC correlations as shown in Fig.2.
Compound 2 has one more chiral center C-2′ than that of 1,and the small coupling constant between H-2′ and H-3′ (J=2.8 Hz)implied that they were also in thecis-relationship and assigned asβ-oriented (in unit A’).The NOESY cross-peaks of 2 highly resembled those of 1 as shown in Fig.S1,indicating that units A and A’in 2 possessed the same relative configurations as those of 1.The same biosynthetic pathways and closely similar ECD curves(Fig.4) of 1 and 2 suggested the absolute configuration of 2 to be 2/2′R,3/3′S,5/5′S,8/8′R,9/9′R,10/10′S,23/23′S.
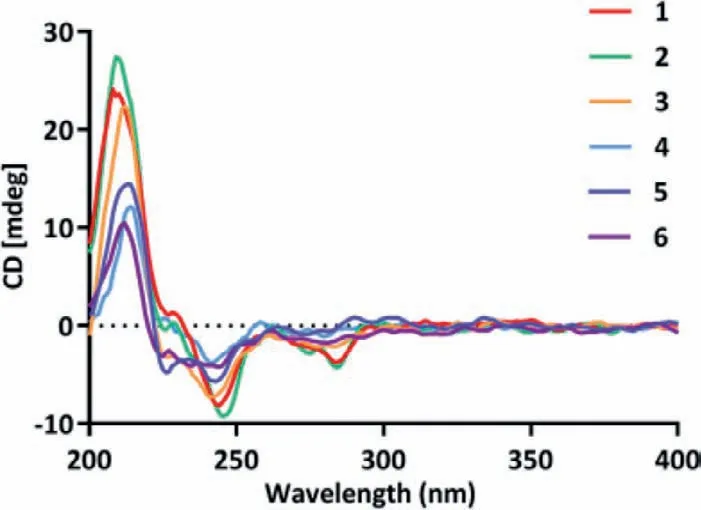
Fig.4.Experimental ECD spectra of 1-6.
Distachydrimane C (3) was deduced to have a molecular formula of C61H76O16,according to the HRESIMS data (m/z1087.5001[M+Na]+,calcd.for 1087.5026),which was the same as that of 2.Comprehensive analysis of the NMR data (Tables S1 and S2)of 3 and 2 suggested that both compounds were structural congeners,except for the part of unit C.Two pairs of carbons with the equal chemical shifts (δC155.1,C-1′′/3′′;δC112.0,C-4′′/6′′) were observed on the benzene ring in unit C,suggesting the replacement of a 4-substituted-5-methyl-1,3-benzenediol moiety in 2 by a 2-substituted-5-methyl-1,3-benzenediol moiety in 3,which can be confirmed by the detailed1H-1H COSY and HMBC correlations as shown in Fig.2.The similar NOE data and ECD curves (Fig.4) of 2 and 3 allowed an explicit assignment of the absolute configuration of 3 as 2/2′R,3/3′S,5/5′S,8/8′R,9/9′R,10/10′S,23R/23′S.
Distachydrimane D (4) had the molecular formula C51H68O12with 18 indices of unsaturation,which was determined by the HRESIMS data (m/z895.4606 [M+Na]+,calcd.for 895.4603).Analysis of the NMR data (Tables S1 and S2) of 4 suggested that it was also a phenylspirodrimane dimer and two phenylspirodrimane monomers of 4 were the same as those of 1,which could be further demonstrated by the HMBC and1H-1H COSY correlations (Fig.2).The only imparity was that a methoxy group (δC51.0) linked to C-23 in 4 replaced a 4-substituted-5-methyl-1,3-benzenediol moiety at the same position in 1,which was confirmed by the key HMBC correlation from OCH3-23 (δH3.23) to C-23 (δC105.5).Similar NOESY data and ECD curves (Fig.4) of 1 and 4 suggested that both compounds shared the identical absolute configuration.To further verify this conclusion by a more reliable method,the recrystallization experiment was attempted with various two-phase or three-phase solvent systems under atmospheric conditions.Luckily,the mixed CH2Cl2-EtOH-H2O (10:10:1,v/v/v) solutions of 4 afforded crystals suitable for X-ray crystallography,and they were explicitly identified as ethoxylate 4a,which could also be supported by the evidence that a C-23 methoxy group disappeared and an ethoxy group (δH3.72,2H,q,J=7.1 Hz;δH1.23,3H,dd,J=7.1,1.8 Hz) existed in 4a (Fig.S56 in Supporting information).The crystallography data [Flack parameter=-0.10(5)]demonstrated the absolute stereochemistry of 4a to be 2R,3S,5S,8R,9R,10S,23R,3′R,5′S,8′R,9′R,10′S,23′S(Fig.3),which was identical to that of 4.Accordingly,the absolute structure of 4 was defined.
Distachydrimane E (5) was assigned to a molecular formula of C55H72O15by the HRESIMS analysis atm/z995.4735[M+Na]+(calcd.for 995.4763).The 1D NMR data (Tables S1 and S2) of 5 showed a close structural resemblance to those of 2,defining that a 4-substituted-5-methyl-1,3-benzenediol moiety in 2 was replaced by a methoxy group (δC51.3) linked to C-23 in 5,which was supported by the key HMBC correlation from the proton of methoxy group to C-23 (Fig.2).The same NOESY correlations of 5 and 2 could be discernible and their ECD spectra showed similar Cotton effects (Fig.4),which suggested the absolute configuration of 5 could also be assigned as 2/2′R,3/3′S,5/5′S,8/8′R,9/9′R,10/10′S,23R/23′S.
Distachydrimane F (6) was deduced as a C-23 epimer of 5 from their identical HRESIMS ion peaks and awfully similar 1D NMR data (Tables S1 and S2),except that the discernible signals C-23(δC107.5) and OCH3-23 (δC56.4) of 6 were distinguished from C-23 (δC105.6) and OCH3-23 (δC51.3) of 5.Crucially,the diagnostic NOESY correlation of the methoxy proton (δH3.51) to H3-14′ further verified theS-configuration of C-23 in 6 (Fig.S1).The absolute configurations of all other chiral centers were deduced to be the same as those of 5 (2R,3S,5S,8R,9R,10S,2′R,3′S,5′S,8′R,9′R,10′S,23′S)by comparing their identical NOESY correlations and ECD curves(Fig.4).
Distachydrimanes A-F (1-6) represent two special classes of dimeric phenylspirodrimanes with unprecedented carbon skeletons.Their plausible biosynthetic pathways are proposed as follows(Scheme 1).A series of phenylspirodrimane monomers including stachybotrysin C,stachybotrysin H,and mer-NF5003E,are derived from orsellinic acid and farnesyl diphosphate (FPP) through key prenylation,cyclization,and redox reactions [26].Further hydroxylation,methylation and dehydration reactions afford intermediates i and ii,which undergo a further Friedel-crafts reactionviaan unexpected C-18-C-23′ bond to generate an intermediate iii.Selective acetylation of rings A/A’of iii could create compounds 4-6.On the other hand,after the removal of methoxy,iii undergoes a key Friedel-crafts reaction with a small molecule orcinolviaa carboncarbon linkage,and further oxidation and acetylation reactions to produce compounds 1-3.
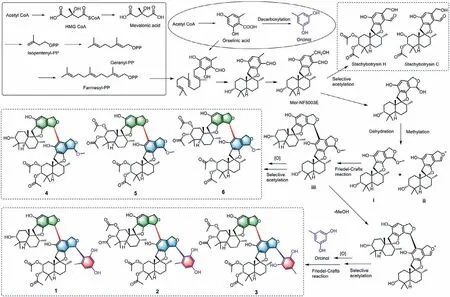
Scheme 1.Plausible biosynthetic pathways for compounds 1-6.
The bioactivity of compounds 1-6 against seven cancer cell lines was initially assessed by a CCK-8 kit,and etoposide (VP16)was used as the positive control.Compounds 5 and 6 showed significant anti-proliferation effect against all cancer cell lines,with IC50values ranging from 2.66 μmol/L to 8.14 μmol/L,and compounds 1,3 and 4 also showed remarkable anti-proliferation effect against most of these cancer cell lines,especially to L1210 (Table S3 in Supporting information;Figs.5A-C).Regarding the yield and novelty of these chemical structures,compounds 1,5 and 6 were chosen to further explore the anti-proliferation effect and mechanism of this kind of compounds.We first detected the signaling of S6 ribosomal protein,which is a marker of cell proliferation,mitogenic stimulation and overexpressed in various cancers [27].Compounds 1,5 and 6 treatments showed no effect on S6,but significantly inhibited its phosphorylated form after 24 h,and even a complete inhibition was found after compound 1 treatment for 48 h (Fig.5D).These results demonstrated that the proliferation inhibition effects of compounds 1,5 and 6 were associated with the down-regulation of S6 phosphorylation.Moreover,cell morphological changes were very obvious.Microscopic imaging showed that,after compounds 1,5 and 6 treatments,some cells shrank and became dense,and some cells featured cytoplasmic swelling and plasma membrane bubbles featured classic apoptotic bodies (Fig.5E),suggesting that these compounds probably induced apoptosis.
Thus,we performed the apoptosis assays.The induction of apoptosis was first evaluated by DAPI nuclear staining,and the results indicated that compounds 1,5 and 6 treatments induced significant stronger blue stain,which labeled the internucleosome fragmentation of genomic DNA during apoptosis (Fig.6A).Apoptosis was further detected and quantified by Annexin V-FITC/PI staining kit and flow cytometer.Compounds 1,5 and 6 (10,10 and 5 μmol/L,respectively),treated for 48 h caused 78.9%,82.6% and 48.6% apoptosis in L1210 cells,respectively (Fig.6B).To further explore the mechanism of apoptosis induction effect of compounds 1,5 and 6,the mitochondrial membrane potential (MMP) of L1210 cells was evaluated.As shown in Fig.6C,compounds 1,5 and 6(especially 1 and 5) treatments decreased MMP of L1210 cells,suggesting that mitochondria may be involved in their apoptotic induction effect.In order to confirm this,proteins of Bcl-2 family as well as the cleaved-caspase 3,PARP were detected.As shown in Fig.6D,treatments of compounds 1,5 and 6 altered the expression levels of PARP,cleaved-caspase 3,Bcl-XL and Bax.The DNA damage marker γ-H2AX also sharply increased after treated with compounds 1,5 and 6.Taken together,these data suggested that compounds 1,5 and 6 induced mitochondrial-mediated apoptosis in L1210 cells.
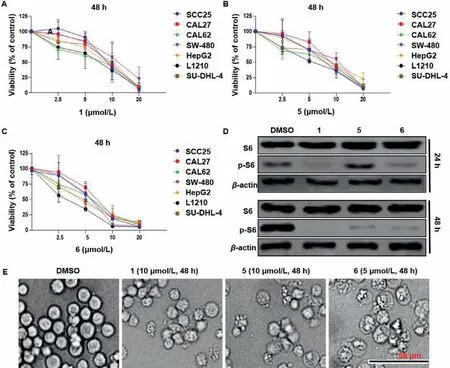
Fig.5.Compounds 1,5 and 6 inhibit cell proliferation and cause morphological changes in L1210 cells.(A-C) Dose-response viability curve of L1210 and other cancer cells after 48 h treatment.Values were mean ± SD of three independent experiments.(D) After treated with compounds 1 (10 μmol/L),5 (10 μmol/L) and 6 (5 μmol/L) for 24 h and 48 h,the expression of S6 and phospho-S6 (Ser235/236) were evaluated by western blot.β-Actin was used as a loading control.(E) Morphological changes in L1210 cells.
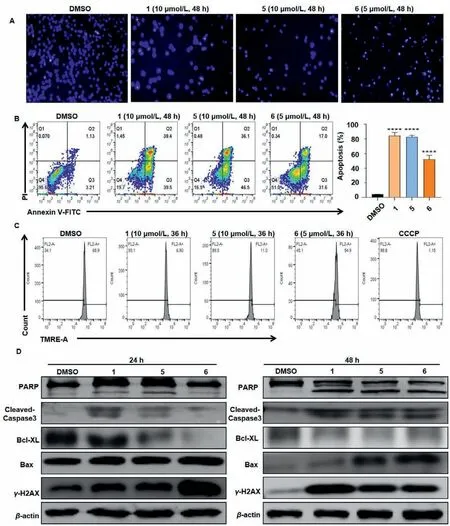
Fig.6.Compounds 1,5 and 6 induce mitochondria-mediated apoptosis in L1210 cells.After treated with compounds 1 (10 μmol/L),5 (10 μmol/L) and 6 (5 μmol/L),cell apoptosis was evaluated by DAPI staining (A) and Annexin V-FITC/PI staining kit followed by flow cytometer.Columns,means of three different experiments;bars,SD,***P<0.001 against DMSO (<0.1%) group (B).(C) Mitochondrial membrane potential of the cells was analysed with a TMRE staining kit after 36 h.(D) Western blot analysis of the apoptosis related proteins after 24 h and 48 h treatment.β-Actin was used as a loading control.
Cell proliferation,DNA damage and apoptosis are closely related to the cell cycle.Our results showed that compounds 1,5 and 6 treated for 24 h induced G0/G1 arrest in L1210 cells (Figs.7A and B).As arrest in the G0/G1 phase of cell cycle has been considered as a marker of senescent cells [28],we next detected cellular senescence with SA-β-gal staining in L1210 cells treated with compounds 1,5 and 6 in low concentrations,and all of them induced cellular senescence (Fig.7C).At the molecular level,treatments of 1,5 and 6 up-regulated the expression levels of checkpoint proteins involved in the regulation of the G1/S transition including cyclin D2,CDK2 and CDK6,and also up-regulated the expression levels of p16,p21 and p53,which are major upstream regulators of the G1/S cell-cycle checkpoint and cellular senescence (Fig.7D)[29].The above data suggested that compounds 1,5 and 6 induced G0/G1 arrest and senescence in L1210 cells and that p53,p21,and p16 may involve in this process.Taken together,compounds 1,5 and 6 inhibited proliferation,induced G0/G1 cell cycle arrest,senescence and mitochondria-mediated apoptosis in L1210 cells.
In conclusion,uncovered by the OSMAC and LC-MS-based molecular networking strategies,six novel phenylspirodrimane dimers and hybrids (1-6) were isolated and characterized from a modified large-scale culture of a coral-derived fungusS.chartarum.Structurally,compounds 1-6 represent a series of skeletally unprecedented dimeric phenylspirodrimanes by an unexpected linkage between C-18 and C-23′,simultaneously compounds 1-3 are uniquely defined by an unexpected 5-methyl-1,3-benzenediol moietyviaa carbon-carbon linkage.The mechanism of action investigation revealed that compounds 1,5 and 6 induced cell proliferation inhibition,G0/G1 phase cell cycle arrest and senescence,leading to mitochondria-mediated apoptosis in L1210 cells.Our current findings provide two novel classes of phenylspirodrimane dimers and hybrids as promising candidate molecules for hematological leukemia treatment research.
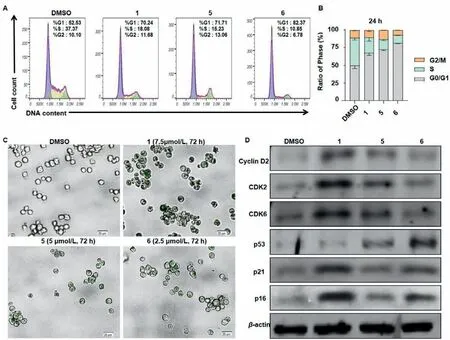
Fig.7.Compounds 1,5 and 6 induced G0/G1 cell cycle arrest and senescence in L1210 cells.(A,B) Effects of compounds 1,5 and 6 on cell cycle progressions of L1210 cells.After treated with compounds 1 (10 μmol/L),5 (10 μmol/L) and 6 (5 μmol/L) for 24 h,cell cycle distribution was analysed with PI staining and FACS.Columns,means of three different experiments;bars,SD,**P<0.01 vs. control group (DMSO<0.1%).(C) Compounds 1 (7.5 μmol/L),5 (5 μmol/L) and 6 (2.5 μmol/L) treated for 72 h induced senescence-associated β-galactosidase (SA-β-gal) activity in L1210 cells.Scale bars: 20 μm.(D) Western blot analysis of G0/G1 cell cycle and senescence related proteins after 48 h treatment.β-Actin was used as a loading control.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
We thank the Analytical and Testing Center at Huazhong University of Science and Technology for measuring ECD,IR,UV and single-crystal X-ray diffraction data.This project was financially supported by the National Natural Science Foundation for Distinguished Young Scholars (No.81725021),the Innovative Research Groups of the National Natural Science Foundation of China (No.81721005),the National Natural Science Foundation of China (No.81573316),the Fundamental Research Funds for the Central Universities (Nos.2020kfyXJJS083,2021yjsCXCY094 and 2172019kfyXJJS166),the Tongji-Rongcheng Center for Biomedicine,Huazhong University of Science and Technology (No.0231514141),the Research and Development Program of Hubei Province (No.2020BCA058),and the Hubei Provincial Natural Science Foundation of China (No.2019CFB646).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.064.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
